Abstract
Purpose
More and more orthopedic procedures are performed in an outpatient setting. A commonly used strategy in pain management is the intra-articular injection of local anesthetics. Recent attention has been drawn to their possible toxic effect on chondrocytes. Local anesthetics, and in particular Lidocaine, are also used for diagnostic joint infiltrations. A controlled laboratory study was performed to investigate the possible toxic effect of Lidocaine on human articular chondrocytes.
Methods
Mature human articular chondrocytes were harvested from the knees of human tissue donors or patients undergoing total knee replacement. The cells were exposed to Lidocaine 1 and 2% with and without epinephrine and to a saline 0.9% control group, with variable exposure times in different experiments. The activity and viability of the cells were assessed by lactate dehydrogenase activity, interleukin-6 production and a live/dead cell count.
Results
After a 1-h exposure, devastating results were seen for Lidocaine 1, 2 and 2% with epinephrine showing cell death rates of 91, 99 and 97%, respectively, compared with 26% in the saline control group (P-values of 0.004, 0.010, 0.006, respectively).
Exposing the chondrocytes to a 50/50 mixture of culture medium and local anesthetics substantially decreased cytotoxicity but still showed high toxicity when compared with the saline group (90% dead cells for Lidocaine 2%, P = 0.047). Lidocaine also showed a time-dependent cytotoxicity with gradually more dead cells after exposure for 15, 30 or 60 min.
Conclusion
In vitro, local anesthetics containing Lidocaine are significantly more toxic to mature human articular chondrocytes than a saline 0.9% control group. The effect of Lidocaine on the viability of human chondrocytes in vivo needs further investigation. However, based on our in vitro results, cautious use of intra-articular Lidocaine in clinical practice is recommended.
Level of evidence
Prospective, comparative therapeutic study, Level II.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
More and more orthopedic surgeries (knee, hip, wrist, ankle and shoulder arthroscopies, arthroscopic anterior cruciate ligament reconstructions and in some hospitals even total knee arthroplasties) are being performed on an outpatient basis [8]. This increasing trend toward day care surgery induced the development of new perioperative pain management protocols, able to give the patient sufficient comfort during his hospital stay. For several years, anesthesiologists used local anesthetics for that reason. Those products are thought to provide an effective and reliable postoperative pain control. Intra-articular injection is routinely used by orthopedic surgeons, mainly due to its simplicity and low cost. It is assumed to be a safe procedure [1, 2]. For the continuous delivery of local anesthetics into the affected joint, pain pumps became available [5, 6, 28].
Single-shot injections of local anesthetics into a joint are widespread in clinical orthopedic practice. Injections of local anesthetics aid the orthopedic surgeon in making the correct diagnosis and set up an efficient treatment. Lidocaine is one of the local anesthetics most commonly used for these diagnostic infiltrations.
Recently, questions were formulated about the safety of local anesthetics [4]. Publications report on the use of local anesthetics as a cause of postarthroscopic chondrolysis. Concerns have arisen about the possible chondrotoxicity of local anesthetics [15, 20]. Case reports of severe chondrolysis after the use of intra-articular pain pumps after shoulder surgery have been published. Some patients ended up with a total shoulder arthroplasty [20, 30]. Case series showed that there probably are multiple factors responsible for postarthroscopic glenohumeral chondrolysis; however, large doses of intra-articular local anesthetics are strongly discouraged [3, 24, 25]. Several publications reported a toxic effect of local anesthetics on articular cartilage in vitro and animal models [9–11, 13, 14, 17, 22, 26, 29, 31]. Chu et al. reported the extreme toxicity of bupivacaine on human chondrocytes in vitro [10]. Publications investigating the effect of Lidocaine are rare. Karpie and Chu in 2007 found a dose- and time-dependent cytotoxic effect on bovine chondrocytes of Lidocaine, but less than bupivacaine [22].
Materials and methods
Human articular chondrocytes were harvested from two different populations. Part of the chondrocytes were obtained from patients undergoing total knee arthroplasty. All patients signed an informed consent form. Perioperatively, only the intact articular cartilage was separated from the subchondral bone, put in a sterile recipient with culture medium (Dulbecco’s modified Eagle’s medium, DMEM, GIBCO BRL, Grand Island, NY, USA) and transported to the laboratory for further processing.
