Abstract
Background
Biomechanical comparison of four different Speed-Bridge configurations with or without medial or lateral row reinforcement. Reinforcement of the knotless Speed-Bridge double-row repair technique with additional medial mattress- or lateral single-stitches was hypothesized to improve biomechanical repair stability at time zero.
Methods
Controlled laboratory study: In 36 porcine fresh-frozen shoulders, the infraspinatus tendons were dissected and shoulders were randomized to four groups: (1) Speed-Bridge technique with single tendon perforation per anchor (STP); (2) Speed-Bridge technique with double tendon perforation per anchor (DTP); (3) Speed-Bridge technique with medial mattress-stitch reinforcement (MMS); (4) Speed-Bridge technique with lateral single-stitch reinforcement (LSS). All repairs were cyclically loaded from 10–60 N up to 10–200 N (20 N stepwise increase) using a material testing device. Forces at 3 and 5 mm gap formation, mode of failure and maximum load to failure were recorded.
Results
The MMS-technique with double tendon perforation showed significantly higher ultimate tensile strength (338.9 ± 90.0 N) than DTP (228.3 ± 99.9 N), LSS (188.9 ± 62.5 N) and STP-technique (122.2 ± 33.8 N). Furthermore, the MMS-technique provided increased maximal force resistance until 3 and 5 mm gap formation (3 mm: 77.8 ± 18.6 N; 5 mm: 113.3 ± 36.1 N) compared with LSS, DTP and STP (P < 0.05 for each 3 and 5 mm gap formation). Failure mode was medial row defect by tendon sawing first, then laterally. No anchor pullout occurred.
Conclusion
Double tendon perforation per anchor and additional medial mattress stitches significantly enhance biomechanical construct stability at time zero in this ex vivo model when compared with the all-knotless Speed-Bridge rotator cuff repair.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arthroscopic double-row repair techniques of the rotator cuff have been shown to provide increased biomechanical strength at time zero when compared with single-row repair [19, 23, 24, 26, 38]. Besides quadruple medial and lateral tendon perforations, transosseous-equivalent techniques such as the Suture Bridge have been proposed [29].
Recently, all-knotless repairs have been introduced as a further development [3, 32, 37]. Among others, the Speed-Bridge technique combines quick arthroscopic application and eliminates medial and lateral knot impingement. Stable biomechanical properties were proposed due to a ‘self-reinforcement’ mechanism of the tendon underneath the sutures [6]. Since the eyelet at the tip of the anchor allows suture limb fixation between anchor thread and bone, repair failure through eyelet breakage seems to be overcome. However, recent biomechanical studies have emphasized the importance of medial row augmentation [2, 8, 31]. Therefore, all-knotless techniques potentially may result in weaker properties of tendon-bone fixation. Possible failure sites for this construct are suture cutting through the tendon, anchor loosening or breakage. Furthermore, the Speed-Bridge technique as originally described is based upon a single tendon perforation per anchor with two broad laces, which may cause remarkable friction and tissue harm. In particular, strong synthetic suture materials tend to threaten or harm attached degenerated soft tissues [12, 21, 28]. This may potentially weaken the medial row where pull transmission to the bone occurs first. Furthermore, increased micro motion of the medial tendon portion may occur since no stabilizing knots attach it to the footprint.
In order to address these potential weaknesses of the knotless Speed-Bridge technique, it is the purpose of the present biomechanical study to investigate the construct strength of three different modifications of the Speed-Bridge technique at time zero, varying in the technique of medial tendon perforation and the use of medial or lateral row augmentation.
We hypothesized that there would be improved resistance to failure and less gap formation with a reinforced tendon grasping technique (medially or laterally) or additional medial mattress stitches when compared with all-knotless Speed-Bridge repairs.
