Abstract
Purpose
The purpose of this study was to evaluate and compare the resulting knee kinematics and stability of an anatomic superficial MCL (sMCL) reconstruction and a non-anatomic sMCL reconstruction.
Methods
In a cadaveric model, normal knee stability and kinematics were compared with sMCL deficient knees and with two experimental sMCL reconstructions. The first reconstruction (AnatRecon) attempted to anatomically reconstruct the sMCL. The second reconstruction (ShortRecon) used a shorter graft to mimic the effect of failing to reproduce the anatomic length of the sMCL. Changes in position of the femur with respect to the tibia were measured with an electromagnetic tracking system during simulated active knee extension and during passive knee stability testing in the sMCL intact knee, the sMCL deficient knee, and the two experimental reconstructions.
Results
Simulated active knee extension demonstrated a significant increase in external tibial rotation of ShortRecon compared to AnatRecon between 30° and 80° of knee flexion (mean difference <3.0° over the range of knee flexion angles; P < 0.008), and a significant increase in external tibial rotation of ShortRecon compared to the intact sMCL was found at 60° and 70° of knee flexion (mean difference <2.0°over the range of knee flexion angles; P < 0.008). Passive joint stability testing demonstrated that division of the sMCL produced approximately 6° of valgus laxity at 30° of knee flexion and increased external tibial rotation of approximately 5° at 30°, 9° at 60°, and 10° at 90° of knee flexion, respectively. AnatRecon restored normal knee kinematics and stability. Additionally, passive stability testing demonstrated a significant increase in external tibial rotation of ShortRecon compared to AnatRecon at 60° (mean difference = 3.7°; P < 0.05) and 90° of knee flexion (mean difference = 4.9°; P < 0.05).
Conclusion
Anatomic reconstruction of the sMCL effectively restored knee kinematics and stability in the sMCL deficient knee. Altering the normal ligament length resulted in measurable changes in knee kinematics and stability. This study suggests that in cases of chronic valgus knee instability, anatomic sMCL reconstruction would provide better results than non-anatomic sMCL reconstruction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Isolated injury of the medial collateral ligament (MCL) of the knee usually heals uneventfully without surgery [1, 5, 13, 14, 21], and combined injury of the anterior cruciate ligament (ACL) and MCL can be treated by non-operative management of the torn MCL [10, 19]. However, combined injury of the MCL, one or both cruciate ligaments, and posterior oblique ligament (POL) can lead to chronic valgus laxity of the knee [11, 12]. In acute cases, persistent valgus instability after cruciate ligament reconstruction can be addressed by repairing the superficial MCL (sMCL) and POL. In chronic cases, however, the sMCL becomes scarred and contracted, and anatomic repair may not be possible. In the knee with chronic functional valgus instability, reconstruction of the sMCL may be indicated, as results of non-operative treatment have been found to be poor [15]. The options for sMCL reconstruction include advancement of local medial capsule, rerouting, and tenodesis of the pes tendons or free tendon graft such as bone-patellar tendon-bone or semitendinosis [3, 16, 18, 20, 24, 30].
When considering sMCL reconstruction, it is important to note the significant contributions of both the sMCL and the POL to valgus and rotational stability of the knee [7, 8, 22, 26]. While it may be possible to advance or imbricate the POL in cases of chronic valgus laxity, it is difficult to anatomically restore the anatomic origins and insertions of the sMCL with tissue advancement. The sMCL is an unusually long ligament which originates on the medial femoral epicondyle and inserts on the proximal medial tibia approximately 60 mm distal to the joint line [25]. Given the length of this ligament, it is apparent that advancement of local scar tissue, rerouting of hamstring tendons or even reconstruction with patellar tendon would not reproduce the anatomic length of the sMCL. As in most cases of ligament reconstruction, failure to mimic normal anatomy may lead to over constraint of a joint and stiffness or attritional failure of the reconstruction and recurrent instability.