The second part of the articular cartilage, used in this study, was harvested from human tissue donors. Informed consent was obtained from the donor’s relatives by the team of transplant coordinators. Thereafter, the bone prelevation team was informed, and during prelevation of the bone, the articular cartilage of the femoral condyles was separated from the subchondral bone, put in a sterile recipient with culture medium (DMEM) and transported to the laboratory for further processing.
The Institutional Review Board and the Ethics Committee of the Ghent University Hospital approved both procedures.
Once in the laboratory, the articular cartilage was minced into particles of 1 mm³. An enzymatic digestion in three steps, using hyaluronidase, pronase and collagenase, was performed to isolate the articular chondrocytes over a 2-day period. After isolation, the cells were resuspended in a mixture of HBSS (Hank’s balanced salt solution) and alginate gel (low-viscosity alginate from Macrocystis pyrifera, Sigma) to a concentration of 5 million cells per milliliter. The alginate was polymerized into alginate beads that were dripped into culture plates that were placed in an incubator at 37°C and 5% CO2. Culture medium was renewed every 3–4 days. The alginate beads were cultured for a minimal of 7–10 days before exposition to the test substances in order to maintain their phenotype and to allow time for the extracellular matrix to form [7, 27]. A detailed description of this protocol can be found in previous scientific reports [16, 19, 32–34].
Exposure and analysis
One day before exposure to the different test substances, the beads were put into 12-well culture plates, each well containing 10 beads and 2 ml of culture medium.
After exposure, the test substance was aspirated, the beads were washed with phosphate-buffered saline (PBS, GIBCO) for 5 min, and 2 ml of fresh culture medium was added.
Twenty-four hours after exposure, a sample of 50 μl of culture medium was aspirated to measure lactate dehydrogenase (LDH) concentration. Another sample of 200 μl was aspirated and freezed at −20°C for later interleukin-6 (IL-6) concentration assessment.
For LDH measurement, the TOX-7 assay kit was used (Sigma–AldrichTM, St. Louis, MO, USA). LDH values represent a light absorption at 492 nm and could be expressed as optical density, representing the number of absorption units, indicating a relative concentration of LDH in the medium surrounding the cells. The interleukin-6 concentration was measured with enzyme-linked immunosorbent assay (ELISA). ELISA analysis allowed us to calculate the amount of IL-6 in the samples in picogram per milliliter. Concentrations that were too low to measure were indicated by 2.34 pg/ml, this being the lowest concentration measurable with IL-6 ELISA.
At 48 h, the same procedure was followed, but at this time point all of the remaining culture medium was aspirated and freezed at −20°C and 2 ml of fresh medium was added to the wells.
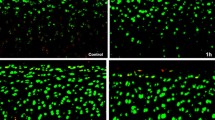
Seven days after exposure, samples for LDH and IL-6 measurement were obtained. Per well, all beads were digested by adding 500 μl of sodium citrate. A 25-μl sample of this cell suspension was used for staining with Trypan Blue, and a live/dead cell count was performed by an experienced and blinded investigator.
Experiments
Human articular cartilage was harvested from four human tissue donors (3 men and 1 woman, between 64 and 70 years of age) and from three patients undergoing a total knee arthroplasty (1 man and 2 women, between 65 and 68 years of age). Three experiments were conducted. Experiment 1 exposed the wells to 2 ml of Lidocaine 1% with and without epinephrine, to 2 ml of Lidocaine 2% with and without epinephrine and to 2 ml of NaCl 0.9% for the duration of 60 min. Experiment 2 exposed the beads to a mixture of 1 ml of culture medium plus 1 ml of our local anesthetics or saline 0.9% (same duration as in experiment 1). This experiment was set up to approach the in vivo dilution with joint fluid more accurately. Because a lack of nutrients may also cause cell death, culture medium was added in this protocol. Experiment 3 exposed the beads to our local anesthetics for the duration of 15, 30 and 60 min to investigate a potential time-dependent effect.
Statistical analysis
A statistical analysis of the results was made using SPSS version 17.0 (Statistical Package for the Social Sciences Inc., Chicago, Illinois, USA).
The analysis of the results was done by means of non-parametric Mann–Whitney U tests to compare the local anesthetics individually to the control group (saline 0.9%). The level of significance was set at P < 0.05.
