Abstract
Purpose
To evaluate magnetic resonance imaging (MRI) graft signal intensity after allograft double-bundle (DB) anterior cruciate ligament (ACL) reconstruction and determine the relationship between signal intensity and time from surgery.
Methods
Twenty-six patients with an intact graft on MRI after anatomic allograft DB ACL reconstruction up to 1 year post-operatively were included. All subjects underwent post-operative MRI using a 1.5-T magnet. Sagittal proton density-weighted images (PDWI) and sagittal T2-weighted images (T2WI) were analysed. Using the region-of-interest (ROI) function on imaging software, the anteromedial (AM) and posterolateral (PL) bundles of the graft and the posterior cruciate ligament (PCL) were outlined. Mean signal intensity of the three ROIs were recorded as absolute signal intensity. Signal intensity (SI ratio) was calculated based on the signal intensity of the PCL. Correlation coefficients were calculated to determine the relationship between signal intensity and time from surgery.
Results
SI ratio of the PL bundle was higher than that of the AM bundle for both the PDWI (1.7 ± 1.5 vs. 2.5 ± 1.7, p < 0.05) and T2WI (1.3 ± 0.4 vs 1.6 ± 0.6, p < 0.05). There were weak correlations between AM SI ratio and time from surgery (r = 0.38, p < 0.05 on PDWI), and moderate correlations between PL SI ratio and time from surgery (r = 0.43, p < 0.05 on PDWI) (r = 0.44, p < 0.05 on T2WI).
Conclusions
The PL bundle displayed increased signal intensity compared to the AM bundle and based on previous studies may indicate a longer healing process. Plain MRI may be useful to assess graft healing after ACL reconstruction.
Level of evidence
Retrospective case series, Level IV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anterior cruciate ligament (ACL) rupture is one of the most frequent types of knee injuries with a yearly incidence of 8 out of 10,000 people [7]. Recently, there has been a push for a better understanding of the complex healing process of the graft after ACL reconstruction to provide more effective care to our patients [6–8]. Graft rupture after ACL reconstruction continues to be of concern to the patient and surgeon; however, no study has scientifically defined the optimum time to return to activity following ACL reconstruction. In a recent review of graft failure after anatomic ACL reconstruction with allograft, van Eck et al. [31] found that most failures occurred 6 to 9 months after surgery, which corresponds with the time most patients are released to return to sports participation. Additionally, several studies have shown an increased risk of graft rerupture in younger patients after allograft ACL reconstruction [28, 30].
Magnetic resonance imaging (MRI) is an established and widely used imaging modality to identify soft tissue pathologies of the knee joint including ACL injuries or to assess outcome after ACL reconstruction including assessment of the graft impingement [19], rotatory laxity [35] and cartilage and meniscus degeneration [20]. MRI graft signal intensity following ACL reconstruction has previously been thought of as a marker of graft healing and maturation. Recent studies have reported vastly different data on graft signal intensity and graft hetero-/homogeneity after ACL reconstruction. Furthermore, no consensus is found on graft visibility and prediction of graft maturation using plain MRI or enhanced imaging technologies [2, 4, 10–14, 23–25, 28–30, 34]. In addition, few studies have investigated the maturation of the ACL graft using MRI in clinical studies [1, 7, 9, 10, 21, 27]. Following ACL reconstruction, little is currently known about the changes in graft signal intensity and whether there is relationship between signal intensity and time from surgery. Also, no study has assessed the differences between signal intensity in the anteromedial (AM) and posterolateral (PL) bundles after anatomic double-bundle (DB) ACL reconstruction.
The purpose of this study was to determine graft signal intensity after anatomic DB ACL reconstruction with allograft on plain MRI and to determine the relationship between graft signal intensity and time from surgery. The results of this study could provide important information regarding graft healing status. We hypothesize that there would be a decrease in signal intensity in the first year after ACL reconstruction. We also hypothesize that there will be a difference in signal intensity between the AM and PL bundles, indicating different maturation rates for each bundle.