To our knowledge, the potential effects of knee motions and valgus stability resulting from reconstruction of the sMCL with differing graft lengths have not been studied. To this end, the purpose of this study was to determine the effect of two common graft lengths used for sMCL reconstruction on knee kinematics and stability, and to determine which reconstruction more closely restored normal knee motions and stability. The effects of sMCL reconstruction graft lengths were evaluated biomechanically using simulated active knee extension and passive joint stability testing in a human cadaver model. It was hypothesized that reconstruction of the sMCL with hamstring tendon grafts to replicate the normal length and attachment of the sMCL would restore normal knee kinematics and stability.
Materials and methods
Twelve human leg specimens extending from mid-thigh to foot were procured from the University of California at Davis donated body program. The specimens had an average age of 67 years (range, 56–76) and frozen at −10°C until experimentation. Five specimens were from women and seven from men. None of the specimens exhibited visual evidence of deformity or prior knee surgery. During inspection of knee structures after experimentation, all intra-articular ligaments appeared normal and only focal areas of mild to moderate chondromalacia were observed on the articular surfaces.
Testing methods and apparatus
The effect of two sMCL reconstruction graft lengths on knee kinematics and stability was evaluated through simulated knee extension and passive joint stability testing. Quadriceps-activated knee extension was used to evaluate knee kinematics during simulated active knee extension of each subject. Passive joint stability testing evaluated knee rotation in response to knee moments applied at the tibia.
An electromagnetic tracking device (Flock of birds, Ascension Technology, Burlington, VT) was used to measure changes in knee flexion angle, internal–external rotation, and varus–valgus angulation during both simulated active knee extension and passive joint stability testing. The device sensed position and orientation of a transmitter in six degrees-of-freedom with respect to a receiving unit. The transmitter for the device was attached to an acrylic square mounted on the most anterior aspect of the tibial tubercle using titanium lag screws (Fig. 1). The orientation of the transmitter, as described by the manufacturer, was visually aligned with the long axis of the tibia. The receiver unit was rigidly mounted on a bench top approximately 30 cm from the transmitter, within the optimal range of 22–64 cm [17]. Additionally, aluminum metals and other common orthopedic alloys were used in the experimental apparatus and testing protocol to minimize electromagnetic signal interference. With these steps, the electromagnetic tracking device has been shown to detect differences of 0.1° in rotation [17]. Motion of the tibia with respect to the femur was calculated using custom Visual C++software (v6.0, Microsoft Corporation, Seattle, WA). The software was used to record data output from the electromagnetic tracking device mounted on the tibia, first in a reference position and then in subsequent positions.
A custom testing apparatus was designed to allow measurement of knee rotations during quadriceps-activated knee extension and passive joint stability testing. The femur and tibia of each specimen were fixed to the apparatus using respective fixation frames. Quadriceps-activated knee extension was simulated using the stepper motor and freeze clamp attached to the quadriceps tendon. Passive joint stability testing was performed by applying a varus–valgus angulation moment about the knee with a force transducer (not shown) at the ankle, or by applying an internal–external rotation moment about the knee with a torque wrench (not shown) at the glide mechanism. Knee rotations were determined using an electromagnetic tracking device (ETD) receiver and transmitter
A custom testing apparatus was designed to allow measurement of knee rotations during simulated active knee extension (Fig. 1). The testing device consisted of a femoral fixation frame that allowed for rigid attachment of the femur to a bench top using two 4-mm transfixion pins inserted through the femur. The design of the apparatus and the femoral alignment allowed the knee, leg, and foot to extend over the edge of the bench top, using gravity to flex the knee 90°, and determine the initial reference position of tibia varus–valgus angulation and internal–external rotation.
Quadriceps-activated knee extension was performed in each specimen to determine knee kinematics during a simulated cycle of knee extension. To simulate active knee extension, the quadriceps tendon was connected to the stepper motor using a liquid nitrogen freeze clamp [23]. Quadriceps-activated knee extension was performed from 90° to 5° flexion by configuring the stepper motor to produce extension at a rate of 0.5° per second. Five degrees of flexion was chosen as the maximum amount of knee extension due to the inability of four knees to be extended beyond this point using the stepper motor. During quadriceps-activated knee extension, knee rotations (flexion–extension, varus–valgus angulation, and internal–external rotation) from the reference position were determined at a rate of two measurements per second.
