Abstract
Purpose
The present study was designed to evaluate the penetration of diclofenac sodium 4 % spray gel in synovial tissue, synovial fluid and blood plasma after topical application in subjects with joint effusions and planned total knee arthroplasty (TKA) due to osteoarthritis.
Methods
A total of 39 patients were randomised to two- or three-times daily application of diclofenac sodium 4 % spray gel to knees requiring surgery over a treatment period of 3 days. Within 8 h after the last application, TKA was conducted, and the diclofenac concentrations in synovial tissue, synovial fluid and blood plasma were measured by liquid chromatography.
Results
The median diclofenac concentration was approximately 10–20-fold higher in synovial tissue (36.2 and 42.8 ng/g) than in synovial fluid (2.6 and 2.8 ng/mL) or plasma (3.9 and 4.1 ng/mL) in both treatment groups. Dose proportionality for any compartment or treatment groups could not be detected. Treatment-related adverse events were noted in two cases and limited to skin reactions.
Conclusion
Diclofenac sodium 4 % spray gel was found to penetrate the skin locally in substantial amounts and thus reach the desired target tissue. Concentrations were not dose-dependent, and application was well tolerated by 97.4 % of patients. Topical application of diclofenac should be considered a valuable alternative to systemic NSAID therapy in the initial treatment of osteoarthritis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Topical non-steroidal anti-inflammatory drugs (NSAIDs) are effective analgesics that are commonly used to treat acute pain and inflammation [16, 20]. They have been recommended as first-line therapy in chronic rheumatologic disorders such as osteoarthritis (OA) before the use of systemic NSAIDs [14, 26, 27] because they have comparable efficacy and are, in general, more tolerated (especially if used in the longer term) [17, 20]. Nevertheless, there is controversy as to whether topical NSAIDs reach target tissues in quantities sufficient for a relevant therapeutic effect and, in the case of OA, the exact identity of the target tissues [2]. Therefore, most orthopaedic clinicians are unsure of the value of topical NSAID therapy and favour systemic NSAIDs for the initial treatment of arthritis pain.
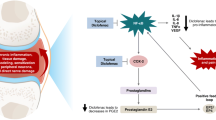
Cartilage does not contain blood vessels or nociceptors. Hence, joint pain cannot arise from it during degeneration but instead must originate from other structures within the joint (e.g., synovial membrane, joint capsule, periarticular muscles and ligaments, periosteum, subchondral bone) [2, 10]. The efficacy of a topical NSAID is influenced by the extent to which the drug penetrates inflamed tissues [4]. Hence, measurement of the drug concentration in synovial fluid and synovial tissue can provide valuable information regarding its likely clinical effect in OA [3]. The ideal scenario is a high concentration of drug at these target tissues, with a low concentration in plasma to minimise adverse events in gastrointestinal, cardiovascular or renal systems [4].
Diclofenac sodium 4 % spray gel (DSSG; Mika Pharma, Speyer, Germany) is a topical NSAID indicated for painful, inflammatory, rheumatic or traumatic complaints in muscles, joints or tendons. If the solution is sprayed onto the skin, evaporation of the alcoholic components causes changes in its viscosity and a low-viscosity gel results, which promptly adheres to the skin. The active substance is absorbed through the skin and permeates into deeper tissue layers. The unique formulation of DSSG was developed to enhance skin penetration and thus improve local delivery of the drug to deeper target tissue layers rather than to the systemic circulation [5]. Several studies have focused on measurement of the concentration of diclofenac in synovial fluid and blood plasma after topical application [25], but it is unclear if the drug reaches its desired target tissue in such treatment.
Therefore, the aim of the present randomised, open-label, parallel-group study was to investigate the penetration of DSSG into the synovial tissue, synovial fluid and blood plasma of patients with joint effusions and planned knee-joint replacement surgery. The hypothesis of the study was that higher concentrations of diclofenac would be found in synovial tissue than in synovial fluid and plasma. This study should enhance knowledge about the efficacy of topical NSAIDs to reach the target tissue and encourage colleagues to consider topical NSAIDs the first-line alternative before the use of systemic drugs.
Materials and methods
This randomised, open-label, parallel-group, single-centre clinical study was approved by the Ethics Committee of the University Hospital Marburg. Informed consent was obtained from all patients. The project was undertaken in accordance with the Helsinki Declaration and Guidelines of Good Clinical Practice (EudraCT number, 2007-003782-40).
