Abstract
Purpose
To assess the mechanical behavior and the histology of collagen fibers after prolotherapy with 12.5% dextrose into rat Achilles tendons and to compare with those of corticosteroid treatment.
Methods
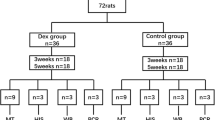
Out of 60 adult female Wistar rats (70 tendons), 15 received 12.5% dextrose (group I); 15 were treated with corticosteroid injection (group II); and 15 were given 0.9% saline injection (group III), all into the right Achilles tendon, whereas 13 animals received no injections (group IV). Three doses of each substance (groups I, II, and III) were given at a 5-day interval. Collagen fiber color was quantitatively assessed in three samples from each group and in five samples from the control group using picrosirius red staining under polarized and nonpolarized light. Twelve tendons from each group treated with the test substance and 20 tendons from the control group were submitted to the tensile strength test.
Results
There was no statistical difference across the groups with respect to maximum load at failure (n.s.) and absorbed energy (n.s.). With respect to tendon rupture, there was no difference between the myotendinous and the tendinous regions (n.s.). However, hematoxylin–eosin staining revealed statistical significance in lymphocytic inflammatory infiltrate (P = 0.008) and in parallel fiber orientation (P = 0.003) when comparing groups to the control group, without significance for either neovascularization (n.s.) or the presence of fibroblasts (n.s.). Likewise, there was no significant difference between the percentage of mature (n.s.) and immature (n.s.) fibers.
Conclusions
Dextrose was not deleterious to the tendinous tissue, as it did not change the mechanical and histological properties of Achilles tendons in rats. The data obtained in this study may help clinicians in their daily work as they suggest that injections of 12.5% dextrose caused no harm to the tendons, although the clinical importance in humans still needs to be defined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tendinopathy is classically defined as a degenerative tendon disorder that has been ascribed to several causes such as local injury, hormones, aging, chemical substances, vascular failure, and overuse [5]. Prolotherapy, or proliferative therapy, is a therapeutic technique widely used in sports medicine, characterized by the injection of growth factors into the injured site in order to promote cell growth, enhancing the natural process of tissue repair [4, 10, 11, 23]. Growth factors have been identified as key to the stimulation of tendons, ligaments, and cartilage [25]. However, they have been mainly utilized in tendinopathies [1].
The administration of 12.5% dextrose for the treatment for tendinopathies in elite athletes has shown remarkable efficacy, with good clinical outcomes [23]. Other researchers have described dextrose treatment as efficacious and safe in sports medicine [16]. In sports medicine, the overall prevalence and incidence of chronic tendon disorders are unknown, but there is evidence that sports play a major role in the development of injuries [1, 2]. In addition, tendinopathy constitutes an important injury that poses a diagnostic and therapeutic challenge [12, 18, 20].
Corticosteroids are potent anti-inflammatory drugs, which are widely used in routine clinical practice, especially in the treatment for sports injuries. Their use in the treatment for overload injuries is still controversial, and their role has not been well established [9].
The effects of dextrose on the biomechanical properties of tendons and the histological analysis are yet unknown. The aim of this study was to assess the mechanical behavior and the histology of collagen fibers after prolotherapy with 12.5% dextrose into rat Achilles tendons, compared with corticosteroid treatment. Our first hypothesis is that intratendinous injection of dextrose does not change the mechanical properties and the histological analysis of tendons, whereas our secondary hypothesis postulates that there are changes in mechanical properties and histological aspects when the corticosteroid injection is used.
Materials and methods
This study protocol was evaluated and approved by the institutional review board (IRB 2007/805) of University of Passo Fundo (UPF). The initial sample comprised 60 healthy 90-day-old white female Wistar rats, weighing on average 195 g, obtained from UPF’s animal research facility. One rat from group II and one from group III were replaced due to anesthesia complications in the first anesthesia. During the experiment, the animals were kept in individual polyethylene cages at UPF’s veterinary hospital, at a temperature between 18° and 22°, under appropriate lighting and ventilation, being fed ad libitum.
