Abstract
Purpose
The purpose of this study was to determine whether there is an association between the critical shoulder angle (CSA) and rotator cuff tears (RCT) as well as osteoarthritis (OA), and to compare the reproducibility and accuracy of CSA values as measured with radiograph and MRI.
Methods
Based on a pre hoc power analysis, three independent reviewers measured CSAs for three groups of 10 age-matched patients each (RCT group, OA group, and non-RCT/non-OA group) using radiographs and MRIs. Inter- and intra-observer agreements of CSAs were determined with intraclass correlation coefficients. ANOVA analysis was performed.
Results
Interobserver and intra-observer agreements on radiographs were 0.87 (95 % CI 0.78–0.93) and 0.91 (95 % CI 0.82–0.96), respectively. Significant differences in mean CSAs by radiographs were found between the groups (RCT 37.3° ± 2.6°; OA 28.7° ± 2.2°; non-RCT/non-OA 32.7° ± 2.5°; p < 0.001). CSAs obtained from MRI scans had interobserver agreement of 0.62 (95 % CI 0.43–0.78) and intra-observer agreement of 0.68 (95 % CI 0.50–0.80). There was a significant correlation between the patients’ pathologies and CSAs measured by radiographs (r = 0.44; p = 0.02) and MRI (r = 0.50; p = 0.01). A significant difference in mean CSAs measured on radiograph versus MRI was seen in the OA group (28.7° ± 2.2° vs. 31.3° ± 4.4°; p = 0.01).
Conclusion
CSA measurements obtained from radiographs demonstrated excellent interobserver agreement with less variability than CSAs from MRI, especially in OA patients. This study confirms an association between the CSA and RCTs as well as OA pathology. Assessing the CSA may be helpful during diagnostic evaluation of patients with shoulder pain and can help predict pathology.
Level of evidence
Retrospective cohort study, Level IV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pathogenesis of rotator cuff tears (RCT) and glenohumeral osteoarthritis (OA) has many intrinsic (genetic) and extrinsic (anatomical) contributing factors, but the diseases are rarely seen in combination. Several extrinsic factors have been proposed in the development of RCT and OA, such as the anterior acromion morphology, the lateral acromial angle, the coracohumeral interval, the glenoid inclination, and most recently the acromion index (AI) [3, 4, 11, 12, 17–19]. Intrinsic factors that may lead to rotator cuff tears are tissue degeneration due to alterations in metabolism and oxidative stress-related change [6, 10], which may play a role in the development of RCTs. Therefore, RCT may have both intrinsic and extrinsic elements [7]. In contrast, the cause of primary glenohumeral OA is largely unknown [2].
Recently, the effect of lateral extension of the acromion in relation to the humeral head, defined as the AI, has been studied in its relationship to rotator cuff disease [19]. Some authors have found a significant association between high AI values and full-thickness RCTs [12, 19, 23], while others have found no association [1, 9]. Miyazaki et al. [15] reported that the AI has a high degree of variability and was associated with RCTs only in certain ethnic groups. Ames et al. [1] indicated that high AI values can affect outcomes after arthroscopic cuff repair and may lead to technical difficulties during cuff repairs because of the large acromion lateral offset.
Recently, Moor et al. [16] introduced the concept of the critical shoulder angle (CSA), which is defined as the angle between the plane of the glenoid fossa and the connecting line to most inferolateral point of the acromion measured on anteroposterior (AP) shoulder radiographs. The authors found that smaller CSA values were associated with OA and larger CSA values with RTCs. Based on these published heterogeneous AI studies, it seemed prudent to demonstrate the reproducibility of the CSA measurement in patients with RCTs and with OA. Furthermore, since both radiographs and MRIs are commonly used to evaluate shoulder pathology [14], comparing the diagnostic accuracy of the CSA when using both imaging modalities may have clinical applications. So far, no study has used MRI for measuring the CSA.
Therefore, the purpose of this study was to determine whether there is an association between the CSA and RCT as well as OA. Additionally, a secondary objective was to compare the reproducibility and accuracy of CSA values when measured on radiographs and MRIs.
Our hypotheses included: First, there is a strong association between CSA values, cuff disease, and OA. Second, the reproducibility and accuracy of CSA values measured by radiograph will be excellent. Finally, the reproducibility and accuracy of CSA values measured by MRI will outperform the reproducibility seen by radiograph.