The custom testing apparatus also allowed measurement of knee rotations during passive joint stability testing (Fig. 1). A flexion–extension jig was constructed to allow precise duplication of knee flexion angles between knee specimens during passive joint stability testing. A tibial fixation frame was mounted to each specimen using two 4-mm transfixion pins inserted into the distal tibia to allow for controlled measurement of tibial rotations. The tibial fixation frame included an extension rod distal to the foot to which an aluminum glide mechanism was attached. The glide mechanism included rollers to allow for low friction varus–valgus angulation and a bushing connection to the extension rod to allow for internal–external rotation. This design permitted isolated measurement of either varus–valgus or internal–external rotations at fixed flexion angles during applied moments.
Passive joint stability testing was performed in each specimen to determine the knee rotations in response to applied moments. At 0° and 30° of knee flexion, varus–valgus moments of 10 Nm about the knee were applied at the ankle with a force transducer. At 0°, 30°, 60°, and 90° of knee flexion, internal–external rotation moments of 5 Nm were applied through the extension rod of the tibial fixation frame using a torque wrench. Tibial rotation measurements (varus–valgus or internal–external rotation, depending on the applied moments) were determined as the difference in rotation between the position the tibia assumed during the applied moment and the position that the tibia naturally assumed initially at the fixed flexion angle. Tibial rotation measurement were repeated twice, and averaged for statistical analysis. Passive stability testing was performed in eight specimens that could be fully extended to 0°.
Experimentation: testing of intact, divided, and reconstructed sMCL
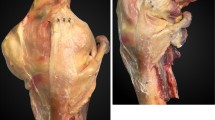
Simulated active knee extension and passive joint stability testing were used to evaluate four experimental conditions: (1) normal knees (intact sMCL), (2) knees with a divided sMCL, (3) knees with a sMCL reconstruction graft length equal to the length of the native sMCL (AnatRecon; Fig. 2), and (4) knees with sMCL reconstruction graft length equal to the length of the patellar tendon (ShortRecon; Fig. 2). At the time of experimentation, the length of the intact sMCL was measured for each specimen from the medial epicondyle of the femur to its proximal tibial insertion beneath the pes anserine tendons. The length of the patellar tendon was measured from the inferior pole of the patella to the proximal insertion on the tibial tuberosity. Additionally, prior to testing, preconditioning of the knee was accomplished through ten manual cycles of passive flexion and extension.
MCL reconstruction with the graft length equal to the length of the patellar tendon (ShortRecon), above, and the length of the anatomic MCL (AnatRecon), below. The graft tendon was transferred to the course of the MCL and fixed to the medial femoral epicondyle with distal fixation on the tibia. Note the divided MCL reflected at its distal insertion
Following simulated active knee extension and passive joint stability testing of the intact sMCL, the sMCL was divided using a standardized protocol. A longitudinal medial incision was used to expose the origin and insertion of the sMCL. The sMCL was sharply divided at the joint line, taking care to avoid injury to the deep MCL or the POL. Simulated active knee extension and passive joint stability testing were then performed on the divided sMCL specimens.
Next, the two experimental sMCL reconstructions were performed, and simulated active knee extension and passive joint stability testing were repeated. The order of reconstructions and testing was randomized for each specimen. The gracilis and semitendinosis tendons were harvested for sMCL reconstruction, obtaining a minimum of 22 cm length for each tendon. For each reconstruction, the tendons were doubled over a post at the medial femoral epicondyle and secured with a spiked ligament washer. For AnatRecon, the hamstring tendon graft was fixed to the proximal tibia with a screw and spiked ligament washer at the anatomic insertion of the sMCL. This resulted in a graft length of 80.5 ± 5.0 mm for AnatRecon. For ShortRecon, the hamstring graft was fixed to the proximal tibia with a screw and spiked ligament washer along the course of the sMCL at a length that corresponded to the length of the patellar tendon. This resulted in a graft length of 38.5 ± 6.0 mm for ShortRecon.