Fifteen animals received dextrose in the right Achilles tendon (group I); another 15 animals were given corticosteroid in the right Achilles tendon (group II); and 15 animals received saline solution (group III). Out of 15 in the control group (group IV), two animals were excluded but not replaced due to injury in the second week. Of the 13 animals in this group, both tendons (right and left) were used, totaling 26 tendons. The tendons from the control group animals were not submitted to any procedures, and therefore, values were considered to be those of healthy tendons. Three samples from groups I, II, and III and five samples from the control group were submitted to quantitative collagen analysis, as one tendon was excluded due to technical conditions during the preparation of staining dyes.
Group I, II, and III animals received three injections of the respective substances at a 5-day interval. Group I was given 0.1 ml of the dilution of 0.1 ml of 50% dextrose and 0.3 ml of 1% lidocaine, with a final concentration of 12.5% dextrose, while group II received 0.1 ml of the dilution of 0.1 ml of betamethasone dipropionate (5 mg) + betamethasone phosphate (2 mg), Diprospan™, and 0.3 ml of 1% lidocaine. Group 3 was given 0.1 ml of 0.9% NaCl (saline solution). Group IV was not given any injection. For the administration of the substances, animals were injected with xylazine hydrochloride 5 mg/kg and ketamine-S 50 mg/kg under anesthesia. They were placed in lateral decubitus, and their paws were kept at 90°. All injections were given by the same researcher (senior author), and U-100 insulin syringe and needle were used for all injections.
The animals were killed and assessed 5 days after receiving the third dose. They were anesthetized prior to euthanasia with 10 mg/ml of IM morphine (0.1 ml/animal). Thereafter, the animals were euthanized with 1.5 ml of sodium thiopental, 100 mg given intraperitoneally to each animal.
Tensile test
The muscle origin was resected, the tibia was sectioned distally, and the muscle–tendon–bone complex was eventually used as the study sample.
Immediately after killing and dissection of hind paws, the tendons were submitted to the tensile test.
The 12 tendons from each group treated with the test substance and the 20 tendons from the control group were submitted to the tensile test using the EMIC-DL® 10000 universal testing machine (reading accuracy of 0.5% and 0.01 mm displacement), provided by the Analytical Testing Laboratory. The results were based on the maximum load needed for tissue failure, i.e., the maximum load that the tissue can withstand before rupture, and on absorbed energy.
A 100-kgf (1,000 N) load cell and a 12 mm/min speed were used on all tests. The muscle belly was wrapped in an 80-grit wet sandpaper (Alcar®) to prevent the specimen from slipping off the upper grip during the test. An initial 5-mm gap was allowed between the grips (Fig. 1).
Following the literature, the tensile test data were normalized, i.e., the maximum load value was divided by the animal’s weight, measured at the time of killing [22].
Histological analysis
The histological section preserved the whole muscle–tendon–bone complex. The specimens were fastened by their ends to a firm base in order to preserve the tendon anatomy and were then soaked in 10% formaldehyde. The tendinous structure was removed from the specimens and embedded in paraffin. Special care was taken to keep the tendon longitudinally placed for the section and mounting of specimens. 5-μm-thick longitudinal sections were obtained, with two histological sections from each block, totaling two slides for each analyzed tendon. One slide was stained with hematoxylin–eosin (HE), and the other slide was stained with picrosirius red [17]. A Zeiss® Axioplan II light microscope equipped with Plan Neofluar objectives (Sliedrecht, The Netherlands) was used.
Hematoxylin–eosin (HE) staining
The qualitative analysis of the HE-stained sections considered the following aspects: lymphocytic infiltrate, presence of fibroblasts, neovascularization, and parallel fiber orientation. ×50, ×100, and ×400 magnifications were used.
Parallel orientation of collagen fibers was assessed by observing the longitudinal sections on a 0–3 scale, where 0—no parallel pattern; 1—slightly parallel; 2—partially parallel; and 3—parallel [3]. For the histological analysis of the tendon, the following parameters were considered: amount and characteristics of fibroblasts and presence of inflammatory infiltrate and neovascularization. A score was established for each characteristic in the histological analysis using a 0–3 scale where 0—absent; 1—mild, 2—moderate, and 3—intense [3].