Materials and methods
In this study, matched-pair cohort study, an a priori power analysis based on the data reported by Moor et al. [16] was performed. Inclusion criteria were age between 40 and 60 years, isolated non-traumatic full-thickness RCTs, isolated non-traumatic OA, and patients with non-RCT and non-OA pathologies (Table 1). Since the goal was to examine anatomical factors related to specific developmental disease states, exclusion criteria were traumatic RCTs, post-traumatic OA, prior surgery, history of shoulder dislocation or subluxation, missing preoperative true AP radiographs, and missing preoperative shoulder MRIs (including low-quality MRIs <1.5 T). Additionally, patients with a combination of both RCT and OA pathologies were excluded. Three age-matched groups of 10 patients each were selected from our data registry with group 1: a non-traumatic full-thickness RCT group, group 2: a non-traumatic and non-inflammatory OA group, and group 3: a non-RCT/non-OA pathology group (Table 1). The size of the RCT was determined using the classification of DeOrio and Cofield [5], the level of tendon retraction was defined using the classification of Patte [21], and the grade of OA was graded using the classification of Outerbridge [20] as documented by the surgeon at the time of surgery.
The mean time between MRI and surgery was 64 days (range 1–161 days), and between radiograph and surgery was 37 days (range 1–161 days). The majority of radiographs and MRIs were within 30 days of surgery. Patients of the non-RCT/non-OA pathology group had mainly biceps pathologies, SLAP tears, or Bankart lesions (Table 1). This group was termed as a negative control and included patients with minor traumatic injuries that were defined as low impact falls with or without glenohumeral dislocations. Patient’s injury history was evaluated with chart review.
All patients underwent standardised preoperative radiographs and a standard clinical 3-T MRI of the shoulder, using a 3.0 T Siemens Magnetom Verio MRI (Siemens Medical Solutions, Erlangen, Germany) and 4-channel dedicated shoulder coil, with the arm positioned in neutral rotation by the patient’s side. The standard shoulder MRI protocol included oblique coronal, oblique sagittal, and oblique axial images oriented to the axes of the glenohumeral joint. Standardised radiographs were taken in all patients including an AP view in standing position and neutral arm rotation by the patient’s side. The AP view was consistently performed perpendicular to the plane of the scapula, so that a true view of the glenohumeral joint could be seen.
Measurement of the critical shoulder angle
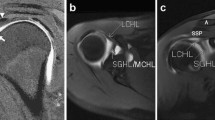
The CSA was calculated using the technique described by Moor et al. [16]. On the AP radiograph, the angle between the glenoid and the lateral border of the acromion was measured (Fig. 1). For the MRIs, the measurements were taken using the coronal–oblique T1 or proton density-weighted image. The MRI slice which depicts the most lateral acromion border was selected. The lateral border of the acromion was marked with the cursor (Fig. 2a). Next, the MRI slice which runs through the centre of glenoid was selected, and the measurement was completed (Fig. 2b). CSA measurements were taken to one decimal place and were taken between April and May 2013 using Stryker OfficePACS Power 4.1 Express Edition (Kalamazoo, MI).
Coronal–oblique proton density-weighted fat-suppressed MRI image of the shoulder showing the CSA defined by MRI. Frequently, the most lateral extent of the acromion is located dorsally of the central glenoid plane. Therefore, the slice presenting the most lateral extent of the acromion is determined and marked with the cursor (a). After scrolling to the plane that includes the acromion centre, the critical shoulder angle is measured (b)
CSA measurement validity
The reproducibility of the CSA measurement was examined with the intraclass correlation coefficient for both interobserver reliability (measurements taken by three different observers) and intra-observer reliability (measurements repeated at different points in time by the same observer) for repeated measurements. For all thirty patients, three independent observers, one board-certified radiologist (WSS), and two board-certified orthopaedic surgeons (PJM, UJS) calculated the CSA for both radiograph and MRI. In addition, for intra-observer reliability testing, the measurements were repeated after a 2-week interval, with the patient order randomised. This study was IRB (Institutional Review Board) approved by the Vail Valley Medical Center IRB, number 2002–2003.
