Abstract
Purpose
To evaluate whether medial open wedge high tibial osteotomy (HTO) results in structural and biochemical changes in the lateral meniscus in adult sheep.
Methods
Three experimental groups with biplanar osteotomies of the right proximal tibiae were tested: (a) closing wedge HTO resulting in 4.5° of tibial varus, (b) open wedge HTO resulting in 4.5° of tibial valgus (standard correction) and (c) open wedge HTO resulting in 9.5° of valgus (overcorrection), each of which was compared to the contralateral knees with normal limb axes. After 6 months, the lateral menisci were macroscopically and microscopically evaluated. The proteoglycan and DNA contents of the red–red and white–white zones of the anterior, middle and posterior third were determined.
Results
Semiquantitative macroscopic and microscopic grading revealed no structural differences between groups. The red–red zone of the middle third of the lateral menisci of animals that underwent overcorrection exhibited a significant 0.7-fold decrease in mean DNA contents compared with the control knee without HTO (P = 0.012). Comparative estimation of the DNA and proteoglycan contents and proteoglycan/DNA ratios of all other parts and zones of the lateral menisci did not reveal significant differences between groups.
Conclusion
Open wedge HTO does not lead to significant macroscopic and microscopic structural changes in the lateral meniscus after 6 months in vivo. Overcorrection significantly decreases the proliferative activity of the cells in the red–red zone of the middle third in the sheep model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High tibial osteotomy (HTO) is a useful therapy for symptomatic medial femorotibial osteoarthritis in patients with varus malalignment of the knee [5, 38]. Particularly for younger and physically active patients with medial knee osteoarthritis, HTO is an excellent alternative to knee arthroplasty [52]. Here, the weight-bearing axis of the leg is shifted away from the medial compartment with the aim of reducing excessive loading and pain and improving joint function. Consequently [1], the load distribution between the medial and lateral compartments of the knee is altered [50]. Loading is transferred towards the lateral tibiofemoral compartment—especially when a valgus overcorrection is performed—while loading of the medial compartment is decreased [1]. Interestingly, its consequences on the tibiofemoral cartilage have been described in cadaver studies focussing on pressure distribution [1] and in patients after HTO determining the distribution of mineralization of the tibial plateau by CT-osteoabsorptiometric investigations [28, 32]. In addition, effects of differences in load distribution on articular cartilage proteoglycans were assessed in models of below-knee amputation or femur valgus osteotomy in guinea pigs [53] or in cartilage samples obtained from patients undergoing total knee replacement [35]. Here, increased load was associated with a decreased proteoglycan concentration [53]. Although these studies found morphological and biochemical changes in the cartilage in relation to loading [35, 53], the influence of increased loading on the lateral meniscus remains unknown.
Here, we hypothesized that medial open wedge HTO results in structural and biochemical changes in the lateral meniscus in a preclinical sheep model and that these changes predominantly occur after overcorrection.
Materials and methods
Study design
Three experimental groups with medial osteotomies of the right tibiae were tested in sheep: (a) a closing wedge HTO resulting in 4.5° of tibial varus (unloaded control), (b) an open wedge HTO resulting in 4.5° of tibial valgus (standard correction) and (c) an open wedge HTO resulting in 9.5° of tibial valgus (overcorrection), each of which was compared to the contralateral left knees that received an arthrotomy only. Six months postoperatively, the animals were killed, and the macroscopic appearance of the lateral meniscus was scored. The menisci underwent histological and immunohistological analysis. The red–red and white–white zones of the anterior, middle and posterior third of the lateral menisci were evaluated for proteoglycan and DNA contents.