Contents and color of collagen fibers (picrosirius red staining)
The histological sections stained with picrosirius red were assessed as to the amount of collagen. Collagen content was calculated as a percentage of the area of each image, expressed in pixels. There might be an inaccurate assessment given that collagen is not the only birefringent material found in the tissues, e.g., fibrin, which is weakly birefringent and appears green when stained with picrosirius red. Thus, the slides were assessed without a polarized light source and under strong polarized light using a magnification of ×50 [7]. The images were captured by a Pixelink PL-A662 camera and sent to the color monitor of a Mac OS X with a 1.5-GHz-power PC G4 processor, digitized, and saved in TIFF format (uncompressed files) (Fig. 2).
The tissue sections were stained with picrosirius red (×50) and viewed under a polarized light and b nonpolarized light. The fibers are mainly red (dotted arrows), indicating mature collagen. In b, the dotted arrow indicates birefringent cells. Some green fibers (bold arrow) can be seen, but they are located in a more central region of the tendon. A triangular region (oval dotted lines), where the needle penetrates the tendon, shows tissue with a disorganized pattern, in addition to fibroblast proliferation
The images captured under nonpolarized light were edited using Scion Image® and the RGB (red, green, blue) system. For each image, the red (mature collagen) and green (immature collagen) colors were selected and then combined into two new images for analysis. The images captured under polarized light were assessed directly. The system calibration was based on the optical density of the pixel resolution of the images. The pixels of each color (green and red) were counted and expressed as percentage value, as previously described. The images were converted into hues of black and gray over the resolution threshold, and the pixels were counted using the same software program mentioned above. All slides were assessed with the same configurations.
Statistical analysis
Parametric and nonparametric tests were carried out. The Kolmogorov–Smirnov test allowed observing the normal distribution of the tensile test variables. Therefore, one-way ANOVA was used for the statistical analysis of the tensile strength. The nonparametric Kruskal–Wallis test was applied for histological comparison of collagen fiber colors, given the small sample size. The chi-square test was used for categorical variables. The significance level was set as P < 0.05. The statistical data were calculated using the SPSS software package version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Tensile test
No statistically significant difference (n.s.) was observed between the mean values obtained for the tensile strength (Table 1). As to absorbed energy, there was no significant difference between the groups (n.s.).
With respect to tendon rupture (Table 2) in groups I and III, 91% occurred in the myotendinous region. In group IV, 89% also occurred at this site. Myotendinous ruptures accounted for 59% of all ruptures observed in group II, but no statistically significant difference was noted between the groups (n.s.).
Histological analysis
The amount of collagenous tissue stained red and green in the tendons from each group is shown in Table 3.
The results of the qualitative histological analysis of the tendons are shown in Table 4.
Discussion
The most important finding of the present study was that the mean tensile strength value for the tendons in the dextrose group was not different from that of the tendons in the saline group and in the control group, indicating that dextrose administration did not reduce the tensile strength in the analyzed period. The energy absorbed before rupture yielded a very similar mean in all groups. The tendons that received corticosteroid injection showed greater strength, but this was not statistically significant. The rupture site of the tendons in the dextrose group was predominantly the myotendinous region, demonstrating good strength of the tendinous region into which the substance was injected.
As to the rupture site on the fatigue strength test, the corticosteroid group had the largest number (five cases) of ruptures in the tendinous region. Although tendon strength was expected to decrease and to be statistically nonsignificant, these five samples showed good strength. These findings are consistent with those described in the literature, which points out that the administration of corticosteroids to rat tendons increases the stability of the collagenous tissue for 15 days and that, during such period, tendon strength does not decrease [15]. Perhaps this increase in the stability of the collagenous tissue is related to the higher strength of tendons in the corticosteroid group, especially if one considers the period in which the assessment was made (5 days after the last injection).
The results of this study confirm those obtained by McWhorter et al. [14], who stated that the administration of corticosteroid to tendons does not cause biochemical changes, and by Hugate et al. [6], who concluded that corticosteroids do not reduce the ultimate tensile strength of Achilles tendon tissues in rabbits. These data contrast with those found by Kapetanos [8], who affirms that corticosteroids inhibit the formation of connective tissue and reduce the tendon mass, thus lowering the ultimate tensile strength of tissues.