Statistical analysis
Sample size calculation was done using a priori power analysis based on the data reported by Moor et al. [16]. With a mean difference of 5° between the groups and standard deviation of 3.3 (ranged from 2.1 to 3.3), a minimum of eight patients per group was needed to achieve statistical power of 0.80 to detect significant differences in the CSA measured on radiograph across all three study groups. From that calculation, the authors decided to include 10 patients per group. The Statistical Package version 17.0 (SPSS) was used (SPSS, Chicago, Illinois). Inter- and intra-observer agreements were measured with the use of the two-way mixed single-measures intraclass correlation coefficient with 95 % confidence intervals reported (95 % CI). The CSA values on both radiograph and MRI measurements were normally distributed. Comparisons of the CSA, pathology groups, and the diagnostic tools were performed with an independent t test using the measurements taken by one observer (UJS). Pearson’s correlation analysis (R) was used for comparison of CSA values and the associated pathologies and RCT sizes with a level of significance p < 0.05. The CSA thresholds (RCT >35°; OA <30°), defined by Moor et al. [16], were used to distinguish the RCT, OA, and non-RCT/non-OA pathology group. The level of significance was set at p < 0.05.
Results
Inter- and intra-observer reliabilities
The CSAs values measured by radiograph had high agreement [13], both for the interobserver [0.869 (95 % CI 0.776–0.930)] and intra-observer [0.909 (95 % CI 0.818–0.956)] correlation coefficients. The CSAs measured by radiograph, for all groups, are shown in Fig. 3. There were significant differences in the mean CSAs among the three groups (p < 0.001).
Box plot representing the average CSA, evaluated by radiograph, per group. The horizontal line indicates the median, the box extends from the 25th to the 75th percentile, and the bars indicate the largest and smallest observed values. RCT rotator cuff tear group, OA osteoarthritis group, and Non-RCT/Non-OA non-rotator cuff and non-osteoarthritis pathology group
In contrast, the MRI measurements showed greater variability (Fig. 4). The interobserver agreement was moderate [0.622 (95 % CI 0.427–0.780)], whereas the intra-observer agreement was poor [0.534 (95 % CI 0.221–0.747)]. The mean CSAs showed a significant difference between the patients with RCTs and the patients with OA and non-RCT/non-OA pathology (p < 0.001 and p < 0.001, respectively), whereas there was no significant difference among patients with OA and non-RCT/non-OA pathology (p = n.s.). There was a significant correlation between the patients’ pathologies and both CSAs measured by radiograph (r = 0.44; p = 0.02) and MRI (r = 0.50; p = 0.01). There was no significant correlation between CSAs and RCT sizes (r = 0.39; p = n.s.) and tendon retraction (r = 0.52; p = n.s.). By excluding patients with RCT, a significant correlation between the pathologies and the CSAs was only seen when the measurement was taken by radiograph (r = 0.69; p = 0.01) and not by MRI (r = 0.07; p = n.s.). No statistical differences were seen between the CSA values of men and women (mean male CSA = 32.8°; mean female CSA = 33.4°; p = n.s.).
Box plot representing the average CSA, evaluated by MRI, per group. The horizontal line indicates the median, the box extends from the 25th to the 75th percentile, and the bars indicate the largest and smallest observed values. RCT rotator cuff tear group, OA osteoarthritis group, and Non-RCT/Non-OA non-rotator cuff and non-osteoarthritis pathology group
Radiographs versus MRI
Comparison of CSAs measured by radiograph versus MRI showed no significant differences in the RCT and non-RCT/non-OA pathology group, 37.3° ± 2.6° versus 36.4° ± 1.9° (p = n.s.) and 32.7° ± 2.5° versus 31.8° ± 2.7° (p = n.s.), respectively. In contrast, a significant difference was observed in the OA group, 28.7° ± 2.2° by radiograph versus 31.3° ± 4.4° by MRI (p = 0.01). The CSAs by MRI in OA cases had a wide distribution of values, far above the 30° threshold (Fig. 5).
Scatter depicts the CSA of all patients with OA in correlation with their CSA. The CSA measured by radiograph is at the top row, and those evaluated by MRI is at the bottom row. The threshold of 30° is marked by a reference line. All values of the CSAs above the threshold are labelled with red colour
Discussion
The most important finding of the present study was the strong association between the CSA and RCT and OA. This finding supports our first hypothesis that larger CSA values were significantly correlated with full-thickness RCT, while small CSA values were significantly correlated with OA, which reaffirms previous work by Moore [16]. In support of our second hypothesis, both the inter- and intra-observer reliabilities of the radiographs were excellent. The MRI showed similar trends but was not as reliable. Therefore, our third hypothesis was rejected.