Statistical analysis
To determine the effect that dividing the sMCL and the effect that reconstructing the sMCL with two different graft lengths had on the kinematics and static stability of the normal knee, the experimental data was analyzed using a two-factor analysis of variance (ANOVA). For simulated active knee extension, a two-factor ANOVA was performed at 10° increments of flexion angle. Treatment was set at four levels (intact sMCL, divided sMCL, AnatRecon, and ShortRecon). Given a significant treatment effect, contrasts between the intact sMCL and each of the other three treatments were performed with differences considered significant at P ≤ 0.05. For passive joint stability testing, a separate two-factor ANOVA was performed at each combination of knee flexion angle (0°, 30°, 60°, and 90°) and knee angulation and rotation (varus–valgus angulation and internal and external rotation). The two factors were specimen with eight levels and treatment with four levels. The contrast between normal and each of the other levels was considered significant at P ≤ 0.05. Using this analysis, our statistical power was capable of detecting significant differences as small as 1.6°.
Results
Quadriceps-activated knee extension
Quadriceps-activated knee extension demonstrated no significant differences between AnatRecon (graft length equal to the length of the intact sMCL; 80.5 ± 5.0 mm) and the intact sMCL. However, significant differences were demonstrated between the divided sMCL and the intact sMCL, ShortRecon (graft length equal to the length of the patellar tendon; 38.5 ± 6.0 mm) and the intact sMCL, and ShortRecon and AnatRecon.
During quadriceps-activated knee extension, a pattern of increased valgus angulation occurred in the divided sMCL compared to the intact sMCL (Fig. 3a). The effect of dividing the sMCL caused a significant increase in valgus at 90° of knee flexion (mean difference = 2.5°; P < 0.01) and a visible trend of less than 1.0° throughout the remaining arc of rotation. Similarly, a pattern of increased internal rotation occurred in the divided sMCL compared to the intact sMCL from 0° to 75° of knee flexion (Fig. 3c). The effect of dividing the sMCL caused a significant increase in external rotation at 90° of knee flexion (mean difference = 3.8°; P < 0.01) and a visible trend of less than 2.0° from 75° to 90° of knee flexion.
Varus–valgus angulation at knee flexion angles during quadriceps-activated extension. a An increase in valgus in the divided MCL knee compared to the intact knee with a significant difference detected at 90° of knee flexion (P < 0.01). b Compares rotation data from both MCL reconstructions (reconstruction 1 or AnatRecon, and reconstruction 2 or ShortRecon) with normal knee showing no statistical differences. Data showing internal–external rotation at knee flexion angles during quadriceps-activated knee extension. c Compares the intact knee and the divided MCL knee. There was a significant increase in external rotation in the divided MCL knee at 90° of knee flexion (P < 0.01). d Compares both MCL reconstructions with the intact MCL knee, where a significant increase was found in external rotation of ShortRecon (reconstruction 2) compared to the intact MCL at 60° and 70° of knee flexion, and compared to AnatRecon (reconstruction 1) between 30° and 80° of knee flexion (P < 0.008)
Quadriceps-activated knee extension measurements of knees with a reconstructed sMCL showed no differences in varus–valgus angulations as compared to the intact sMCL over the 90° to 5° range of flexion angles (Fig. 3b). A noticeable, but not significant, increase of approximately 1.3° in valgus angulation of the divided sMCL from the intact sMCL occurred at 15° and 20° of knee flexion. However, quadriceps-activated knee extension measurements of knees with a reconstructed sMCL did show a difference between graft types in internal–external rotation (Fig. 3d). Whereas no significant differences were found in internal–external rotation between AnatRecon and the intact sMCL during knee flexion, a significant increase in external rotation of ShortRecon compared to AnatRecon was found between 30° and 80° of knee flexion (mean difference <3.0° over the range of knee flexion angles; P < 0.008). Further, a significant increase in external rotation of ShortRecon compared to the intact sMCL was found at 60° and 70° of knee flexion (mean difference <2.0° over the range of knee flexion angles; P < 0.008). Additionally, a noticeable, but not significant, increase of less than 3.0° in internal rotation of ShortRecon from the intact sMCL occurred at 15° and 20° of knee flexion.