Results
Experiment 1
At 24 h, higher values of LDH were noted for all different Lidocaine products compared with saline 0.9% (Table 1). All of these results were statistically significant.IL-6 measurements for Lidocaine 1% with epinephrine showed statistically significant higher values than saline 0.9% (P = 0.032). Concentrations for Lidocaine 1%, Lidocaine 2% with and without epinephrine were statistically lower than saline (P = 0.003, 0.015, 0.002). The concentration of Lidocaine 2% was too low to measure (Table 2).
The second evaluation at 48 h showed similar results for LDH measurements as those at 24 h postexposure (Table 1). All of our local anesthetics showed higher values than the saline control group. All were statistically significant, except for Lidocaine 1% with epinephrine (n.s.). Concerning IL-6 concentrations at 48 h, values for Lidocaine 1%, Lidocaine 2% with and without epinephrine at 48 h were still very low or undetectable. Lidocaine 1% with epinephrine again showed higher values than Lidocaine 1%: 189.76 vs. 3.71 (P = 0.005) (Table 2).
At 7 days postexposure, the LDH analysis showed only a statistically significant difference for Lidocaine 2%, which showed lower median values than the saline 0.9% group (P = 0.047) (Table 1).
IL-6 measurements at day 7 confirmed the trend: extremely low values for Lidocaine 1%, Lidocaine 2% with and without epinephrine. Lidocaine 1% with epinephrine showed values that were comparable to saline (Table 2).
The microscopic live/dead cell count after Trypan Blue staining showed a survival of 74.26% of the cells in the saline control group (interquartile range (IQR) = 55.74–85.19). For Lidocaine 1%, Lidocaine 2% with and without epinephrine, nearly all cells were dead at 7 days after 1-h exposure. The median percentage of viable cells at 7 days was 9.22% (IQR = 0–14.63), 2.93% (IQR = 0–16.95) and 1.40% (IQR = 0–3.64), respectively. After exposure to Lidocaine 1% with epinephrine, generally more than 50% of the cells were still alive after 7 days in culture medium (median = 61.99%, IQR = 43.14–66.04) (Table 3).
Experiment 2
For LDH measurements, the differences that were seen following experiment 1 were still present, but not as apparent. At 24 h, none of the differences could be proven statistically (P-values between 0.076 and 0.602). Lidocaine 2% showed a clear trend toward statistical significance (P = 0.076). At 48 h, results for LDH were comparable to those at 24 h. Lidocaine 2% gave statistically significant higher values than saline: 0.53 versus 0.27, respectively (P = 0.021). For Lidocaine 1%, a significant trend was noticeable compared with saline (P = 0.081). LDH analysis at 7 days postexposure could not show any statistically significant difference (Table 4).
IL-6 measurements at 24 h showed statistically significant higher values for Lidocaine 1% with epinephrine (P = 0.009). Lidocaine 2% with epinephrine, Lidocaine 1 and 2% showed higher values but with a wide spread and without significant difference with the control group. At 48 h, Lidocaine 1% with epinephrine kept showing significant higher values compared with saline (P = 0.009) At day 7, only the values for Lidocaine 2% were significantly lower than the saline controls (P = 0.047) (Table 5).
Staining with Trypan Blue at day 7 revealed a median survival of 70% for the saline control group, comparable with the survival of 68% of the cells exposed only to culture medium (DMEM). The highest mortality was seen after exposure to Lidocaine 2% with and without epinephrine with cell death rates of 70 and 89.86% after a 1-h exposure. Only the latter was statistically significant compared with saline (P = 0.047) (Table 6).
Experiment 3
The longer the exposure to any Lidocaine containing solution, the greater the number of dead cells at 7 days postexposure. For Lidocaine 2%, nearly all cells were dead after 7 days for all three exposure times (15, 30 and 60 min). All Lidocaine 2% values were statistically significant lower than the saline group (P = 0.019, 0.028, 0.032). Fifteen-minute exposure to Lidocaine 1% already resulted in 49% dead cells at day 7. This increased to 60 and 94% after 30 and 60 min of exposure time. Percentages of dead cells for Lidocaine 1% with epinephrine were lower with 19, 27 and 50% after exposure for 15, 30 and 60 min. Lidocaine 2% with epinephrine resulted in 49, 30 and 98% dead cells for 15-, 30- and 60-min exposure.
Exposure to Lidocaine 1% with epinephrine appeared to be the least toxic of all, indicating a possible positive effect of epinephrine on the biology of human articular chondrocytes. There was a trend toward a significant difference between 15 and 60 min of exposure (P = 0.083).