Materials and methods
Shoulder dissection
Thirty-six fresh-frozen porcine shoulders (right side, age: 6 months, gender: male) were stored at −20°C until thawed at room temperature 5 h prior to use. All soft tissues were dissected to isolate the infraspinatus tendon attached to the humeral head. The infraspinatus tendon was then sharply detached from its bony footprint insertion on the greater tuberosity (with no humeral fibrocartilage attached) in order to mimick a full-thickness tear of the human supraspinatus tendon [21, 23, 28, 31]. Footprint dimensions and tendon thickness/caliper at the proximal and distal end of the bony insertion were obtained using a digital caliper gauge and documented. To prevent tissue dehydration, immediate processing and testing of all shoulders were ensured and tendon tissue was kept moist using sprayed isotonic saline (0.9% sodium chloride).
Preparation
All reconstruction techniques consisted of identical number and kind of anchors (two medially, two laterally) and were performed by a single investigator.
Medial row
Speed-Bridge repair consisted of two medial anchors (Bio-SwiveLock C 4.75 mm, PLLA, loaded with one 2 mm broad, lace-like FiberTape; Arthrex, Naples, FL). These were inserted to the medial aspect of the footprint, about 3 mm lateral to the articular surface, in a 45° angle (deadman’s angle [4]) after using an initiator. The tendon was reduced and perforated 12–14 mm medially using the Scorpion Suture Passer (Arthrex, Naples, FL): either once per anchor with simultaneous passage of both FiberTape-limbs or twice per anchor, approximately 3 mm apart horizontally, with separate passage of each limb. Each technique was performed in nine specimens, respectively.
Technique 1
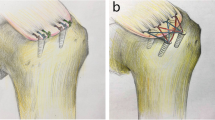
Speed-Bridge technique with single tendon perforation per anchor (STP, all knotless): No knot was tied medially. After crossing the FiberTapes, both were fixed laterally using another Bio-SwiveLock C 4.75 mm (Fig. 1).
Technique 2
Speed-Bridge technique with double tendon perforation per anchor (DTP, all knotless): The same technique as described previously only varying in a separate passage of each FiberTape-limb, approximately 3 mm apart horizontally (Fig. 2).
Technique 3
Speed-Bridge technique with medial mattress-stitch reinforcement (MMS): Prior to separate tendon perforation for each FiberTape-limb medially as described previously, one additional mattress stitch just 3 mm medially was prepared as measured with a digital caliper gauge. The #2 Fiberwire (as preloaded with the anchor for eyelet security) penetrated the tendon 4 mm apart in a horizontal fashion (Fig. 3).
To achieve maximum loop and knot security, fisherman’s sliding knot was used and reinforced with three reversed-post half-hitches to maximize knot holding capacity [5, 9] after completion of the lateral row.
Technique 4
Speed-Bridge technique with lateral single-stitch reinforcement (LSS): Besides separate tendon perforation for each FiberTape-limb medially as described previously, one additional simple stitch was added laterally (Fig. 4). In clinical practice, this procedure minimizes the formation of ‘dog-ears’ or bulging of the lateral tendon aspect. For this purpose, the #2 Fiberwire as provided with the lateral anchor was used and fisherman’s sliding knot with three reversed-post half-hitches was applied again [5, 9].
Biomechanical testing
For biomechanical testing, the medial free limb of the tendon was wrapped with a cotton bandage to increase friction. Subsequently, it was attached to a steel-wired extension hull (‘chinese finger trap’), then cross-suture secured (No. 5 Fiberwire, Arthrex, Naples, FL) to avoid tendon slippage. In a previous study, this fixation method revealed solid tendon fixation up to 600 N and superior interface grip when compared with other soft tissue fixation techniques [34].
The humerus was then sawed and adjusted to vertical load application in order to simulate physiological load conditions as seen in human supraspinatus tendon [12]. It was fixed to biomechanical testing moulds using bone cement (Beracryl, Fa. Troller, Fulenbach, Switzerland).
Prior to biomechanical testing, ultraviolet light reflecting trackballs were needle pinned to the tendon-bone construct: (a) medially to the medial row, (b) in between both suture rows, (c) to the greater tuberosity and (d) to the fixation mould as described previously [31]. An optical 3D-tracking device with an accuracy of 0.1 mm three dimensionally was used for the detection of gap formation (Qualisys® AB, Gothenburg, Sweden).