Materials and methods
This cohort study included patients who underwent anatomic DB ACL reconstruction and who had post-operative MRIs after allograft ACL reconstruction with the same 1.5-T MRI magnet between 2007 and 2010. Patients with evidence of a graft rupture at the time of the MRI, concomitant knee injuries and/or MRI which used different sequence parameters were excluded. All subjects had undergone MRI of the knee using a 1.5-T open-bore magnet (GE signa, GE Healthcare, USA), and standard sagittal images were used for analysis. Twenty-six consecutive patients met these inclusion criteria (15 male, 11 female; mean age, 25.5 ± 11 years; range, 15–49 years) and were included in the analysis. Different allograft sources were used in this study group. In 18 cases, the tibialis anterior tendons (TA) were used, in one case a tibialis posterior tendon (TP), one semitendinosus tendon (ST) and one Achilles tendon, respectively. There were combinations of graft types such as combination of TA/TP in two cases and TA/ST in two cases.
Measurement of signal intensity
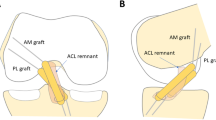
Proton density-weighted images (PDWI) and T2-weighted images (T2WI) with 3-mm slice thickness were used for analysis. The repetition time was 2,000–2,223.2 and 4,040–5,190 ms and echo time was 18 and 95 ms for PDWI and T2WI, respectively. Images were obtained from the Stentor imaging system. Using the sagittal images, the best one slice that demonstrated the AM bundle and the best one slice that demonstrated the PL bundle were selected for analysis. Images were saved as JPEG-images in a deidentified manner. Using the region-of-interest (ROI) function on ImageJ (NIH 1.43, USA), the AM and PL bundles of the ACL-graft and the native posterior cruciate ligament (PCL) were outlined using the ROI function (Fig. 1). Mean signal intensity and standard deviations were recorded based of image pixels as absolute signal intensity (absolute SI) with a measurement accuracy of one decimal. Using the absolute SI as numerator, we calculated the signal intensity ratio (SI ratio) as the ratio of absolute SI to the signal intensity of the PCL.
To assess inter-observer reliability, the images were independently measured in a blinded fashion by 2 observers, both being orthopaedic sports medicine research fellows (M.M. and D.H.). To assess intra-observer reliability, one observer (M.M.) made all measurements twice 8 weeks apart.
Exempt IRB approval was obtained for this study to use data from an IRB-approved research registry (University of Pittsburgh, Institutional Review Board, IRB-Number: PRO07090066). Informed consent was obtained to enrol subjects in the research registry, but additional informed consent was not required to include subjects in the current exempt study.
Statistical analysis
Pearson correlation coefficients were used to determine the relationship between the absolute SI and SI ratio in the AM and PL bundles with the time from surgery. Paired t tests were used to compare the absolute SI between the AM and PL bundles. Statistical significance was defined as p < 0.05. Intra-class correlation coefficients including 95 % confidence interval [95 % CI] were calculated to determine inter-and intra- observer reliability. All data were analysed using SPSS version 16 (SPSS Inc, Chicago, Illinois, USA).
Results
The intra-tester reliability and inter-tester reliability for the measurements of absolute signal intensity for the AM and PL bundles and PCL were strong; however, the reliability for the ratio of the signal intensity of the AM or PL bundles to the PCL was lower (see Table 1).
Absolute SI of the PL bundle was higher than that of the AM bundle for the PDWI (34.5 ± 15.8 vs. 23.4 ± 13.9, p < 0.05), and SI ratio of the PL bundle was higher than that of the AM bundle for both the PDWI (1.7 ± 1.5 vs. 2.5 ± 1.7, p < 0.05) and T2WI (1.3 ± 0.4 vs. 1.6 ± 0.6, p < 0.05) (Table 1, Fig. 2 ).