Passive joint stability testing
Passive joint stability testing demonstrated no significant differences between AnatRecon and the intact sMCL. However, significant differences were demonstrated between the divided sMCL and the intact sMCL, as well as between ShortRecon and AnatRecon.
Passive joint stability testing of varus–valgus angulation demonstrated no significant differences between the divided and intact sMCL at 0° of knee flexion (Fig. 4a). Further, AnatRecon and ShortRecon showed no differences from the intact sMCL at 0° and 30° of knee flexion. However, division of the sMCL caused a significant increase in valgus angulation compared to the intact sMCL at 30° of knee flexion (mean difference = 5.8°; P < 0.005; Fig. 4b).
a Varus and valgus stability testing at 0° of knee flexion. No statistically significant difference was measured for the divided MCL or either reconstruction (reconstruction 1 or AnatRecon, and reconstruction 2 or ShortRecon) of the MCL compared with the intact MCL. Errors bars indicate ± one standard deviation. b Varus and valgus stability testing at 30° of knee flexion. There was a significant increase in valgus in the divided MCL compared to the intact MCL (P < 0.005). Both reconstructions (reconstruction 1 or AnatRecon, and reconstruction 2 or ShortRecon) restore stability. Errors bars indicate ± one standard deviation
In internal rotation (Fig. 5a), passive joint stability testing demonstrated a significant increase in internal rotation of the divided sMCL compared to the intact sMCL at 30° (mean difference = 3.2°; P < 0.005) and 60° (mean difference = 3.4°; P < 0.005).
a Internal rotation stability testing at 0°, 30°, 60°, and 90° of knee flexion. At 30° and 60° of knee flexion, there was a significant increase in internal rotation in the divided MCL compared to the intact MCL (P < 0.005). This increase in rotation is stabilized using either reconstruction (reconstruction 1 or AnatRecon, and reconstruction 2 or ShortRecon). Errors bars indicate ± one standard deviation. b External rotation stability testing at 0°, 30°, 60°, and 90° of knee flexion. At 30°, 60°, and 90° of knee flexion, there was a significant increase in external rotation in the divided MCL compared to the intact MCL (P < 0.005). This increase in rotation is stabilized using either reconstruction, however, AnatRecon (reconstruction 1) offers more resistance to external rotation than ShortRecon (reconstruction 2), with a significant difference found at 90° of knee flexion (P < 0.05). Errors bars indicate ± one standard deviation
In external rotation (Fig. 5b), passive joint stability testing demonstrated a significant increase in external rotation of the divided sMCL compared to the intact sMCL at 30° (mean difference = 5.3°; P < 0.005), 60° (mean difference = 9.4°; P < 0.005), and 90° (mean difference = 9.7°; P < 0.005). Additionally, passive joint stability testing demonstrated a significant increase in external rotation of ShortRecon compared to the AnatRecon at 60° (mean difference = 3.7°; P < 0.05) and 90° (mean difference = 4.9°; P < 0.05).
Discussion
This study evaluated the kinematic and passive stability of two sMCL reconstructions and compared them to the sMCL intact knee. The important finding of this study is that failing to reconstruct the anatomic sMCL length causes measurable alterations in joint kinematics and passive rotational stability of the knee. As would be expected, restoration of the anatomic origin and insertion of the sMCL effectively restores knee kinematics and stability.
Isolated injuries to the sMCL usually heal uneventfully with appropriate non-operative management [1, 5, 13, 14, 21]. Complete rupture of the MCL, including the posterior oblique ligament, can result in chronic valgus laxity of the knee. Non-operative treatment of these more severe MCL injuries can result in poor long-term outcomes [15]. Surgical treatment of severe medial knee injuries is indicated when chronic valgus laxity persists despite an appropriate course of non-operative treatment.
Ligament reconstruction should not only strive to restore joint stability, but also to reproduce normal joint kinematics. The concept of anatomic MCL reconstruction has recently gained popularity [2, 4, 6]. In order to evaluate the effectiveness of an anatomic reconstruction of the sMCL, this study tested static knee stability and simulated active knee extension before and after ligament reconstruction, and compared these measurements to normal knee motions and stability. A non-anatomic MCL reconstruction as an experimental control was also tested.