Several studies have examined the impact of anatomical variations in the shoulder as risk factors for the development of RCT or OA. The AI and the glenoid inclination have been two of the more common ones studied [1, 8–10, 14, 19]. Both models share the same biomechanical theories on the pathogenesis of RCT and OA [12, 19], whereby the deltoid has either a greater upward force resulting in RCTs or a greater compressive force leading to OA. With a high CSA, the upward pressure of the humeral head on the overlying rotator cuff tendons contributes to atraumatic RCTs. Nyffler et al. [19] supported their theory by finding significant higher values of the AI in patients with RCT as compared to patients without rotator cuff pathology. Hughes et al. [11] also showed that a higher upward force could be caused by an increased inclination of the glenoid, leading to a reduced ability of the glenoid to resist the deltoid contraction force. In support of this theory, the authors found significant increases in glenoid inclination in eight specimens with RCTs compared to the uninjured contralateral side. Both theories of an increased upward force of the humeral head are logical from a biomechanical point of view, leading to the assumption that both theories may play roles in the pathogenesis of RCTs. The different mechanisms might explain the variability of the association of the AI on the detection of RCTs across different ethnic groups [15].
Similarly, the theory of the pathogenesis of glenohumeral OA can be explained by similar biomechanical models [11, 19]. It is conceivable that a smaller lateral extension of the acromion, which leads to a lower CSA, results in a higher compressive force component of the deltoid muscle. This compressive force may lead to excess loading on the glenohumeral joint and subsequent OA [16]. Moreover, an inferiorly inclined glenoid can contribute to a lower CSA. The presence of an inferiorly inclined glenoid can lead to higher deltoid compressive forces on the glenohumeral joint. These higher compressive forces can contribute to the development of OA.
Interestingly, Ames et al. [1] were unable to find any significant association between the AI and the size of full-thickness RCTs but reported a positive association between large acromial indices and the number of tendons torn. Additionally, they found higher preoperative disabilities scores in their patients with full-thickness RCTs and high acromion indices. Furthermore, Ames et al. [1] postulated that a high AI increases the technical challenge associated with a cuff repair and argued that a large lateral extension of the acromion could interfere with anchor position as the acromion could effectively block the ideal anchor placement. They described a technique for applying inferolateral traction to the affected arm to attempt to overcome this. Moreover, they concluded that the AI was a useful tool that may assist in treating physician in these cases. However, in this study, the glenoid inclination was not taken into consideration.
The CSA, described by Moor et al. [16], combines both these anatomical models, the lateral extent of the acromion and the glenoid inclination. These factors may be additive or may even have synergistic effects on disease development and progression. In support of this, Moor et al. [16] showed significant associations between high CSA values of more than 35° and RCTs as well as low CSA values of <30° and OA, with high interclass correlation coefficients. Our results confirm their findings in all aspects, including the excellent interclass correlation and the significant associations between large CSA values and RCTs and between low CSA values and OA. Additionally, the threshold values of the CSA are consistent across both studies. Thus, by incorporating both independent morphologic models, the AI and the glenoid inclination, the risk of developing OA or RCTs might be evaluated predictably in the future.
Since CSA evaluation is dependent on the radiograph quality and technique, additional measurements of the CSA by the more sophisticated MRI image were obtained to determine whether they would be more reliable. We speculated that by using a three-dimensional shoulder imaging technique, we would reduce sources of error from conventional radiographs, such as arm malrotation or other patient positioning issues. It has been shown that a malrotation in the conventional radiographs exceeding 20° leads to substantial overlap between the anterior and posterior glenoid rims, decreasing the reproducibility [16]. By incorporating the MRI in the evaluation of the CSA, we hypothesised that it might be possible to further improve the diagnostic accuracy.