Animal experiments
Animal experiments were conducted under an Institution Animal Studies Committee–approved protocol. Animals had a weight of 66.6 ± 5.0 kg. Radiographs were taken prior to the experiments to exclude osteoarthritis. Biplanar osteotomies of the proximal tibiae were carried out using an anteromedial approach with an oscillating saw, leaving the contralateral cortical bone intact as described elsewhere [39]. The osteotomies underwent gradual opening using flat chisels, resulting in standardized openings [37]. A small stature TomoFix plate fixator (Synthes, Tuttlingen, Germany) was applied. The following experimental groups with medial osteotomies of the right tibiae were tested: (a) closing wedge HTO resulting in 4.5° of tibial varus (range, 2.0–6.0; unloaded control; n = 5), (b) open wedge HTO resulting in 4.5° of tibial valgus (range, 2.0–7.5°; valgus standard correction; n = 5), and (c) open wedge HTO resulting in 9.5° of valgus (range, 7.5–13.0°; overcorrection; n = 9), each of which was compared to the contralateral left knees that received an arthrotomy only. Postoperatively, all animals were immediately allowed full weight-bearing. Six months postoperatively, the animals were killed, and the lateral menisci were evaluated.
Macroscopic analysis
The macroscopic appearance of the lateral meniscus was evaluated using a newly developed score (Table 1), which grades colour (0–1), quality of the inner meniscal rim (0–3), central meniscal degeneration (0–2) and meniscal insertion (0–2).
Histological and immunohistochemical analyses
Menisci were fixed in 4 % phosphate-buffered formalin. Paraffin-embedded coronal [19] sections (5 μm) were stained with haematoxylin and eosin to detect cells and safranin O/fast green to detect proteoglycans according to the routine histological protocols [8, 25, 26]. Histological analysis was performed in a blinded fashion as described by Pauli et al. [40] with the modification that matrix staining was inversely scored. Immunostaining for collagen type I was performed as described [20]. All immunoreactivities were assessed as follows: 0, no immunoreactivity; 1, significantly weaker immunoreactivity; 2, moderately weaker immunoreactivity; 3, similar immunoreactivity; 4, stronger immunoreactivity compared with the positive control (sheep subchondral bone). A total of 114 sections were scored.
Biochemical analyses
For biochemical evaluations, samples were taken from the red–red zone and the white–white zone of the anterior, middle and posterior third of the menisci. Proteoglycan contents were measured by binding to the DMMB dye, and DNA contents were monitored using Hoechst 33258 as previously described [29, 34]. All data were normalized to the protein contents, as determined using a Bradford assay (Pierce, Rockford, IL, USA). Measurements were performed with a GENios spectrophotometer/fluorometer (Tecan, Crailsheim, Germany). A total of 38 lateral menisci were processed.
Statistical analysis
Mean values and SD were assessed for all evaluated criteria. A Wilcoxon signed-rank test was applied to compare the treatment versus control groups (SPSS, version 17.0; Chicago, IL). P values <0.05 were considered statistically significant.
Results
Six months postoperatively, all osteotomies healed uneventfully. Semiquantitative macroscopic evaluation of the lateral menisci (Fig. 1) revealed a trend towards worse scores for overcorrected knees (2.9-fold; n.s.) than for knees that received a standard correction (1.4-fold; n.s.) or a varus correction (1.3-fold; n.s.) compared with the unoperated contralateral control without reaching statistical significance (Table 2).
Macroscopic analysis of the lateral menisci following high tibial osteotomy. Semiquantitative grading revealed a trend towards worse scores for overcorrected knees with increased pressure load on the lateral meniscus (9.5° tibial valgus; d) than for knees with standard (4.5° tibial valgus; c) or varus correction (b; P > 0.15). Images were selected from menisci having a macroscopic rating equal to the mean score for its respective treatment group. Scale bars 5.0 mm
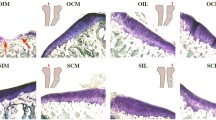
Microscopic analysis of the lateral menisci showed no significant differences between groups with respect to surface, cellularity, matrix organization, matrix staining and the average total histological score (Fig. 2a–h; Table 3).