Loading evaluation
Preload was set at 10 N, followed by cyclic loading of all reconstructions with n = 50 cycles of 10–60 N on a material testing device (Model 1455, Zwick, Ulm, Germany). Subsequently, load was increased with 20 N stepwise for another n = 50 cycles, respectively (50× 10–80 N, 50× 10–100 N, 50× 10–120 N,…) until 50× 10–200 N. Specimen surviving 50 cycles of 10–200 N were tested until maximum load to failure [23]. Pulling speed was set to 350 mm/min for regular test cycles and to 500 mm/min for ultimate tensile strength beyond 200 N [31].
Parameters
The definition of failure was gap formation of both 3.0 and 5.0 mm between the medial tendon marker and the marker at the bony tuberosity [2, 20, 22, 25, 31].
Definition of ultimate failure was either complete tendon tear or loss of load applied of greater than 50% (as obtained by the material testing device). Mode and place of failure were documented.
Statistical analysis
Tendon thickness as well as the force until gap formation load to failure was analysed with nonparametric overall Kruskal–Wallis tests. In case of significance, pairwise post hoc examinations were conducted using the Mann–Whitney U test. A power analysis using Pitman’s asymptotic relative efficiency revealed that a large pairwise effect size (equivalent to Cohen’s d = 1.3) can be detected with a significance level α = 0.05 and a power >0.80 with nine specimens per reconstruction.
Statistical analysis was performed using PASW 17 for Windows software (SPSS Inc, Chicago, IL).
Results
Tendon thickness and footprint dimensions
There was no significant difference between all four groups with respect to footprint dimensions or tendon thickness (n.s.; Table 1).
Mode of failure
In all cases, the repair failed by suture cutting through the tendon, initially at the medial row and progressing laterally until lateral row failure. No anchor pullout was observed.
Different numbers of reconstructed units survived all test cycles until 10–200 N and were subsequently tested until load to failure: n = 0 units from STP-group, n = 2 shoulders from DTP-group, n = 7 from MMS-group and n = 1 shoulders from LSS-group.
Gap formation 3/5 mm
The average force to create 3 mm gap formation between the medial tendon reflector and the humeral head differed overall between the reconstruction techniques (\( \chi_{(3)}^{2} \) = 11.0, P = 0.01) and was significantly higher for MMS-repair (77.8 ± 18.6 N) compared with LSS (62.2 ± 6.7 N, Mann–Whitney U Test: Z = 2.1, P = 0.04), DTP (60.0 ± 0 N, Z = 2.5, P = 0.01) and STP (62.2 ± 6.7 N, Z = 2.1, P = 0.04; Fig. 5a).
The average force to create 5 mm gap formation between the medial tendon reflector and the humeral head again differed between the reconstruction techniques (\( \chi_{(3)}^{2} \) = 10.8, P = 0.01) and was also significantly higher for MMS-repair (113.3 ± 36.1 N) compared with LSS (80.0 ± 20.0 N, Z = 2.0, P = 0.04), DTP (73.3 ± 20.0 N, Z = 2.5, P = 0.01) and STP (68.9 ± 14.5 N, Z = 2.7, P < 0.01; Fig. 5b).
The average load of 68.9 N at 5 mm gap formation in the classic STP-technique was still below the average load at 3 mm gap formation in the MMS-technique. Hence, separate tendon perforation and addition of a medial mattress suture prevented 2 mm gap formation at 70 N load.