There were moderate correlations between time from surgery and absolute SI of AM (r = 0.61, p < 0.05) and PL (r = 0.64, p < 0.05) on PDWI, and PL (r = 0.51, p < 0.05) on T2WI, and a weak correlation with the absolute SI of AM (r = 0.45, p < 0.05) on T2WI. There were weak correlations between time from surgery and the AM-SI (r = 0.38, p < 0.05) and PL–SI ratios (r = 0.43, p < 0.05) on PDWI. There was no correlation between time from surgery and the AM-SI ratio, but a weak correlation with the PL–SI ratio (r = 0.44, p < 0.05) on T2WI. Examples of SI ratio are shown in Fig. 3.
Discussion
The most significant results of this study are that there is a weak to moderate correlation between absolute SI of the graft and time from ACL reconstruction within first year following surgery, indicating increased graft signal intensity with increasing time from surgery for up to 1 year. This finding did not support our hypothesis that signal intensity would decrease within 1 year after surgery. It was also found that the PL bundle has higher absolute SI and SI ratio than the AM bundle on plain MRI. To our knowledge, this is the first study to measure signal intensity of each bundle following anatomic DB ACL reconstruction with a simple objective method and to establish the relationship between signal intensity and time from surgery.
Previous studies have demonstrated that the graft undergoes a process of revascularization during the maturation process [5, 6, 8, 10, 17, 25, 27, 32, 33]. Weiler et al. compared graft signal intensity as a marker of revascularization and morphologic characteristics on MRI with biomechanical and histological parameters in an animal study with autografts [31, 33]. They found a relationship between time from ACL reconstruction and signal intensity using the signal-to-noise quotient (SNQ), with higher signal intensity on MRI correlating with decreased biomechanical properties of the graft during early phase of remodelling. Furthermore, there was a negative linear correlation between SNQ and load to failure, stiffness and tensile strength of the graft. They found maximal signal intensity 3 months after surgery, and signal intensity decreased starting 6 months after surgery and reached a minimum 2 years after surgery. They concluded that MRI signal intensity might be a useful tool to observe the graft remodelling process. Recently, Biercevicz et al. studied the correlation between 3D volume/grayscale value (signal intensity) of allograft on T2* WI and structural properties in porcine model [3, 28, 30]. They found volume and grayscale from T2* MRI was predictive of structural properties of the healing ligament or graft. Human studies on the relationship between graft signal intensity and structural integrity of the graft cannot feasibly be done, and therefore, animal studies provide us with the best evidence at this time.
The current study found that there was increasing absolute SI and SI ratio on MRI up to 12 months after anatomic DB ACL reconstruction with allograft, and therefore, based on the results of Weiler et al. and Biercevicz et al., there continues to be a structurally weakened allograft that may be at increased risk for failure compared to autograft.
Ntoulia et al. studied autologous bone–tendon–bone ACL graft healing process with contrast-enhanced MRI for up to two years following surgery. They evaluated axial images perpendicular to the ACL graft and found that the amount of revascularization tissue influences the MRI characteristics of the graft and the time interval after surgery. They found that reduced vascularity was associated with the end stages of healing and maturation which coincides with the homogeneously low signal intensity of the graft by 2 years post-operatively [2, 4, 10–14, 23–25, 27–30, 34].
The current study also found a difference between the absolute SI of the AM and PL bundles, with a higher absolute SI of the PL bundle compared to the AM bundle. Theoretically, the most likely reason for this finding is that the two bundles are exposed to different mechanical loads as the AM bundle is under tension throughout the physiologic range of motion of the knee, whereas the PL bundle is tight in extension and loose in flexion. Therefore, the potential exists for healing at different rates, with different stages of revascularization [16]. Another possible reason for these findings might be the difference in MRI sectioning based on the oblique intra-articular trajectory of the PL bundle. Kiekara et al. [18] reported that PL graft courses obliquely in all standard MRI sequences, leading to the possibility of volume-averaging the PL graft signal with surrounding intermediate-signal material.