Histological evaluation of changes in the middle thirds of the lateral menisci following high tibial osteotomy. Large images A1–H1 and j–k show coronal cross-sections of the middle third (pars intermedia) of the entire lateral meniscus, whereas small images display magnified views of the red–red zone (A2–H2) and the white–white zone (A3–H3). Rectangles in image A1 represent the standardized locations for analysis of the red–red (left, vertical) and white–white (right, horizontal) zones. According to safranin O/fast green staining (a–d), proteoglycan distribution (coloured in red) was unaffected by the change in limb axis and the resulting alteration in pressure load on the lateral menisci following the different tibial osteotomies. Haematoxylin/eosin staining e–h did not reveal variations in cell density or cellular organization between the three treatment groups (F1–F3, G1–G3, H1–H3) and the normal controls (E1–E3). Likewise, none of the treatments provoked changes in the immunoreactivity to a monoclonal mouse anti-human type I collagen IgG (j–m) in the lateral menisci. Sections were taken from menisci having a histological rating equal to the mean score for its respective treatment group. Scale bars 2.0 mm (A1–H1 and j–m) and 0.2 mm (A2–H2 and A3–H3)
Immunoreactivity to type I collagen was located in the femoral and tibial superficial zones of the menisci and also in central parts of the lateral menisci (Fig. 2j–m). No difference in immunoreactivity to type I collagen was seen between the four groups (Table 4).
Biochemical evaluations revealed a significant decrease in the DNA and the proteoglycan contents from the white–white zone compared with the red–red zone in all menisci (Fig. 3). Between the experimental groups, there were no differences in the proteoglycan contents in each zone of the menisci (Fig. 4). However, the lateral menisci of animals that underwent overcorrection (9.5° of valgus) exhibited a significant 0.7-fold decrease in mean DNA contents in the red–red zone of the middle third (pars intermedia) of the samples compared with the control knee without HTO (12.3 ± 3.3 and 8.5 ± 3.8 μg DNA/mg total protein, respectively; P = 0.012) (Fig. 3). Interestingly, the proteoglycan contents of this region were also reduced compared with control condition, although without reaching statistical significance (Fig. 4). The proteoglycans-to-DNA ratios remained unchanged (Fig. 5). Comparative estimation of the proteoglycan contents of all other parts and zones of the lateral menisci did not reveal significant differences between groups.
DNA contents (μg DNA/mg total protein) of the different zones of the lateral menisci. Blue boxes control (left knees). Green boxes HTO groups (right knees): a 4.5° varus: unloaded control. b 4.5° valgus: standard correction. c 9.5° valgus: overcorrection. Bottom and top of the boxes show the 25th and 75th percentile; the median is shown as a black band in the box. Whiskers are defined as the lowest value still within 1.5 interquartile range (IQR) of the lower quartile and the highest value still within 1.5 IQR of the upper quartile
Proteoglycan contents (μg proteoglycans/mg total protein) of the different zones of the lateral menisci. Blue boxes control (left knees). Green boxes HTO groups (right knees): a 4.5° varus: unloaded control. b 4.5° valgus: standard correction. c 9.5° valgus: overcorrection. Bottom and top of the boxes show the 25th and 75th percentile; the median is shown as a black band in the box. Whiskers are defined as the lowest value still within 1.5 interquartile range (IQR) of the lower quartile and the highest value still within 1.5 IQR of the upper quartile
Proteoglycan/DNA ratios (μg proteoglycans/μg DNA) of the different zones of the lateral menisci. Blue boxes control (left knees). Green boxes HTO groups (right knees): a 4.5° varus: unloaded control. b 4.5° valgus: standard correction. c 9.5° valgus: overcorrection. Bottom and top of the boxes show the 25th and 75th percentile, the median is shown as a black band in the box. Whiskers are defined as the lowest value still within 1.5 interquartile range (IQR) of the lower quartile and the highest value still within 1.5 IQR of the upper quartile
Discussion
The main finding of the present study was that open wedge HTO was not associated with significant macroscopic and microscopic structural changes in the lateral meniscus 6 months after surgery in the preclinical sheep model. Standard correction (with 4.5° tibial valgus) does not lead to mid-term morphological alterations and differences in the DNA and proteoglycan content of the lateral menisci. Overcorrection (with 9.5° tibial valgus) significantly reduced cell numbers in the middle third of the red–red zone of the lateral menisci, as indicated by the reduced DNA contents compared with control knees without HTO, but does not lead to detectable mid-term morphological alterations after 6 months. Interestingly, this effect was not associated with inferior proteoglycan contents in this region. It is possible that the increased lateral contact pressure as a result of overcorrection may lead to structural changes in the red–red zone of the lateral meniscus in the long-term.