Maximum load to failure
The average force until total failure differed between the reconstruction techniques (\( \chi_{(3)}^{2} \) = 20.8, P < 0.01) and was lowest for STP-repair (122.2 ± 33.8 N), for LSS (188.9 ± 62.5 N) and for DTP-technique (228.3 ± 99.9 N). With an average load of 338.9 ± 90.0 N, MMS-technique revealed significantly higher resistance compared with LSS and STP (Z > 2.9, P < 0.01), and a trend towards higher resistance compared with DTP (Z = 1.9, n.s.). Only by separate instead of simultaneous tendon perforations (DTP vs. STP), DTP-repair showed a significantly higher resistance than STP-technique (Z = 3.1, P < 0.01; Fig. 6).
Discussion
The present study found that separate tendon perforation as well as additional medial mattress stitches significantly improve biomechanical stability of a knotless double-row Speed-Bridge repair at time zero in an animal cadaver study.
With only little additional effort in terms of all-knotless repairs, separate medial tendon perforation for each suture limb (DTP) resulted in enhanced load to failure. However, no significant effect towards gap formation was observed when compared with all-knotless single medial tendon perforation per anchor (STP).
Medial row augmentation using horizontal mattress stitches (MMS) improved both maximum loads to failure as well as force resistance to 3 and 5 mm gap formation. It revealed nearby triple load to failure when compared with regular STP-repair. Though leaving the concept of knotless double-row repair, this technique requires no additional suture expenses when compared with regular STP-repair type. As a clinical limitation, additional knot tying medially may cause knot impingement when compared with knotless medial row repairs.
However, lateral addition of simple stitches (LSS, meant to reduce lateral ‘dog ear’-deformities) did not improve repair stability at all.
These findings demonstrate the important biomechanical contribution of medial row tendon fixation in rotator cuff repair. This observation is in accordance to previous biomechanical studies regarding knot-type double-row repairs [2, 31]. Busfield et al. found completely knotless double-row repairs (Suture-Bridge) to be significantly weaker when compared with regular knot-type Suture-Bridge repair (human cadaver study) [8].
In a previous study, several Suture-Bridge repair techniques were investigated by our study group [31]. The present MMS-technique with one mattress stitch per anchor medially was biomechanically weaker (F max 338.9 N, 3 mm: 77.8 N, 5 mm: 113.3 N) when compared with Suture-Bridge repair with two medial mattress sutures per anchor (F max 368 N; 3 mm: 90 N; 5 mm: 128 N) [31]. Both studies followed an identical biomechanical protocol, and both techniques consist of comparable lateral knotless tendon fixation. In contrast, Spang et al. found no significant biomechanical difference between both techniques after these had been strengthened with one additional medial mattress per medial anchor (ovine cadaver study) [35]. Both broad FiberTapes were passed through the tendon simultaneously, which may cause friction and a broad intratendinous canal. However, the present study found that passing both suture limbs separately with a horizontal distance of 3–4 mm significantly increases biomechanical stability when compared with simultaneous application.
Hence, a major biomechanical contribution of medial row fixation must be assumed in these comparable settings. Even enhanced single rows with six simple stitches medially (using two triple-loaded anchors) had proven better biomechanical properties than current double-row repair techniques [2].
Apart from biomechanical cadaver investigations, clinical observations have found structural defects of the tendon medially to the footprint in revision cases following double-row repair [36, 39]. Radiographic follow-up examinations of Suture-Bridge double-row repairs at 6–24 months postoperatively described remnant, apparently well-fixed cuff tissue at the insertion site [11]. In contrast to single-row techniques, retears occurred more medially to the repair construct towards the musculotendinous junction. These sites of failure match with tear patterns as observed in previous biomechanical cadaver studies [31].
Presently, failure or tear of reconstructed tendon-bone units occurred medially first at the site of load transmission from tendon to bone. Subsequently, strong synthetic suture materials were combing the tendon towards the lateral row until total failure. From our observations, the close tendon fixation against the footprint medially was crucial to delay tendon slippage around the strong synthetic sutures as long as possible. Once tendon started to migrate medially, sutures began sawing and early construct failure was inevitable.