Muramatsu et al. [26] compared post-operative SNQ of allografts and autografts and found that allografts show a significantly lower SNQ during 1 year after ACL reconstruction when compared to autografts, indicating a slower onset and rate of revascularization of allografts. Furthermore, they reported that the SNQ of allografts increases until 18–24 months after surgery, whereas the SNQ of autografts peaked at 4–6 months. Li et al. [21] also evaluated signal intensity of the intra-articular graft and found that allografts had significantly higher SNQ compared with that of autografts at 30 months after surgery. They concluded that allografts might have inferior graft maturity compared with autograft. Although this study only looked at allograft ACL reconstruction, the results are consistent with the literature of continued hyper-intense signal intensity up to 12 months. In the present study, there was no peak in absolute SI and SI ratio for both the AM and the PL bundle, and continued follow-up MRIs are planned in this patient population to continue to evaluate changes in the allograft signal intensity over time.
There are several limitations of this study. First, we evaluated signal intensity of different patients at different time points. Longitudinally following patients with serial MRIs after ACL reconstruction would provide the best method of evaluating changes in graft signal intensity over time; however, this is challenging from a clinical standpoint due to the time and cost associated with such a protocol. Secondly, we had a small sample size secondary to exclusion of patients with different MRI sequence parameters and the small number of patients receiving clinically relevant post-operative MRI. Thirdly, clinical MRI data were only available for short follow-up, less than 1 year, and we could only investigate the phase where the graft signal intensity is increased. Ideally, patients would be followed longitudinally over the course of time to determine when the signal intensity rises, plateaus and falls. If our subjects were followed over a longer period of time, we might have been able to detect additional changes in graft signal intensity that would have provided additional information related to graft healing and maturation. The echo time of images we evaluated was pre-determined based on our standard clinical imaging protocol. Other pulse parameters may have been more optimal to image revascularization and healing of the graft. Additionally, our results may not be directly comparable to those obtained with other pulse parameters. In addition, signal intensity of the grafts depends on echo time or proton density of the graft [15, 22].
Conclusion
Weak to moderate correlations which were statistically significant were found between signal intensity of the allograft and time from surgery after anatomic double-bundle ACL reconstruction. Furthermore, there was statistically significant difference between the AM and PL bundles, with the PL bundle having increased signal intensity. Our findings suggest that after anatomic double-bundle allograft ACL reconstruction, based on the increasing signal intensity over the first 12 months of surgery, the graft may be at increased risk of failure if exposed to high loads that are associated with return to strenuous sports activities and participation. Additionally, the two grafts to reconstruct the AM and PL bundles appear to revascularize and heal at different rates. The increased signal intensity of the PL bundle suggests that it may be at increased risk for failure if the knee is subjected to high rotational loads within the first 12 months after surgery. In the future, sequential MRI after ACL reconstruction may be useful to monitoring healing and maturation of the graft and may provide objective evidence to facilitate return-to-sports decisions.