To ensure that the collection of samples for the determination of diclofenac concentration would not involve additional interventions and consequent risk, patients with joint effusions and planned knee-joint replacement surgery were selected. The exclusion criteria were severe concomitant gastrointestinal, renal, hepatic or coagulation disorders likely to prohibit the use of diclofenac; intra-articular injections (e.g., corticosteroid, hyaluronic acid) into the target knee <3 months before enrolment; any form of diclofenac administration (e.g., oral, intramuscular, intra-articular, soft-tissue injections or topical) <2 weeks before enrolment; skin disease located on the study knee to be treated with the study drug.
The study involved four site visits. The first involved an initial screening visit for evaluation of eligibility. The second was a baseline visit for the enrolment of subjects with documentation of: demographic data; the diagnosis; medical history; confirmation of inclusion/exclusion criteria; blood sampling for determination of pre-study plasma concentration of diclofenac; first dosing of the study drug. The third visit was for the end of treatment—after 3 days (or earlier in the case of a premature discontinuation), any remaining study drug was collected, patients were checked for compliance and adverse events recorded after knee surgery. The final visit was <8 h from the last application of the study; samples of synovial tissue and synovial fluid as well as further blood tests for plasma levels of diclofenac were obtained during planned knee arthroplasty. The total length of the study was 5 ± 2 days (including 3 days of treatment with the study drug).
Intervention
Patients were randomised (computer-generated by an independent statistician) to two- or three-times daily application of DSSG over a treatment period of 3 days until surgery. Instructions were given to apply the drug at about the same time every day and at intervals of about 12 or 8 h, respectively, with the last application <8 h before surgery. Patients were advised to gently spread and rub in the applied amount of spray gel onto intact skin and wait 3–5 min until absorption was complete. Application of the study drug was done 1 h before washing the study knee. Patients were given a subject diary at enrolment to document application of the study drug. The drug was supplied in bottles containing 25 g of solution. One dose (five full pump strokes, 1 g of solution) equaled 40 mg diclofenac sodium for topical application. Therefore, patients received 80 mg (group 1) or 120 mg (group 2) diclofenac sodium per day and either 240 mg (group 1) or 360 mg (group 2) diclofenac sodium over the entire treatment period. A dose of 40 mg was calculated after a review of the literature [5, 22, 25] and was doubled in one group to detect the possible dose dependency of pharmacologic effects. Concomitant local treatment other than the study medication was not allowed for application on either knee. This included the application of any other topical and intra-articular formulations of herbal agents, other analgesics, NSAIDs, muscle relaxants, corticosteroids, anticoagulants as well as physical treatment.
Sample analyses
During knee-replacement surgery, two tissue samples of synovial tissue (approximately the size of a pea), a sample of synovial fluid (about 2.5–5.0 mL) and a blood sample (about 5.0 mL) were collected. Samples were stored at −20 °C until analyses for determination of diclofenac concentration. Diclofenac concentrations in synovial tissue, synovial fluid and blood plasma were determined according to Good Laboratory Practice using validated (Pharmakin GmbH, Ulm, Germany) liquid chromatography-mass spectrometry methods (LC–MS/MS) with lower limits of quantification (LLOQ) of ≤0.15 ng/mL (plasma and synovial fluid) and ≤1.5 ng/g (synovial tissue).
Efficacy (pharmacokinetic)
The primary pharmacokinetic variable was the diclofenac concentration measured <8 h from the final application in plasma and in the synovial fluid and synovial tissue of the knee treated for 3 days with the study drug. The tolerability of DSSG was evaluated by monitoring the nature, severity and frequency of adverse events and serious adverse events.
Statistical analyses
Due to the exploratory character of the study, an estimation of sample size was not carried out. However, the sample size selected for the present study is in general agreement with similar studies [22, 23].
The study populations were the intention-to-treat (ITT) population (including all patients with at least one dose of the study drug) and the per-protocol (PP) population (involving patients in the ITT population without major deviations in protocols).
Descriptive statistics were used to summarise the diclofenac concentrations in synovial tissue and synovial fluid as well as the diclofenac concentrations in blood plasma. Concentrations below the LLOQ were imputed with half of the LLOQ. Descriptive statistics of concentrations were calculated if at least two-thirds of the individual values were equal or above the LLOQ. The measured diclofenac concentrations did not have a normal distribution in the study population, so median diclofenac concentrations and ranges are shown. Differences in concentrations between treatments were described with the p value of the exact Wilcoxon 2 samples test at p = 0.05. The correlation between the diclofenac concentration in synovial tissue, synovial fluid and blood plasma and the compliance of the subjects were investigated using the Spearman correlation coefficient (p value of test “coefficient” = 0).