All-knotless double-row repair of the rotator cuff potentially harbours several beneficial aspects when compared with knot-type repairs as with the Suture-Bridge technique [29]. Another all-knotless double-row repair technique consisting of FiberChain suture material (Arthrex, Naples, FL) showed equivalent stability when compared with a double-row reconstruction, suggesting a self-reinforcing mechanism when tension is applied (human cadaver study) [6].
Furthermore, knotless surgical repair as with the Speed Bridge is quicker and facilitated in terms of easier suture limb management when compared with Suture-Bridge repair. As a further development based upon the latter, the Speed-Bridge technique consisting of 2-mm broad fibre laces is furthermore supposed to better distribute pressure to the underlying tendon tissue. The SwiveLock-anchor features its eyelet at the tip, fixing the suture limb between anchor thread and bone and hence minimizing repair failure through eyelet breakage.
Along with numerous other biomechanical studies, the present study represents a time zero ex vivo analysis. Obtained porcine footprint dimensions of the infraspinatus are comparable to human supraspinatus dimensions [13, 14]. Neither tendon nor footprint dimensions differed significantly between the tested techniques, which allows objective biomechanical comparison.
However, comparable anatomic references do not allow immediate transfer to human conditions. In particular, degenerated tendon and bone tissue strength of elderly human patients may not be represented by juvenile swines with strong tendon and trabecular bone. Histologic evidence of tendon degeneration as observed in elderly patients is associated with reduced tensile strength [33]. Anchor loosening must be assumed to be more often in osteopenic human bone as a consequence of inactivity in chronic tears [27]. The correlation of bone-mass density and gap formation in human cadaver studies was described [3]. On the other hand, comparable bone density was described in 8-month-old porcine and middle-aged human shoulders [23]. As a strength, the present model focused on the tendon-suture interface for comparing biomechanical stability since the anchor-bone interface as a potential failure site was excluded. Standardized comparison of different surgical techniques was feasible with less variation in age and tissue quality than in human cadavers.
Improved biomechanical techniques in double-row reconstructions do not necessarily translate into superior clinical performance. Despite recent biomechanical approaches, clinical and radiographic studies found only marginal or no advantage with double-row reconstructions (knot-type repairs) [1, 7, 10, 16, 18, 30].
This effect may be explained by tendon tissue strangulation through strong adaption against the bony insertion [15, 17]. Maximized biomechanical construct stability at time zero may endanger microcirculation and biological regeneration, which is mandatory for durable tendon-bone reintegration.
Conclusion
Separate tendon perforation and additional medial mattress stitches significantly enhance biomechanical construct stability at time zero in this porcine ex vivo model when compared with the all-knotless Speed-Bridge rotator cuff repair. In the clinical setting, possible technical advantages such as time-saving (but weaker) knotless repair must be counterbalanced with this technically more demanding procedure for the sake of maximized initial stability.
References
Aydin N, Kocaoglu B, Guven O (2010) Single-row versus double-row arthroscopic rotator cuff repair in small- to medium-sized tears. J Should Elbow Surg 19:722–725
Barber FA, Herbert MA, Schroeder FA, Aziz-Jacobo J, Mays MM, Rapley JH (2010) Biomechanical advantages of triple-loaded suture anchors compared with double-row rotator cuff repairs. Arthroscopy 26:316–323