The menisci are indispensable components of the knee joint, as they allow for maximum congruence between the incongruent surfaces of the flattened tibial plateau and curved femoral condyles, have energy dissipating capacities and distribute loads [12, 51]. A meniscal tear can lead to knee osteoarthritis, but knee osteoarthritis can also lead to a spontaneous meniscal tear [9, 10, 31]. The subsequent increase in contact pressure and compression over time induces the development of osteoarthritis [23],
The lateral meniscus [3] is of specific importance, since the peak contact stress and maximum shear stress in the articular cartilage increased 200 % more after a lateral than a medial meniscectomy under axial femoral compressive loads, as shown in a three-dimensional finite element model of the human tibiofemoral joint [41]. The delicate balance between the lateral meniscus and the articular cartilage [15] is underlined by the clinical observation that cartilage lesions proceed much faster after lateral than after medial meniscectomy and that the clinical outcomes of lateral meniscectomy are significantly worse than after medial meniscectomy [4, 17, 24, 43]. Loss of meniscal tissue, such as resulting from injury or partial meniscectomy, significantly alters the biomechanical environment of the knee joint [15]. A recent clinical study on the effect of microfracture and medial open wedge HTO in patients with varus knee osteoarthritis demonstrated that those with injury of the medial meniscus have a higher likelihood of later undergoing total knee arthroplasty than patients without meniscal damage [44].
While it has been shown that meniscal resection leads to a disturbance of the contact between tibial and femoral cartilage [22], the mid-term effect on the lateral meniscus of an increase in loading following HTO has not, to the best of our knowledge, been demonstrated to date. When the knee is normally aligned, the centre of pressure passes slightly through the medial side of the knee [36]. Varus malalignment abnormally distributes the load towards the medial tibiofemoral compartment [46]. Similarly, valgus malalignment increases load in the lateral compartment. The effect of axial malalignment and subsequent overload on the articular cartilage and the subchondral bone has been well described [2, 14, 15, 45]. Despite this clear correlation, little is known on the effect of axial malalignment on the lateral meniscus. The data of the present study show that in the sheep model, no significant macroscopic and microscopic structural changes occur in the lateral meniscus at 6 months after surgery.
This study also supports the finding of region-specific differences within the lateral meniscus [16, 30, 49]. The red–red and white–white zone of the sheep meniscus express different patterns of genes [11, 48], and the central part of the meniscus of sheep is more cartilaginous (e.g. containing more glycosaminoglycans) than the peripheral part [7, 13]. Of note, we found a significant decrease in cell proliferation in the red–red zone of the middle third in overcorrected knees compared with non-operated control knees. This suggests an inhibitory effect of the increased compression on the lateral compartment including the meniscus as a result of the valgus overcorrection. Ex vivo, it has already been proposed that the dynamic compressive behaviour of human meniscus correlates with its extra-cellular matrix composition [6]. When meniscus tissue explants in radial confinement were subjected to in vitro compressive overload, cell lysis increased with peak injury force and loading rate. In contrast, the content and release of glycosaminoglycans, together with mechanical properties, did not significantly vary with loading rate. Also, after 9 days in vitro, the tissue displayed little to no macroscopic damage [6]. These results, together with the present in vivo findings, indicate that meniscal cell damage may be present without immediate physical or compositional changes in meniscal tissue [33]. Whether this plays a role in the development of early osteoarthritis [27] remains to be determined.
Many in vitro studies have demonstrated that the homoeostatic balance between collagen biosynthesis and catabolism of meniscal cells is altered by static and dynamic compression and that the biosynthetic response of the meniscus to mechanical stimuli is regulated, in part, at the transcriptional level [17, 18, 47]. The data of the present study suggest that a standard correction (with 4.5° tibial valgus) does not lead to detectable mid-term morphological alterations and differences in the DNA and proteoglycan content of the lateral menisci in all of the six meniscal regions evaluated.
These findings contradict the hypothesis that medial open wedge HTO results in structural and biochemical changes in the lateral meniscus in a preclinical sheep model. Thus, from the viewpoint of this preclinical large animal model, this indicates that such standard correction is safe. The data of the present study also show that overcorrection (with 9.5° tibial valgus) does not lead to detectable mid-term morphological alterations, but to a significant decrease in the DNA content of the middle third of the red–red zone of the lateral menisci. This result supports the hypothesis that overcorrection leads to biochemical changes in the lateral meniscus. Consequently, it warrants further long-term studies to determine whether this reduction in the cell numbers will translate to structural changes of the meniscus.