Nevertheless, Shrier et al. [19] conclude that published data are not enough to determine the risks and benefits of corticosteroid injection into tendons and that lower tendon strength in animal studies suggests that the rupture might be a potential complication after several weeks of administration. Wang et al. [24]. mention that the effects of corticosteroids on the tendons are still unclear, and they recommend that controlled and rigorous studies be conducted to demonstrate the actual effect of corticosteroid injection into tendons.
Moreover, the dextrose group revealed a larger amount of immature collagen in the tendons, i.e., neocollagen; on the other hand, a long-term study should be undertaken to corroborate this tendency (n.s.). All groups injected with some substance showed absence of parallel fiber orientation, and this is probably due to needle trauma rather than to the injected substance (P = 0.003).
Martin et al. [13] assert that slight biomechanical changes occur in tendons after corticosteroid injection and that, besides being statistically nonsignificant, these changes disappear within 14 days. In addition, the same author found that corticosteroids cause fibroblasts to proliferate. However, we did not perceive this proliferative capacity in three injections over a 20-day period.
In contrast to what Junqueira [7] proposes, neither are red fibers type I collagen, nor are green fibers type III. This occurs due to fibroplasia, a term that denotes the proliferation and migration of fibroblasts and the development of collagenous and noncollagenous matrices. Fibroblasts produce and organize the major extracellular components of the granulation tissue which, after entering the wound, synthesize hyaluronic acid, fibronectin, and collagenase type I and III, which constitute the initial extracellular matrix. As the scar matures, type I collagen becomes the main component and proteoglycan deposition occurs. Nonetheless, this likely represents the presence of immature collagen fiber, as with the early healing phase (green), and of mature collagen fiber (red) [17, 21]. This could be the very reason why we did not find any statistically significant difference in the presence of fibroblasts, as the tendons had not been previously injured. On the other hand, the parallel fiber pattern was lost, and even though it was not specific to a given group, it was present in all of them, when compared to the control group. Acute lymphocytic inflammatory infiltrate was found in HE-stained specimens and also in those which received saline injection in the short-term assessment.
Physicians know quite well all the recommendations concerning corticosteroids, chiefly their precautions and contraindications. Therefore, physicians must be familiar with any restrictions before prescribing this therapy.
One of the limitations of our study was the short time period for the analyses. This might have influenced the results, especially regarding the effects of intratendinous corticosteroid injection, as we used a long-acting one. Furthermore, we did not assess the deleterious systemic effects of corticosteroids. Also, in our study, the tendons did not have previous injuries, but the sample was homogenous in all groups. The data obtained in this study may help clinicians in their daily work as they suggest that injections of 12.5% dextrose caused no harm to the tendons.
Conclusion
Therefore, it is assumed that dextrose injection did not show a deleterious effect on the tensile test or on the constitution of collagen in healthy tendons. Given that this injection has been often prescribed in sports medicine, it is suggested that further studies be carried out in animals with induced tendinopathy and that biomarkers be used to detect the healing rate and its role in the cure of tendinopathies. On the other hand, the assumption that corticosteroid injections are deleterious within this time frame and at the concentrations used is not confirmed; consequently, the secondary hypothesis has been rejected.
Accordingly, the results indicate that intratendinous dextrose injection at the concentration used, given in three doses, was not deleterious to the tendinous tissue.