This current experimental model confirmed the importance of the sMCL as a significant valgus and rotational stabilizer in the flexed knee. During static stability testing, a 5.8° increase in valgus angulation in the sMCL deficient knee flexed to 30° was found, compared to an MCL intact knee. sMCL insufficiency also resulted in a significant increase in internal rotation of the tibia with the knee flexed 30° or 60° and a significant increase in external rotation of the tibia with the knee flexed 30°, 60°, or 90°. Laceration of the sMCL did not, however, result in a significant change in valgus or rotational stability in a fully extended knee. These results confirm previous studies that show the sMCL is an important valgus and rotational stabilizer of the flexed knee, and that the sMCL does not contribute significantly to the stability of an extended knee when the cruciates and POL are intact [7–9, 22, 26]. Furthermore, by demonstrating the expected valgus and rotational stability in the sMCL deficient knee, the ability of the electromagnetic joint tracking system utilized in this study to reproducibly measure angular and rotational changes in our cadaveric knee model was validated.
In addition to static knee stability testing, this study showed that sMCL deficiency results in kinematic alterations during simulated active knee extension. An increase in knee valgus alignment during active knee extension, free of applied valgus load was measured. Likewise, the sMCL deficient knees showed a significant increase in tibial internal rotation from 5° to 70° of knee flexion and a significant increase in tibial external rotation at 90° of flexion. This represents the first demonstration of altered knee motions secondary to sMCL insufficiency, independent of applied valgus or rotational loads to the knee.
Next, the ability of two different sMCL reconstructions to restore normal knee motions and valgus and rotational stability of the knee was evaluated. The first reconstruction attempted to reproduce the anatomic position and length of the native sMCL. No significant difference in the simulated active knee kinematics when comparing the anatomic sMCL reconstruction with the intact sMCL knee kinematics was found. Additionally, static valgus and rotational stability of the anatomically reconstructed knee were similar to that found in the sMCL intact knee.
The second reconstruction that was tested was made purposefully non-anatomic. The origin and course of the second reconstruction were identical to the first reconstruction, but the insertion was made more proximal, by using a shorter tendon graft. The non-anatomic sMCL reconstruction resulted in measurable alterations in knee kinematics and static stability. There was a significant increase in tibial external rotation during simulated active knee extension and during static stability testing in the flexed knee. No difference was found with respect to valgus stability when the non-anatomic reconstruction was compared with either the anatomic reconstruction or the intact sMCL knee.
Several techniques have been described to operatively restore medial knee stability. The majority of these techniques are non-anatomic repairs or reconstructions. The advancement or imbrication of the torn ligaments and capsule has previously been advocated [18, 20]. Another described surgery involves re-routing the semitendinosis, using the distal insertion of the semitendinosis as the non-anatomic insertion of the reconstructed sMCL [3, 16, 30]. Given the altered knee motions and rotational stability measured in this study after a subtle alteration in sMCL anatomy, one could predict that the previously described non-anatomic sMCL repairs/reconstructions would either fail through attrition or overly constrain a knee joint.
More recently, anatomic reconstruction of the MCL has been proposed and evaluated in a cadaveric model [4]. Static knee stability was restored to near normal in the study by Coobs et al., but active motion was not assessed and a non-anatomic reconstruction was not performed for comparison. This study showed that active knee kinematics in addition to static stability could be normalized with an anatomic sMCL reconstruction. Additionally, this study showed the potentially deleterious effects of non-anatomic MCL reconstruction.
The primary focus of this study was to isolate the effect that altering sMCL graft length has on knee kinematics and stability. In reality, severe medial instability of the knee also involves the deep MCL and the POL. The deep MCL certainly contributes to the stability of the medial side of the knee, but based on the results of other studies, it plays a secondary role compared to the sMCL and POL [29]. It is unknown whether the deep MCL should be repaired or reconstructed during medial knee stabilization. The POL, however, is clearly an important element in medial knee stability and its effects are seen more in the extended knee. The relationship between the sMCL and the POL have been described as reciprocal and complementary [27, 28] and repair or reconstruction of the POL, in addition to the sMCL, should always be considered when faced with severe valgus knee instability. Coobs et al. [4] have recently described an anatomic medial knee reconstruction that includes both the sMCL and POL to address severe valgus instability.