Limitations of this study include the different lateral meniscus morphology of sheep compared with humans [7, 42]. Although the sheep is an important experimental model for meniscal repair, tissue engineering and regeneration [21], the degree of postoperative weight-bearing in quadruped animals is difficult to control. Moreover, this study did not evaluate possible pre-existing (e.g. surgically induced) lateral meniscal lesions, as sometimes present in the clinical situation.
Conclusion
Valgization following HTO with a subsequent increase in pressure load does not result in major mid-term macroscopic and microscopic changes in the lateral meniscus in sheep at 6 months postoperatively.
References
Agneskirchner JD, Hurschler C, Wrann CD, Lobenhoffer P (2007) The effects of valgus medial opening wedge high tibial osteotomy on articular cartilage pressure of the knee: a biomechanical study. Arthroscopy 23(8):852–861
Baliunas AJ, Hurwitz DE, Ryals AB, Karrar A, Case JP, Block JA, Andriacchi TP (2002) Increased knee joint loads during walking are present in subjects with knee osteoarthritis. Osteoarthritis Cartilage 10(7):573–579
Beaufils P, Hardy P, Chambat P, Clavert P, Djian P, Frank A, Hulet C, Potel JF, Verdonk R (2006) Adult lateral meniscus. Rev Chir Orthop Reparatrice Appar Mot 92(5 Suppl):2S169–162S194
Bolano LE, Grana WA (1993) Isolated arthroscopic partial meniscectomy. Functional radiographic evaluation at five years. Am J Sports Med 21(3):432–437
Brinkman JM, Lobenhoffer P, Agneskirchner JD, Staubli AE, Wymenga AB, van Heerwaarden RJ (2008) Osteotomies around the knee: patient selection, stability of fixation and bone healing in high tibial osteotomies. J Bone Joint Surg Br 90(12):1548–1557
Bursac P, Arnoczky S, York A (2009) Dynamic compressive behavior of human meniscus correlates with its extra-cellular matrix composition. Biorheology 46(3):227–237
Chevrier A, Nelea M, Hurtig MB, Hoemann CD, Buschmann MD (2009) Meniscus structure in human, sheep, and rabbit for animal models of meniscus repair. J Orthop Res 27(9):1197–1203
Cucchiarini M, Thurn T, Weimer A, Kohn D, Terwilliger EF, Madry H (2007) Restoration of the extracellular matrix in human osteoarthritic articular cartilage by overexpression of the transcription factor SOX9. Arthritis Rheum 56(1):158–167
Englund M, Guermazi A, Lohmander LS (2009) The meniscus in knee osteoarthritis. Rheum Dis Clin North Am 35(3):579–590
Englund M, Guermazi A, Lohmander SL (2009) The role of the meniscus in knee osteoarthritis: a cause or consequence? Radiol Clin North Am 47(4):703–712
Esparza R, Gortazar AR, Forriol F (2012) Cell study of the three areas of the meniscus: effect of growth factors in an experimental model in sheep. J Orthop Res. doi:10.1002/jor.22110
Fithian DC, Kelly MA, Mow VC (1990) Material properties and structure-function relationships in the menisci. Clin Orthop Relat Res 252:19–31
Fuller ES, Smith MM, Little CB, Melrose J (2012) Zonal differences in meniscus matrix turnover and cytokine response. Osteoarthritis Cartilage 20(1):49–59
Griffin TM, Guilak F (2005) The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev 33(4):195–200
Heijink A, Gomoll AH, Madry H, Drobnic M, Filardo G, Espregueira-Mendes J, Van Dijk CN (2012) Biomechanical considerations in the pathogenesis of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc 20(3):423–435
Hennerbichler A, Moutos FT, Hennerbichler D, Weinberg JB, Guilak F (2007) Repair response of the inner and outer regions of the porcine meniscus in vitro. Am J Sports Med 35(5):754–762
Hoser C, Fink C, Brown C, Reichkendler M, Hackl W, Bartlett J (2001) Long-term results of arthroscopic partial lateral meniscectomy in knees without associated damage. J Bone Joint Surg Br 83(4):513–516