References
Almekinders L (1998) Tendinitis and other chronic tendinopathies. J Am Acad Orthop Surg 6(3):157–164
Fullerton BD (2008) High-resolution ultrasound and magnetic resonance imaging to document tissue repair after prolotherapy: a report of 3 cases. Arch Phys Med Rehabil 89(2):377–385
Hirshberg A, Lib M, Kozlovsky A, Kaplan I (2007) The influence of inflammation on the polarization colors of collagen fibers in the wall of odontogenic keratocyst. Oral Oncol 43(3):278–282
Hiti CJ, Stevens KJ, Jamati MK, Garza D, Matheson GO (2011) Athletic osteitis pubis. Sports Med 41(5):361–376
Hsu RW, Hsu WH, Tai CL, Lee KF (2004) Effect of shock-wave therapy on patellar tendinopathy in a rabbit model. J Orthop Res 22(1):221–227
Hugate R, Pennypacker J, Saunders M, Juliano P (2004) The effects of intratendinous and retrocalcaneal intrabursal injections of corticosteroid on the biomechanical properties of rabbit Achilles tendons. J Bone Joint Surg Am 86-A(4):794–801
Junqueira LC, Bignolas G, Brentani RR (1979) Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 11(4):447–455
Kapetanos G (1982) The effect of the local corticosteroids on the healing and biomechanical properties of the partially injured tendon. Clin Orthop Relat Res 163:170–179
Khan KM, Cook JL, Taunton JE, Bonar F (2000) Overuse tendinosis, not tendinitis part 1: a new paradigm for a difficult clinical problem. Phys Sportsmed 28(5):38–48
Khan SA, Kumar A, Varshney MK, Trikha V, Yadav CS (2008) Dextrose prolotherapy for recalcitrant coccygodynia. J Orthop Surg (Hong Kong) 16(1):27–29
Kim WM, Lee HG, Jeong CW, Kim CM, Yoon MH (2010) A randomized controlled trial of intra-articular prolotherapy versus steroid injection for sacroiliac joint pain. J Altern Complement Med 16(12):1285–1290
Majewski M, Ochsner PE, Liu F, Fluckiger R, Evans CH (2009) Accelerated healing of the rat Achilles tendon in response to autologous conditioned serum. Am J Sports Med 37(11):2117–2125
Martin DF, Carlson CS, Berry J, Reboussin BA, Gordon ES, Smith BP (1999) Effect of injected versus iontophoretic corticosteroid on the rabbit tendon. South Med J 92(6):600–608
McWhorter JW, Francis RS, Heckmann RA (1991) Influence of local steroid injections on traumatized tendon properties. A biomechanical and histological study. Am J Sports Med 19(5):435–439
Oxlund H (1982) Long term local cortisol treatment of tendons and the indirect effect on skin. An experimental study in rats. Scand J Plast Reconstr Surg 16(1):61–66
Reeves KD, Hassanein K (2000) Randomized, prospective, placebo-controlled double-blind study of dextrose prolotherapy for osteoarthritic thumb and finger (DIP, PIP, and trapeziometacarpal) joints: evidence of clinical efficacy. J Altern Complement Med 6(4):311–320
Rich L, Whittaker P (2005) Collagen and picrosirius red staining: a polarized light assessment of fibrillar hue and spatial distribution. Braz J Morphol Sci 22:97–104
Scarpone M, Rabago DP, Zgierska A, Arbogast G, Snell E (2008) The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med 18(3):248–254
Shrier I, Matheson GO, Kohl HW 3rd (1996) Achilles tendonitis: are corticosteroid injections useful or harmful? Clin J Sport Med 6(4):245–250
Speed CA (2001) Fortnightly review: corticosteroid injections in tendon lesions. BMJ 323(7309):382–386
Szendroi M, Vajta G, Kovacs L, Schaff Z, Lapis K (1984) Polarization colours of collagen fibres: a sign of collagen production activity in fibrotic processes. Acta Morphol Hung 32(1):47–55
Tipton CM, Schild RJ, Flatt AE (1967) Measurement of ligamentous strength in rat knees. J Bone Joint Surg Am 49(1):63–72
Topol GA, Reeves KD, Hassanein KM (2005) Efficacy of dextrose prolotherapy in elite male kicking-sport athletes with chronic groin pain. Arch Phys Med Rehabil 86(4):697–702
Wang JH, Iosifidis MI, Fu FH (2006) Biomechanical basis for tendinopathy. Clin Orthop Relat Res 443:320–332
Woo SL, Hildebrand K, Watanabe N, Fenwick JA, Papageorgiou CD, Wang JH (1999) Tissue engineering of ligament and tendon healing. Clin Orthop Relat Res 367(Suppl):S312–S323
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martins, C.A.Q., Bertuzzi, R.T., Tisot, R.A. et al. Dextrose prolotherapy and corticosteroid injection into rat Achilles tendon. Knee Surg Sports Traumatol Arthrosc 20, 1895–1900 (2012). https://doi.org/10.1007/s00167-011-1789-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-011-1789-1