Brown BS, Cooper AD, McIff TE, Key VH, Toby EB (2008) Initial fixation and cyclic loading stability of knotless suture anchors for rotator cuff repair. J Should Elbow Surg 17:313–318
Burkhart SS (1995) The deadman theory of suture anchors: observations along a south Texas fence line. Arthroscopy 11:119–123
Burkhart SS (2000) A stepwise approach to arthroscopic rotator cuff repair based on biomechanical principles. Arthroscopy 16:82–90
Burkhart SS, Adams CR, Burkhart SS, Schoolfield JD (2009) A biomechanical comparison of 2 techniques of footprint reconstruction for rotator cuff repair: the SwiveLock-FiberChain construct versus standard double-row repair. Arthroscopy 25:274–281
Burks RT, Crim J, Brown N, Fink B, Greis PE (2009) A prospective randomized clinical trial comparing arthroscopic single- and double-row rotator cuff repair: magnetic resonance imaging and early clinical evaluation. Am J Sports Med 37:674–682
Busfield BT, Glousman RE, McGarry MH, Tibone JE, Lee TQ (2008) A biomechanical comparison of 2 technical variations of double-row rotator cuff fixation: the importance of medial row knots. Am J Sports Med 36:901–906
Chan KC, Burkhart SS, Thiagarajan P, Goh JC (2001) Optimization of stacked half-hitch knots for arthroscopic surgery. Arthroscopy 17:752–759
Charousset C, Grimberg J, Duranthon LD, Bellaiche L, Petrover D (2007) Can a double-row anchorage technique improve tendon healing in arthroscopic rotator cuff repair?: a prospective, nonrandomized, comparative study of double-row and single-row anchorage techniques with computed tomographic arthrography tendon healing assessment. Am J Sports Med 35:1247–1253
Cho NS, Yi JW, Lee BG, Rhee YG (2010) Retear patterns after arthroscopic rotator cuff repair: single-row versus suture bridge technique. Am J Sports Med 38:664–671
Cummins CA, Strickland S, Appleyard RC, Szomor ZL, Marshall J, Murrell GA (2003) Rotator cuff repair with bioabsorbable screws: an in vivo and ex vivo investigation. Arthroscopy 19:239–248
Curtis AS, Burbank KM, Tierney JJ, Scheller AD, Curran AR (2006) The insertional footprint of the rotator cuff: an anatomic study. Arthroscopy 22:609, e601
Dugas JR, Campbell DA, Warren RF, Robie BH, Millett PJ (2002) Anatomy and dimensions of rotator cuff insertions. J Should Elbow Surg 11:498–503
Flatow EL (1996) Tendon-healing in goats. J Bone Joint Surg Am 78:1785–1786
Franceschi F, Ruzzini L, Longo UG, Martina FM, Zobel BB, Maffulli N, Denaro V (2007) Equivalent clinical results of arthroscopic single-row and double-row suture anchor repair for rotator cuff tears: a randomized controlled trial. Am J Sports Med 35:1254–1260
Gerber C, Schneeberger AG, Beck M, Schlegel U (1994) Mechanical strength of repairs of the rotator cuff. J Bone Joint Surg Br 76:371–380
Grasso A, Milano G, Salvatore M, Falcone G, Deriu L, Fabbriciani C (2009) Single-row versus double-row arthroscopic rotator cuff repair: a prospective randomized clinical study. Arthroscopy 25:4–12
Kim DH, Elattrache NS, Tibone JE, Jun BJ, DeLaMora SN, Kvitne RS, Lee TQ (2006) Biomechanical comparison of a single-row versus double-row suture anchor technique for rotator cuff repair. Am J Sports Med 34:407–414
Klinger HM, Steckel H, Spahn G, Buchhorn GH, Baums MH (2007) Biomechanical comparison of double-loaded suture anchors using arthroscopic Mason-Allen stitches versus traditional transosseous suture technique and modified Mason-Allen stitches for rotator cuff repair. Clin Biomech (Bristol, Avon) 22:106–111
Koganti AK, Adamson GJ, Gregersen CS, Pink MM, Shankwiler JA (2006) Biomechanical comparison of traditional and locked suture configurations for arthroscopic repairs of the rotator cuff. Am J Sports Med 34:1832–1838
Lee S, Mahar A, Bynum K, Pedowitz R (2005) Biomechanical comparison of bioabsorbable sutureless screw anchor versus suture anchor fixation for rotator cuff repair. Arthroscopy 21:43–47