Results
Overall, the demographic and baseline characteristics between the two treatment groups were comparable (Table 1). All patients were Caucasian, and 67 % were non-smokers; 82 % were ≥60 years of age. All 39 patients were included in the ITT group. The nature and frequency of prior and concomitant illnesses were as expected in elderly patients, and there was no relevant difference between the two treatment groups. The most common prior/concomitant illnesses were hypertension, allergies, hypercholesterolemia and diabetes mellitus (DM). Prior and concomitant medications related to these diseases were common. Other common medications were given during the study as part of the routine surgical procedure (including a standard anaesthetic as well as antibiotic prophylaxis to prevent infection).
Protocol deviations considered to have a relevant effect on the results occurred in eight patients, so 31 patients completed the study and were included in the PP group. One patient discontinued due to an adverse event and did not have a final assessment. Major protocol deviations occurred in four patients. Two patients received >120 % of the intended diclofenac concentration and two patients <60 % of the intended diclofenac dose. These deviations were considered to have a relevant effect on the results and are not included in the PP-population.
At enrolment, low plasma concentrations of diclofenac were present in both application groups. After treatment, measurable diclofenac concentrations were detected in all three target compartments (Table 2), and the concentration of diclofenac was approximately 10–20-fold higher in synovial tissue than in synovial fluid or blood plasma. There were large variations in diclofenac concentrations between individual patients, with no clear dose proportionality for any compartment or treatment group.
Two patients (5 %) experienced adverse events: one with a rash at the administration site and a severe adverse event in another patient who had a stroke. The rash was assessed to be mild and probably related to the study product because it resolved after the discontinuation of treatment (the patient removed himself from the study). The stroke occurred after the completion of treatment and was assessed as being unrelated to diclofenac and possibly due to underlying comorbidities of the patient. Therefore, only one patient (2.6 %) experienced a probable drug-related adverse event. No other severe adverse events were reported during the study (Fig. 1).
Discussion
The most important finding of the present study was that topically applied Diclofenac sodium 4 % spray gel can penetrate the skin in substantial amounts and reach the synovial membrane. The median diclofenac concentration was 10–20-fold higher in synovial tissue than synovial tissue or blood plasma. Among patients receiving topical diclofenac, only one patient developed skin irritation.
The rate and extent of drug permeation of a topical drug are dependent upon several factors. These include its formulation [12, 24] and specific characteristics [4], which can influence its distribution in inflamed tissue in conditions such as OA. Diclofenac sodium is not readily absorbed upon dermal application [21], and many strategies have been suggested to overcome the low permeability of the drug through the skin [23]. Once it has been absorbed cutaneously, diclofenac sodium must reach the target tissues in therapeutic concentrations. Diclofenac is strongly bound to protein, has a short plasma half-life, a low volume of distribution and is weakly acidic. These characteristics, combined with changes in the hemodynamics of inflamed tissues, mean that diclofenac should be distributed to areas of inflammation rather than to plasma. A diclofenac formulation with a high degree of skin permeation and preferential distribution to the affected compartment would be useful not only for the treatment of locally inflamed skin tissues, but also inflammatory and painful deeper tissues surrounding the joints [9].
Several studies have demonstrated that, after using various formulations of topical diclofenac, greater concentrations of diclofenac are found in the synovial fluid and synovial tissue than in plasma [3, 7, 8, 11, 15]. Penetration of diclofenac into deeper skin layers using the DSSG formulation has been established in vivo [5] in healthy subjects. The present study demonstrated that, after topical application of DSSG in patients with OA about to undergo knee surgery, diclofenac could penetrate the skin to reach the synovial tissue in concentrations that were 10–20-fold higher than in plasma or synovial fluid. The concentration of diclofenac in synovial fluid is similar to that in plasma, as described recently by Miyatake et al. [18]. An explanation may be that synovial fluid concentrations reflect plasma concentrations because this is a highly vascularised compartment [19]. The concentrations that were observed in the synovial fluid are in accordance with those reported by Arcangeli et al. [1]. The estimated minimal effective concentration in synovial fluid is 100–500 to 20–30 ng/mL [15, 22]; the lower concentrations being based on that which can induce 20–60 % suppression of PGE2 and TXB2 [15]. However, the synovial tissue concentration is of greater importance than the plasma concentration because this is the target tissue. The observed synovial tissue concentrations were in the estimated therapeutic range and should persist longer than fluid concentrations. This may explain why efficacy can be obtained with topical diclofenac application even with very low systemic availability [22]. Large inter-individual differences are often reported in in vivo studies of the pharmacokinetics of topical drugs [5]: this was true for the present study. Given the variability between patients, no correlation between dose and diclofenac concentrations were expected or observed. Diclofenac concentrations were not dose-proportional, and similar levels of diclofenac were found in synovial tissue and fluid for two- and three-times daily administrations.