Imler SM, Doshi AN, Levenston ME (2004) Combined effects of growth factors and static mechanical compression on meniscus explant biosynthesis. Osteoarthritis Cartilage 12(9):736–744
Kambic HE, McDevitt CA (2005) Spatial organization of types I and II collagen in the canine meniscus. J Orthop Res 23(1):142–149
Kaul G, Cucchiarini M, Arntzen D, Zurakowski D, Menger MD, Kohn D, Trippel SB, Madry H (2006) Local stimulation of articular cartilage repair by transplantation of encapsulated chondrocytes overexpressing human fibroblast growth factor 2 (FGF-2) in vivo. J Gene Med 8(1):100–111
Kon E, Filardo G, Tschon M, Fini M, Giavaresi G, Marchesini Reggiani L, Chiari C, Nehrer S, Martin IP, Salter D, Ambrosio L, Marcacci M (2012) Tissue engineering for total meniscal substitution. Animal study in sheep model: results at 12 months. Tissue Eng Part A. doi:10.1089/ten.TEA.2011.0572
Lee SJ, Aadalen KJ, Malaviya P, Lorenz EP, Hayden JK, Farr J, Kang RW, Cole BJ (2006) Tibiofemoral contact mechanics after serial medial meniscectomies in the human cadaveric knee. Am J Sports Med 34(8):1334–1344
Lohmander LS, Englund PM, Dahl LL, Roos EM (2007) The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med 35(10):1756–1769
Macnicol MF, Thomas NP (2000) The knee after meniscectomy. J Bone Joint Surg Br 82(2):157–159
Madry H, Cucchiarini M, Stein U, Remberger K, Menger MD, Kohn D, Trippel SB (2003) Sustained transgene expression in cartilage defects in vivo after transplantation of articular chondrocytes modified by lipid-mediated gene transfer in a gel suspension delivery system. J Gene Med 5(6):502–509
Madry H, Kaul G, Cucchiarini M, Stein U, Zurakowski D, Remberger K, Menger MD, Kohn D, Trippel SB (2005) Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I). Gene Ther 12(15):1171–1179
Madry H, Luyten FP, Facchini A (2012) Biological aspects of early osteoarthritis. Knee Surg Sports Traumatol Arthrosc 20(3):407–422
Madry H, van Dijk CN, Mueller-Gerbl M (2010) The basic science of the subchondral bone. Knee Surg Sports Traumatol Arthrosc 18(4):419–433
Madry H, Zurakowski D, Trippel SB (2001) Overexpression of human insulin-like growth factor-I promotes new tissue formation in an ex vivo model of articular chondrocyte transplantation. Gene Ther 8(19):1443–1449
Mauck RL, Martinez-Diaz GJ, Yuan X, Tuan RS (2007) Regional multilineage differentiation potential of meniscal fibrochondrocytes: implications for meniscus repair. Anat Rec 290(1):48–58
McDermott ID, Amis AA (2006) The consequences of meniscectomy. J Bone Joint Surg Br 88(12):1549–1556
Muller-Gerbl M, Putz R, Kenn R (1992) Demonstration of subchondral bone density patterns by three-dimensional CT osteoabsorptiometry as a noninvasive method for in vivo assessment of individual long-term stresses in joints. J Bone Miner Res 7(Suppl 2):S411–S418
Nishimuta JF, Levenston ME (2012) Response of cartilage and meniscus tissue explants to in vitro compressive overload. Osteoarthritis Cartilage 20(5):422–429
Orth P, Zurakowski D, Wincheringer D, Madry H (2011) Reliability, reproducibility and validation of five major histological scoring systems for experimental articular cartilage repair in the rabbit model. Tissue Eng Part C Methods 18(5):329–339
Otsuki S, Nakajima M, Lotz M, Kinoshita M (2008) Hyaluronic acid and chondroitin sulfate content of osteoarthritic human knee cartilage: site-specific correlation with weight-bearing force based on femorotibial angle measurement. J Orthop Res 26(9):1194–1198
Paley D, Pfeil J (2000) Principles of deformity correction around the knee. Orthopade 29(1):18–38