However, the CSA measured by MRI showed a higher variability and lower correlation, particularly in cases of glenohumeral OA. Moreover, the interobserver reliability was moderate, and the intra-observer reliability was poor. High CSAs correlated significantly with RCT when measurements were taken with both radiograph and MRI. In contrast, a significant correlation between low CSA values and glenohumeral OA was only seen when the measurements were taken by radiograph. Many factors may contribute to the greater variability of the CSA measures by MRI. The MRI coronal images are cross-sectional tomographic oblique coronal images taken along the axis of the glenohumeral joint. The inferior glenoid margin and lateral acromial margin needed for the measurement of CSA may typically be on different slices that are oblique to each other in the anterior-to-posterior axis. In contrast, AP radiographs are projections with both the inferior glenoid and lateral acromial margins fixed in the same projection. Evaluating CSA by comparing the inferior glenoid and lateral acromial margins on different obliquely orientated MRI slices compared with a single AP projection radiograph would inherently be subject to higher inter- and intra-observer variabilities.
Due to disadvantages of MRI (especially with fat-suppressed MRI) in bone imaging of the glenoid compared to radiographs and CT [22], the upper and lower borders of glenoid in cases of OA were difficult to define in many cases (Fig. 6), contributing to high inter- and intra-observer variabilities and poor sensitivity for the detection of OA when measuring the CSA by MRI. Additionally, similar to evaluation by radiographs, evaluation of CSA by MRI is also dependent on image quality and technique including, MRI slice orientation, and patient positioning.
Future studies are warranted to evaluate the causality of various shoulder pathologies. Presently, it is unknown as to whether the anatomical factors are stable creating a CSA that leads to the pathologic processes of RCT or OA, or whether the pathologies themselves might lead to dynamic remodelling processes that result in the various CSA values. Just recently, Gerber et al. [8] reported of inferior long-term results in patients with large CSAs after latissimus dorsi tendon transfers for the treatment of irreparable rotator cuff tears. Surgical procedures which decrease the CSA, such as inferolateral acromial resection, lateral acromioplasties, advancement techniques, or glenoid osteotomies, could also be developed theoretically to prevent progression of RCTs or OA.
Several limitations of this study have to be mentioned. The first limitation was that the power analysis was done based on the data reported by Moor et al. [16], which only included radiograph measurements. Additionally, patient history was evaluated using a prospective registry and chart review. While this might be associated with recall bias, it seems unlikely that any traumatic causes were included. A further limitation might be the time delay between MRI and radiograph and surgery. Thus, the real CSA values on surgery day may have been different than the measured ones leading to statistical bias. However, since the time period was <6 months in all cases and usually <30 days, bony morphological changes would not be expected. Another limitation was that scapular rotation, which might influence the CSA, was not considered in this study for practical reasons. The MRIs and all radiographs were performed with a standardised neutral arm position to minimise differences in scapular rotation. Another consideration is that the CSA only considers the coronal force couple, the transverse force couple is not considered in the described biomechanical theories. Anatomical variations in the axial plane are not measured by the CSA. However, both pathologic transverse force couples and pathologies of the axial plane have been associated with OA. There are many factors that could influence the development of OA or cuff disease, which could lead to a false assumption of causality between CSA values and RCT and OA. However, the results are similar to the results published by Moor et al. [16]. When results are independently duplicated, it is greater support for a specific scientific hypothesis. While CT scan might be more accurate for bone morphology, it remains debatable if it is worthwhile to perform CT scanning to measure the CSA due to the radiation exposure risks and increase in costs.
Assessing the CSA can be helpful during diagnostic evaluation of patients with shoulder pain and can help predict pathology. Addressing the bony morphology of the acromion by performing a lateral decompression or lateral resection in cases of rotator cuff tears or cases of impingement with high CSA might be an additional tool in the future. However, further research is warranted.
Conclusions
CSAs measurements obtained from radiographs demonstrated excellent interobserver agreement with less variability than CSAs from MRI, especially in OA patients. This study confirms an association between the CSA and RCTs as well as OA pathology. Assessing the CSA may be helpful during diagnostic evaluation of patients with shoulder pain and can help predict pathology.