Despite relatively fast elimination from plasma, diclofenac also has a long persistence at sites of inflammation, which might explain its prolonged therapeutic effects [4, 7]. The effect could be prolonged further by virtue of sustained concentrations in target tissues through slow release of the drug through different skin layers after topical administration.
In this short-term study (3-day treatment), topical diclofenac was tolerated in all but one instance. One suspected drug-related adverse event (2.6 % of patients) was observed (mild rash at the administration site which resolved after treatment cessation). Measurable concentrations of diclofenac were detected in plasma, thereby demonstrating limited systemic distribution and the potential for systemic adverse events, but systemic adverse events were not observed in the present study. This is a relevant finding because the study population was relatively elderly (87 % were aged >60 years). With longer-term use of oral NSAIDs, such patients often experience intolerable gastrointestinal adverse events [6, 13], so an effective topical NSAID that is well tolerated would be of great benefit. The favourable skin penetration and distribution to target tissues (and hence therapeutic efficacy), combined with a low potential for systemic adverse events, suggest that DSSG is a rational alternative to oral diclofenac formulations for the treatment of chronic rheumatologic conditions such as OA.
The present study had limitations which must be considered. The study length of 3 days may not be long enough to evaluate adverse events related to long-term NSAID therapy. All patients receiving topical diclofenac should undergo an individual risk assessment and ongoing monitoring should be conducted. Longer-term studies with more patients are therefore needed to assess differences between different doses of topical diclofenac sodium. The present study suggested that the therapeutic use of DSSG may be a valuable alternative to initial treatment with systemic NSAIDs. The latter are known to generate a 50-fold higher bioavailability throughout the whole organism [5] and thus may have considerably more side effects.
Conclusion
After topical application of Diclofenac sodium 4 % spray gel onto the knee, the drug penetrates the skin and reaches the target tissue in therapeutic amounts, as demonstrated by synovial-tissue concentrations that were 10–20-fold higher than those of synovial fluid or plasma. The concentration of diclofenac was not dose-dependent. Diclofenac sodium 4 % spray gel had excellent tolerability and may be a low-cost alternative with fewer side effects in the treatment of arthritic pain before the use of other systemic NSAIDs.
References
Arcangeli P, Andreotti L, Palazzini E (1996) Effective treatment of osteoarthritis with a 150 mg prolonged-release of diclofenac sodium. Riv Eur Sci Med Farmacol 18(5–6):217–223
Barron MC, Rubin BR (2007) Managing osteoarthritic knee pain. J Am Osteopath Assoc 107(10 Suppl 6):21–27
Benson MD, Aldo-Benson M, Brandt KD (1985) Synovial fluid concentrations of diclofenac in patients with rheumatoid arthritis or osteoarthritis. Semin Arthritis Rheum 15(2 Suppl 1):65–67
Brune K (2007) Persistence of NSAIDs at effect sites and rapid disappearance from side effect compartments contributes to tolerability. Curr Med Res Opin 23(12):2985–2995
Brunner M, Dehghanyar P, Seigfried B, Martin W, Menke G, Muller M (2005) Favourable dermal penetration of diclofenac after administration to the skin using a novel spray gel formulation. Br J Clin Pharmacol 60(5):573–577
Courtney P, Doherty M (2002) Key questions concerning paracetamol and NSAIDs for osteoarthritis. Ann Rheum Dis 61(9):767–773
Davies NM, Anderson KE (1997) Clinical pharmacokinetics of diclofenac. Therapeutic insights and pitfalls. Clin Pharmacokinet 33(3):184–213
Elmquist WF, Chan KK, Sawchuk RJ (1994) Transsynovial drug distribution: synovial mean transit time of diclofenac and other nonsteroidal antiinflammatory drugs. Pharm Res 11(12):1689–1697
Escribano E, Calpena AC, Queralt J, Obach R, Domenech J (2003) Assessment of diclofenac permeation with different formulations: anti-inflammatory study of a selected formula. Eur J Pharm Sci 19(4):203–210
Felson DT, Neogi T (2004) Osteoarthritis: is it a disease of cartilage or of bone? Arthritis Rheum 50(2):341–344