Pape D, Dueck K, Haag M, Lorbach O, Seil R, Madry H (2012) Wedge volume and osteotomy surface depend on surgical technique for high tibial osteotomy. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-012-1913-x
Pape D, Filardo G, Kon E, van Dijk CN, Madry H (2010) Disease-specific clinical problems associated with the subchondral bone. Knee Surg Sports Traumatol Arthrosc 18(4):448–462
Pape D, Madry H (2012) The preclinical sheep model of high tibial osteotomy relating basic science to the clinics: standards, techniques and pitfalls. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-012-2135
Pauli C, Grogan SP, Patil S, Otsuki S, Hasegawa A, Koziol J, Lotz MK, D’Lima DD (2011) Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthritis Cartilage 19(9):1132–1141
Pena E, Calvo B, Martinez MA, Palanca D, Doblare M (2006) Why lateral meniscectomy is more dangerous than medial meniscectomy. A finite element study. J Orthop Res 24(5):1001–1010
Proffen BL, McElfresh M, Fleming BC, Murray MM (2011) A comparative anatomical study of the human knee and six animal species. Knee 19(4):493–499
Scheller G, Sobau C, Bulow JU (2001) Arthroscopic partial lateral meniscectomy in an otherwise normal knee: clinical, functional, and radiographic results of a long-term follow-up study. Arthroscopy 17(9):946–952
Sterett WI, Steadman JR (2004) Chondral resurfacing and high tibial osteotomy in the varus knee. Am J Sports Med 32(5):1243–1249
Tanamas S, Hanna FS, Cicuttini FM, Wluka AE, Berry P, Urquhart DM (2009) Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum 61(4):459–467
Tetsworth K, Paley D (1994) Malalignment and degenerative arthropathy. Orthop Clin North Am 25(3):367–377
Upton ML, Chen J, Guilak F, Setton LA (2003) Differential effects of static and dynamic compression on meniscal cell gene expression. J Orthop Res 21(6):963–969
Upton ML, Chen J, Setton LA (2006) Region-specific constitutive gene expression in the adult porcine meniscus. J Orthop Res 24(7):1562–1570
Upton ML, Guilak F, Laursen TA, Setton LA (2006) Finite element modeling predictions of region-specific cell-matrix mechanics in the meniscus. Biomech Model Mechanobiol 5(2–3):140–149
Van Thiel GS, Frank RM, Gupta A, Ghodadra N, Shewman EF, Wang VM, Bach BR, Verma NN, Cole BJ, Provencher MT (2011) Biomechanical evaluation of a high tibial osteotomy with a meniscal transplant. J Knee Surg 24(1):45–53
Verdonk R (2010) The meniscus: past, present and future. Knee Surg Sports Traumatol Arthrosc 19(2):145–146
W-Dahl A, Robertsson O, Lohmander LS (2012) High tibial osteotomy in Sweden, 1998–2007. Acta Orthop. 83(3):244–248
Wei L, Hjerpe A, Brismar BH, Svensson O (2001) Effect of load on articular cartilage matrix and the development of guinea-pig osteoarthritis. Osteoarthritis Cartilage 9(5):447–453
Acknowledgments
We are indebted to Dr. vet. Altmann and her team, Bad Langensalza, Thuringia, Germany, for their excellent support in the treatment and care of the animals. This study was supported in part by an AGA Research Grant (Forschungsförderung 29/2008) to HM and DP and by a grant of the Department of Orthopaedic Surgery, Saarland University, Homburg/Saar. Henning Madry, Patrick Orth, Dieter Kohn, Magali Cucchiarini and Dietrich Pape are partners in the Cartilage Net of the Universität der Großregion/Université de la Grande Région (UGR), supported by the INTERREG IV Programme of the European Union.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madry, H., Ziegler, R., Orth, P. et al. Effect of open wedge high tibial osteotomy on the lateral compartment in sheep. Part I: analysis of the lateral meniscus. Knee Surg Sports Traumatol Arthrosc 21, 39–48 (2013). https://doi.org/10.1007/s00167-012-2176-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-012-2176-2