References
Ames JB, Horan MP, Van der Meijden OA, Leake MJ, Millett PJ (2012) Association between acromial index and outcomes following arthroscopic repair of full-thickness rotator cuff tears. J Bone Joint Surg Am 94:1862–1869
Bhatia S, Hsu A, Lin EC, Chalmers P, Ellman M, Cole BJ, Verma NN (2012) Surgical treatment options for the young and active middle-aged patient with glenohumeral arthritis. Adv Orthop 2012:1–8
Bigliani LU, Ticker JB, Flatow EL, Soslowsky LJ, Mow VC (1991) The relationship of acromial architecture to rotator cuff disease. Clin Sports Med 10:823–838
Davidson PA, Elattrache NS, Jobe CM, Jobe FW (1995) Rotator cuff and posterior-superior glenoid labrum injury associated with increased glenohumeral motion: a new site of impingement. J Shoulder Elbow Surg 4:384–390
DeOrio JK, Cofield RH (1984) Results of a second attempt at surgical repair of a failed initial rotator-cuff repair. J Bone Joint Surg Am 66:563–567
Drӧge W (2003) Oxidative stress and aging. Adv Exp Med Biol 543:191–200
Funakoshi T, Iwasaki N, Kamishima T, Nishida M, Ito Y, Kondo M, Minami A (2010) In vivo visualization of vascular patterns of rotator cuff tears using contrast-enhanced ultrasound. Am J Sports Med 38:2464–2471
Gerber C, Rahm SA, Catanzaro S, Farshad M, Moor BK (2013) Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears: long-term results at a minimum follow-up of 10 years. J Bone Joint Surg Am 95:1920–1926
Hamid N, Omid R, Yamaguchi K, Steger-May K, Stobbs G, Keener JD (2012) Relationship of radiographic acromial characteristics and rotator cuff disease: a prospective investigation of clinical, radiographic, and sonographic findings. J Shoulder Elbow Surg 10:1289–1298
Hashimoto T, Nobuhara K, Hamada T (2003) Pathologic evidence of degeneration as a primary cause of rotator cuff tear. Clin Orthop Relat Res 415:111–120
Hughes RE, Bryant CR, Hall JM, Wening J, Huston LJ, Kuhn JE, Carpenter JE, Blasier RB (2003) Glenoid inclination is associated with full-thickness rotator cuff tears. Clin Orthop Relat Res 407:86–91
Kim JR, Ryu KJ, Hong IT, Kim BK, Kim JH (2012) Can a high acromion index predict rotator cuff tears? Int Orthop 36:1019–1024
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Lenza M, Buchbinder R, Takwoingi Y, Johnston RV, Hanchard NC, Faloppa F (2013) Magnetic resonance imaging, magnetic resonance arthrography. Cochrane Database Syst Rev 24(9):CD009020
Miyazaki AN, Itoi E, Sano H, Fregoneze M, Santos PD, da Silva LA, Sella Gdo V, Martel EM, Debom LG, Andrade ML, Checchia SL (2011) Comparison between the acromion index and rotator cuff tears in the Brazilian and Japanese populations. J Shoulder Elbow Surg 20:1082–1086
Moor BK, Bouaicha S, Rothenfluh DA, Sukthankar A, Gerber C (2013) Is there an association between the individual anatomy of the scapula and the development of rotator cuff tears or osteoarthritis of the glenohumeral joint? A radiological study of the critical shoulder angle. Bone Joint J 95-B:935–941
Neer CS 2nd (1972) Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. J Bone Joint Surg Am 54:41–50
Nove-Josserand L, Edwards TB, O’Connor DP, Walch G (2005) The acromiohumeral and coracohumeral intervals are abnormal in rotator cuff tears with muscular fatty degeneration. Clin Orthop Relat Res 433:90–96
Nyffeler RW, Werner CM, Sukthankar A, Schmid MR, Gerber C (2006) Association of a large lateral extension of the acromion with rotator cuff tears. J Bone Joint Surg Am 88:800–805
Outerbridge RE (1961) The etiology of chondromalacia patellae. J Bone Joint Surg Br 43-B:752–757
Patte D (1990) Classification of rotator cuff lesions. Clin Orthop Relat Res 254:81–86
Steinbach LS (2008) MRI of shoulder instability. Eur J Radiol 68:57–71
Zumstein MA, Jost B, Hempel J, Hodler J, Gerber C (2008) The clinical and structural long-term results of open repair of massive tears of the rotator cuff. J Bone Joint Surg Am 90:2423–2431
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spiegl, U.J., Horan, M.P., Smith, S.W. et al. The critical shoulder angle is associated with rotator cuff tears and shoulder osteoarthritis and is better assessed with radiographs over MRI. Knee Surg Sports Traumatol Arthrosc 24, 2244–2251 (2016). https://doi.org/10.1007/s00167-015-3587-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-015-3587-7