References
Ahn JH, Lee SH, Choi SH, Lim TK (2010) Magnetic resonance imaging evaluation of anterior cruciate ligament reconstruction using quadrupled hamstring tendon autografts: comparison of remnant bundle preservation and standard technique. Am J Sports Med 38:1768–1777
Autz G, Goodwin C, Singson RD (1991) Magnetic resonance evaluation of anterior cruciate ligament repair using the patellar tendon double bone block technique. Skeletal Radiol 20:585–588
Biercevicz AM, Miranda DL, Machan JT, Murray MM, Fleming BC (2013) In situ, noninvasive, T2*-weighted MRI-derived parameters predict ex vivo structural properties of an anterior cruciate ligament reconstruction or bioenhanced primary repair in a porcine model. Am J Sports Med 41:560–566
Cheung Y, Magee TH, Rosenberg ZS, Rose DJ (1992) MRI of anterior cruciate ligament reconstruction. J Comput Assist Tomogr 16:134–137
Deehan DJ, Cawston TE (2005) The biology of integration of the anterior cruciate ligament. J Bone Joint Surg Br 87:889–895
Figueroa D, Melean P, Calvo R, Vaisman A, Zilleruelo N, Figueroa F, Villalón I (2010) Magnetic resonance imaging evaluation of the integration and maturation of semitendinosus-gracilis graft in anterior cruciate ligament reconstruction using autologous platelet concentrate. Arthroscopy 26:1318–1325
Frobell RB, Lohmander LS, Roos EM (2007) The challenge of recruiting patients with anterior cruciate ligament injury of the knee into a randomized clinical trial comparing surgical and non-surgical treatment. Contemp Clin Trials 28:295–302
Gianotti SM, Marshall SW, Hume PA, Bunt L (2009) Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport 12:622–627
Gohil S, Annear PO, Breidahl W (2007) Anterior cruciate ligament reconstruction using autologous double hamstrings: a comparison of standard versus minimal debridement techniques using MRI to assess revascularisation. A randomised prospective study with a one-year follow-up. J Bone Joint Surg Br 89:1165–1171
Howell SM, Clark JA, Blasier RD (1991) Serial magnetic resonance imaging of hamstring anterior cruciate ligament autografts during the first year of implantation. A preliminary study. Am J Sports Med 19:42–47
Howell SM, Clark JA, Farley TE (1991) A rationale for predicting anterior cruciate graft impingement by the intercondylar roof. A magnetic resonance imaging study. Am J Sports Med 19:276–282
Howell SM, Clark JA, Farley TE (1992) Serial magnetic resonance study assessing the effects of impingement on the MR image of the patellar tendon graft. YJARS 8:350–358
Howell SM, Knox KE, Farley TE, Taylor MA (1995) Revascularization of a human anterior cruciate ligament graft during the first two years of implantation. Am J Sports Med 23:42–49
Ihara H, Miwa M, Deya K, Torisu K (1996) MRI of anterior cruciate ligament healing. J Comput Assist Tomogr 20:317–321
Ilaslan H, Sundaram M, Miniaci A (2005) Imaging evaluation of the postoperative knee ligaments. Eur J Radiol 54:178–188
Jackson DW, Grood ES, Cohn BT, Arnoczky SP, Simon TM, Cummings JF (1991) The effects of in situ freezing on the anterior cruciate ligament. An experimental study in goats. J Bone Joint Surg Am 73:201–213
Janssen RPA, van der Wijk J, Fiedler A, Schmidt T, Sala HAGM, Scheffler SU (2011) Remodelling of human hamstring autografts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 19:1299–1306
Kiekara T, Järvelä T, Huhtala H, Paakkala A (2011) MRI of double-bundle ACL reconstruction: evaluation of graft findings. Skeletal Radiol 41:835–842
Kropf EJ, Shen W, Eck CF, Musahl V, Irrgang JJ, Fu FH (2013) ACL–PCL and intercondylar notch impingement: magnetic resonance imaging of native and double-bundle ACL-reconstructed knees. Knee Surg Sports Traumatol Arthrosc 21:720–725
Lee YS, Jeong YM, Sim JA, Kwak JH, Kim KH, Nam SW, Lee BK (2013) Specific compartmental analysis of cartilage status in double-bundle ACL reconstruction patients: a comparative study using pre- and postoperative MR images. Knee Surg Sports Traumatol Arthrosc 21:702–707