Lorbach O, Bachelier F, Vees J, Kohn D, Pape D (2008) Cyclic loading of rotator cuff reconstructions: single-row repair with modified suture configurations versus double-row repair. Am J Sports Med 36:1504–1510
Mahar A, Tamborlane J, Oka R, Esch J, Pedowitz RA (2007) Single-row suture anchor repair of the rotator cuff is biomechanically equivalent to double-row repair in a bovine model. Arthroscopy 23:1265–1270
Mazzocca AD, Millett PJ, Guanche CA, Santangelo SA, Arciero RA (2005) Arthroscopic single-row versus double-row suture anchor rotator cuff repair. Am J Sports Med 33:1861–1868
Meier SW, Meier JD (2006) The effect of double-row fixation on initial repair strength in rotator cuff repair: a biomechanical study. Arthroscopy 22:1168–1173
Meyer DC, Fucentese SF, Koller B, Gerber C (2004) Association of osteopenia of the humeral head with full-thickness rotator cuff tears. J Should Elbow Surg 13:333–337
Milano G, Grasso A, Zarelli D, Deriu L, Cillo M, Fabbriciani C (2008) Comparison between single-row and double-row rotator cuff repair: a biomechanical study. Knee Surg Sports Traumatol Arthrosc 16:75–80
Park HB, Keyurapan E, Gill HS, Selhi HS, McFarland EG (2006) Suture anchors and tacks for shoulder surgery, part II: the prevention and treatment of complications. Am J Sports Med 34:136–144
Park JY, Lhee SH, Choi JH, Park HK, Yu JW, Seo JB (2008) Comparison of the clinical outcomes of single- and double-row repairs in rotator cuff tears. Am J Sports Med 36:1310–1316
Pauly S, Kieser B, Schill A, Gerhardt C, Scheibel M (2010) Biomechanical comparison of 4 double-row suture-bridging rotator cuff repair techniques using different medial-row configurations. Arthroscopy 26:1281–1288
Redziniak DE, Hart J, Turman K, Treme G, Lunardini D, Miller MD, Diduch DR (2009) Arthroscopic rotator cuff repair using the Opus knotless suture anchor fixation system. Am J Sports Med 37:1106–1110
Sano H, Ishii H, Yeadon A, Backman DS, Brunet JA, Uhthoff HK (1997) Degeneration at the insertion weakens the tensile strength of the supraspinatus tendon: a comparative mechanical and histologic study of the bone-tendon complex. J Orthop Res 15:719–726
Schottle P, Goudakos I, Rosenstiel N, Hoffmann JE, Taylor WR, Duda GN, Heller MO (2009) A comparison of techniques for fixation of the quadriceps muscle-tendon complex for in vitro biomechanical testing of the knee joint in sheep. Med Eng Phys 31:69–75
Spang JT, Buchmann S, Brucker PU, Kouloumentas P, Obst T, Schroder M, Burgkart R, Imhoff AB (2009) A biomechanical comparison of 2 transosseous-equivalent double-row rotator cuff repair techniques using bioabsorbable anchors: cyclic loading and failure behavior. Arthroscopy 25:872–879
Trantalis JN, Boorman RS, Pletsch K, Lo IK (2008) Medial rotator cuff failure after arthroscopic double-row rotator cuff repair. Arthroscopy 24:727–731
Vaishnav S, Millett PJ (2010) Arthroscopic rotator cuff repair: scientific rationale, surgical technique, and early clinical and functional results of a knotless self-reinforcing double-row rotator cuff repair system. J Should Elbow Surg 19:83–90
Waltrip RL, Zheng N, Dugas JR, Andrews JR (2003) Rotator cuff repair. A biomechanical comparison of three techniques. Am J Sports Med 31:493–497
Yamakado K, Katsuo S, Mizuno K, Arakawa H, Hayashi S (2010) Medial-row failure after arthroscopic double-row rotator cuff repair. Arthroscopy 26:430–435
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pauly, S., Fiebig, D., Kieser, B. et al. Biomechanical comparison of four double-row speed-bridging rotator cuff repair techniques with or without medial or lateral row enhancement. Knee Surg Sports Traumatol Arthrosc 19, 2090–2097 (2011). https://doi.org/10.1007/s00167-011-1517-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-011-1517-x