There are additional limitations to this study. True active knee kinematics can only be estimated with a cadaveric model. The quadriceps-activated knee extension in this study is an attempt to mimic the kinematics of active open chain knee extension. This model fails to include the possible influence that resting tension or co-contraction of the hamstring tendons may have on knee kinematics and static stability. Additionally, this study focused on medial knee stability and kinematics at time zero after ligament reconstruction. The longer, more anatomic reconstruction of the sMCL normalized knee kinematics and stability at time zero in this study. The longer graft may theoretically have a higher tendency to stretch or loosen when faced with cyclic loading compared with a shorter graft. This is something that this study did not evaluate. Another limit to cadaveric studies, in general, is their inability to demonstrate the safety and efficacy of new techniques when applied clinically.
There is currently a paucity of clinical data on the outcomes of anatomic MCL reconstructions. The clinical scenario in which MCL reconstruction is indicated is uncommon. Currently, MCL reconstruction in the knee can only be recommended when more conventional treatments such as bracing and cruciate ligament reconstruction (when indicated) have failed to relieve symptoms of valgus instability. In such circumstances, the results of this study suggest that anatomic sMCL reconstruction would be recommended over previously described medial capsule advancement and non-anatomic reconstruction procedures. Future clinical work needs to be done to help identify when sMCL, POL or deep MCL reconstruction is indicated. Ideally, clinical trials will eventually compare the results of non-operative treatment of medial knee instability with the results of ligament repair and ligament reconstruction.
Conclusion
This current study presents further in vitro evidence supporting anatomic reconstruction over non-anatomic reconstruction of the sMCL when attempting to restore knee kinematics and stability to a sMCL deficient knee. Furthermore, this study demonstrates that even slight deviations in sMCL anatomy, such as ligament length, result in measurable alterations in knee motions and stability.
References
Ballmer PM, Jakob RP (1988) The non operative treatment of isolated complete tears of the medial collateral ligament of the knee. A prospective study. Arch Orthop Trauma Surg 107:273–276
Borden PS, Kantaras AT, Caborn DN (2002) Medial collateral ligament reconstruction with allograft using a double-bundle technique. Arthroscopy 18:E19
Bosworth DM (1952) Transplantation of the semitendinosus for repair of laceration of medial collateral ligament of the knee. J Bone Joint Surg Am 34:196–202
Coobs BR, Widjicks CA, Armitage BM et al (2010) An in vitro analysis of an anatomical medial knee reconstruction. Am J Sports Med 38:339–347
Ellsasser JC, Reynolds FC, Omohundro JR (1974) The non-operative treatment of collateral ligament injuries of the knee in professional football players. An analysis of seventy-four injuries treated non-operatively and twenty-four injuries treated surgically. J Bone Joint Surg Am 56:1185–1190
Feeley BT, Muller MS, Allen AA et al (2009) Biomechanical comparison of medial collateral ligament reconstructions using computer-assisted navigation. Am J Sports Med 37:1123–1130
Griffith CJ, LaPrade RF, Johansen S et al (2009) Medial knee injury: part 1, static function of the individual components of the main medial knee structures. Am J Sports Med 37:1762–1770
Grood ES, Noyes FR, Butler DL, Suntay WJ (1981) Ligamentous and capsular restraints preventing straight medial and lateral laxity in intact human cadaver knees. J Bone Joint Surg Am 63:1257–1269
Haimes JL, Wroble RR, Grood ES, Noyes FR (1994) Role of the medial structures in the intact and anterior cruciate ligament-deficient knee. Limits of motion in the human knee. Am J Sports Med 22:402–409