Li H, Tao H, Cho S, Chen S, Yao Z, Chen S (2012) Difference in graft maturity of the reconstructed anterior cruciate ligament 2 years postoperatively: a comparison between autografts and allografts in young men using clinical and 3.0-T magnetic resonance imaging evaluation. Am J Sports Med 40:1519–1526
May DA, Disler DG, Jones EA, Balkissoon AA, Manaster BJ (2000) Abnormal signal intensity in skeletal muscle at MR imaging: patterns, pearls, and pitfalls. Radiographics 20:S295–S315
Maywood RM, Murphy BJ, Uribe JW, Hechtman KS (1993) Evaluation of arthroscopic anterior cruciate ligament reconstruction using magnetic resonance imaging. Am J Sports Med 21:523–527
Munk PL, Vellet AD, Fowler PJ, Miniaci T, Crues JV (1992) Magnetic resonance imaging of reconstructed knee ligaments. Can Assoc Radiol J 43:411–419
Murakami Y, Sumen Y, Ochi M, Fujimoto E, Adachi N, Ikuta Y (1998) MR evaluation of human anterior cruciate ligament autograft on oblique axial imaging. J Comput Assist Tomogr 22:270–275
Muramatsu K, Hachiya Y, Izawa H (2008) Serial evaluation of human anterior cruciate ligament grafts by contrast-enhanced magnetic resonance imaging: comparison of allografts and autografts. Arthroscopy 24:1038–1044
Ntoulia A, Papadopoulou F, Zampeli F, Ristanis S, Argyropoulou M, Georgoulis A (2012) Evaluation with contrast-enhanced magnetic resonance imaging of the anterior cruciate ligament graft during its healing process: a two-year prospective study. Skeletal Radiol 42:541–552
Pallis M, Svodoba SJ, Cameron KL, Owens BD (2012) Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med 40:1242–1246
Rak KM, Gillogly SD, Schaefer RA, Yakes WF, Liljedahl RR (1991) Anterior cruciate ligament reconstruction: evaluation with MR imaging. Radiology 178:553–556
Spindler KP, Huston LJ, Wright RW, Kaeding CC, Marx RG, Amendola A, Parker RD, Andrish JT, Reinke EK, Harrell FE, MOON Group, Dunn WR (2011) The prognosis and predictors of sports function and activity at minimum 6 years after anterior cruciate ligament reconstruction: a population cohort study. Am J Sports Med 39:348–359
van Eck CF, Schkrohowsky JG, Working ZM, Irrgang JJ, Fu FH (2012) Prospective analysis of failure rate and predictors of failure after anatomic anterior cruciate ligament reconstruction with allograft. Am J Sports Med 40:800–807
Vogl TJ, Schmitt J, Lubrich J, Hochmuth K, Diebold T, Del Tredici K, Südkamp N (2001) Reconstructed anterior cruciate ligaments using patellar tendon ligament grafts: diagnostic value of contrast-enhanced MRI in a 2-year follow-up regimen. Eur Radiol 11:1450–1456
Weiler A, Peters G, Mäurer J, Unterhauser FN, Südkamp NP (2001) Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast-enhanced magnetic resonance imaging. A two-year study in sheep. Am J Sports Med 29:751–761
Yamato M, Yamagishi T (1992) MRI of patellar tendon anterior cruciate ligament autografts. J Comput Assist Tomogr 16:604–607
Zaffagnini S, Marcheggiani Muccioli GM, Lopomo N, Signorelli C, Bonanzinga T, Musiani C, Vassilis P, Nitri M, Marcacci M (2012) Can the pivot-shift be eliminated by anatomic double-bundle anterior cruciate ligament reconstruction? Knee Surg Sports Traumatol Arthrosc 20:743–751
Acknowledgments
The authors would like to acknowledge Maha Torabi, MD, Bethany C. Casagranda, DO and Cynthia Britton, MD from Department of Radiology, University of Pittsburgh Medical Center for their assistance with this study. Statistical guidance completed with the assistance of Carola F. van Eck, MD. from Department of Orthopaedic Surgery, University of Pittsburgh Medical Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miyawaki, M., Hensler, D., Illingworth, K.D. et al. Signal intensity on magnetic resonance imaging after allograft double-bundle anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 22, 1002–1008 (2014). https://doi.org/10.1007/s00167-014-2856-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-014-2856-1