Fowler PD, Shadforth MF, Crook PR, John VA (1983) Plasma and synovial fluid concentrations of diclofenac sodium and its major hydroxylated metabolites during long-term treatment of rheumatoid arthritis. Eur J Clin Pharmacol 25(3):389–394
Hamad M, Metwally S, El-Shafey A, Geneidi A (1994) Comparative percutaneous absorption of diclofenac emulgel penetrations in normal volunteers. J Drug Res 21:133–141
Henry D, Lim LL, Rodriguez LAG, Gutthann SP, Carson JL, Griffin M, Savage R, Logan R, Moride Y, Hawkey C, Hill S, Fries JT (1996) Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: results of a collaborative meta-analysis. BMJ 312(7046):1563–1566
Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JW, Dieppe P, Gunther K, Hauselmann H, Herrero-Beaumont G, Kaklamanis P, Lohmander S, Leeb B, Lequesne M, Mazieres B, Martin-Mola E, Pavelka K, Pendleton A, Punzi L, Serni U, Swoboda B, Verbruggen G, Zimmerman-Gorska I, Dougados M (2003) EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 62(12):1145–1155
Liauw HL, Ku E, Brandt KD, Benson MD, Aldo-Benson MA, Waiter SL, Lee W, Chan K, Vyas K (1985) Effects of Voltaren on arachidonic acid metabolism in arthritis patients. Agents Actions Suppl 17:195–199
Mason L, Moore RA, Edwards JE, Derry S, McQuay HJ (2004) Topical NSAIDs for acute pain: a meta-analysis. BMC Fam Pract 5:10–18
Mason L, Moore RA, Edwards JE, Derry S, McQuay HJ (2004) Topical NSAIDs for chronic musculoskeletal pain: systematic review and meta-analysis. BMC Musculoskelet Disord 5:28–35
Miyatake S, Ichiyama H, Kondo E, Yasuda K (2008) Randomized clinical comparisons of diclofenac concentration in the soft tissues and blood plasma between topical and oral applications. BJCP 67(1):125–129
Moore RA (2004) Topical nonsteroidal antiinflammatory drugs are effective in osteoarthritis of the knee. J Rheumatol 31(10):1893–1895
Moore RA, Tramer MR, Carroll D, Wiffen PJ, McQuay HJ (1998) Quantitative systematic review of topically applied non-steroidal anti-inflammatory drugs. BMJ 316(7128):333–338
Nishihata T, Kotera K, Nakano Y, Yamazaki M (1987) Rat percutaneous transport of diclofenac and influence of hydrogenated soya phospholipids. Chem Pharm Bull (Tokyo) 35(9):3807–3812
Radermacher J, Jentsch D, Scholl MA, Lustinetz T, Frolich JC (1991) Diclofenac concentrations in synovial fluid and plasma after cutaneous application in inflammatory and degenerative joint disease. Br J Clin Pharmacol 31(5):537–541
Riess W, Schmid K, Botta L, Kobayashi K, Moppert J, Schneider W, Sioufi A, Strusberg A, Tomasi M (1986) The percutaneous absorption of diclofenac. Arzneimittelforschung 36(7):1092–1096
Sherertz EF, Sloan KB, McTiernan RG (1987) Use of theoretical partition coefficients determined from solubility parameters to predict permeability coefficients for 5-fluorouracil. J Invest Dermatol 89(2):147–151
Taylor RS, Fotopoulos G, Maibach H (2011) Safety profile of topical diclofenac: a meta analysis of blinded, randomized, controlled trials in musculoskeletal conditions. Curr Med Res Opin 27(3):605–622
Zhang W, Doherty M, Leeb BF, Alekseeva L, Arden NK, Bijlsma JW, Dincer F, Dziedzic K, Hauselmann HJ, Herrero-Beaumont G, Kaklamanis P, Lohmander S, Maheu E, Martin-Mola E, Pavelka K, Punzi L, Reiter S, Sautner J, Smolen J, Verbruggen G, Zimmermann-Gorska I (2007) EULAR evidence based recommendations for the management of hand osteoarthritis: report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 66(3):377–388
Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, Dougados M, Hochberg M, Hunter DJ, Kwoh K, Lohmander LS, Tugwell P (2007) OARSI recommendations for the management of hip and knee osteoarthritis, part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthr Cartil 15(9):981–1000
Acknowledgments
The authors thank Kate McFarlane for her support in preparing the manuscript and proofreading.
Conflict of interest
T.E. and M.D.S. are consultants to Smith and Nephew, Endoscopy. The study was supported by MIKA Pharma.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Efe, T., Sagnak, E., Roessler, P.P. et al. Penetration of topical diclofenac sodium 4 % spray gel into the synovial tissue and synovial fluid of the knee: a randomised clinical trial. Knee Surg Sports Traumatol Arthrosc 22, 345–350 (2014). https://doi.org/10.1007/s00167-013-2408-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-013-2408-0