Hillard-Sembell D, Daniel DM, Stone ML et al (1996) Combined injuries of the anterior cruciate and medial collateral ligaments of the knee. Effect of treatment on stability and function of the joint. J Bone Joint Surg Am 78:169–176
Hughston JC (1994) The importance of the posterior oblique ligament in repairs of acute tears of the medial ligaments in knees with and without an associated rupture of the anterior cruciate ligament. Results of long-term follow-up. J Bone Joint Surg Am 76:1328–1344
Hughston JC, Eilers AF (1973) The role of the posterior oblique ligament in repairs of acute medial (collateral) ligament tears of the knee. J Bone Joint Surg Am 55:923–940
Indelicato PA (1983) Non-operative treatment of complete tears of the medial collateral ligament of the knee. J Bone Joint Surg Am 65:323–329
Indelicato PA, Hermansdorfer J, Huegel M (1990) Nonoperative management of complete tears of the medial collateral ligament of the knee in intercollegiate football players. Clin Orthop Relat Res 256:174–177
Kannus P (1988) Long-term results of conservatively treated medial collateral ligament injuries of the knee joint. Clin Orthop Relat Res 226:103–112
Kim SJ, Choi NH, Shin SJ (2001) Semitendinosus tenodesis for medial instability of the knee. Arthroscopy 17:660–663
Milne AD, Chess DG, Johnson JA, King GJ (1996) Accuracy of an electromagnetic tracking device: a study of the optimal range and metal interference. J Biomech 29:791–793
Nicholas JA (1973) The five-one reconstruction for anteromedial instability of the knee. Indications, technique, and the results in fifty-two patients. J Bone Joint Surg Am 55:899–922
Noyes FR, Barber-Westin SD (1995) The treatment of acute combined ruptures of the anterior cruciate and medial ligaments of the knee. Am J Sports Med 23:380–389
O’Donoghue DH (1973) Reconstruction for medial instability of the knee. J Bone Joint Surg Am 55:941–954
Reider B, Sathy MR, Talkington J et al (1994) Treatment of isolated medial collateral ligament injuries in athletes with early functional rehabilitation. A 5-year follow-up study. Am J Sports Med 22:470–477
Robinson JR, Bull AM, Thomas RR, Amis AA (2006) The role of the medial collateral ligament and posteromedial capsule in controlling knee laxity. Am J Sports Med 34:1815–1823
Sharkey NA, Smith TS, Lundmark DC (1995) Freeze clamping musculo-tendinous junctions for in vitro simulation of joint mechanics. J Biomech 28:631–635
Slocum DB, Larson RL (1968) Pes anserinus transplantation. A surgical procedure for control of rotatory instability of the knee. J Bone Joint Surg Am 50:226–242
Warren LF, Marshall JL (1979) The supporting structures and layers on the medial side of the knee: an anatomical analysis. J Bone Joint Surg Am 61:56–62
Warren LF, Marshall JL, Girgis F (1974) The prime static stabilizer of the medical side of the knee. J Bone Joint Surg Am 56:665–674
Wijdicks CA, Griffith CJ, LaPrade RF et al (2009) Medial knee injury: part 2, load sharing between the posterior oblique ligament and superficial medial collateral ligament. Am J Sports Med 37:1771–1776
Wijdicks CA, Griffith CJ, Johansen S et al (2010) Injuries to the medial collateral ligament and associated medial structures of the knee. J Bone Joint Surg Am 92:1266–1280
Wijdicks CA, Ewart DT, Nuckley DJ et al (2010) Structural properties of the primary medial knee ligaments. Am J Sports Med 38:1638–1646
Yoshiya S, Kuroda R, Mizuno K et al (2005) Medial collateral ligament reconstruction using autogenous hamstring tendons: technique and results in initial cases. Am J Sports Med 33:1380–1385
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Van den Bogaerde, J.M., Shin, E., Neu, C.P. et al. The superficial medial collateral ligament reconstruction of the knee: effect of altering graft length on knee kinematics and stability. Knee Surg Sports Traumatol Arthrosc 19 (Suppl 1), 60–68 (2011). https://doi.org/10.1007/s00167-011-1519-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-011-1519-8