Discussion
The most important finding of the present study was the devastating cytotoxic effect of Lidocaine on articular human chondrocytes in vitro, which exhibited both a time-dependent and dose-dependent effect.
As the technique of intra-articular injection of local anesthetics is becoming more widespread, more and more questions arise about the safety of these procedures on the long term. Single-shot injection of local anesthetics into a joint has mainly been thought of as safe with little side effects being reported. One has to bear in mind that the follow-up was usually limited to the first 48 postoperative hours. As already stated above, recent literature describes several case reports of severe chondrolysis after intra-articular injection of local anesthetics weeks–months after the initial surgery.
As this had, to our knowledge, never been tested before on human tissue when we initiated the study, we set up a series of experiments to test a possible toxic effect of Lidocaine on cultivated human chondrocytes.
In our experiments, LDH and IL-6 concentrations were measured. The LDH assay is a means of measuring the membrane integrity as a function of the amount of cytoplasmic LDH released into the medium [12, 23]. The more cells die, the higher the concentration of LDH in the culture medium surrounding the cells. In response to physiologic and inflammatory stimuli, chondrocytes have been shown to produce IL-6 [18, 21]. IL-6 is a regulator of inflammatory and immunological processes. The higher the concentration of IL-6 found in the culture medium, the more cells that had been biologically activated by exposure to the local anesthetics.
In our first experiment, devastating results were found for Lidocaine 2%, Lidocaine 2% with epinephrine and Lidocaine 1% with statistically significant higher LDH concentrations at 24 and 48 h and almost complete cell death after Trypan Blue staining at 7 days. In other words, they are highly cytotoxic to human articular chondrocytes in vitro. Surprisingly, the results of Lidocaine 1% with epinephrine were very different. Based on the results of the other Lidocaine formulations, one would have expected a high toxicity as well. Instead, we found a significantly higher survival of chondrocytes after the 1-h exposure to Lidocaine 1% with epinephrine. In the in vitro setting, epinephrine seems to have a protective effect on human articular chondrocytes, possibly by interacting with the local anesthetic itself or with the chondrocytes or both.
For IL-6 after Lidocaine 1%, Lidocaine 2% with and without epinephrine exposure, no concentrations could be measured. This might be explained by the extreme cytotoxicity of those products, resulting in almost immediate cell death after exposure, which left no cells behind to produce IL-6. The addition of epinephrine to Lidocaine showed a higher activation of chondrocytes, resulting in higher IL-6 concentrations, further adding to the evidence that epinephrine may activate human articular chondrocytes.
The exact implications of these chondrocyte reactions should further be investigated to determine whether it is beneficial and protective or a sign of inflammatory response.
The second experiment was set up to mimic in vivo dilution and presence of nutrients. The toxic effects of Lidocaine 1%, Lidocaine 2% and Lidocaine 2% with epinephrine, which were seen in the first experiment, were still noticeable, but less apparent. High death rates were seen for Lidocaine 2% with epinephrine and Lidocaine 2%, still indicating a highly cytotoxic effect. Lidocaine 1% with epinephrine did not show the same trend anymore and was comparable to saline 0.9%. Intermediate cytotoxicity was seen for Lidocaine 1%. IL-6 concentrations followed the same trend. Of course, adding the culture medium not only supplied the cells with extra nutrients but also halved the concentrations of all LA, making them less toxic. On the other hand, cell death rates of 70–90% were still seen with Lidocaine 2% with epinephrine and Lidocaine 2%, which still confirms their highly cytotoxic effects.
The third experiment did show a dose- and time-dependent cytotoxicity with Lidocaine 1% being less toxic than Lidocaine 2% and more cell death with longer exposure times. A protective effect for epinephrine was also observed. IL-6 analysis confirmed the results seen with LDH assessment and Trypan Blue staining. The dose- and time-dependent effects of Lidocaine 1% found by Karpie et al. [22] were similar to the results found in this study, although for Lidocaine 2% a much higher toxicity was observed. The detrimental toxic effect of epinephrine shown in literature was not reflected in the results of this study. In contrary, Lidocaine with epinephrine showed higher survival rates of chondrocytes compared with Lidocaine without epinephrine, suggesting a protective effect of epinephrine in vitro.
Taking a closer look at the origin of the chondrocytes, we found that cells from patients undergoing TKA seemed to be much more sensitive for the toxic effect of the local anesthetic, although we could not show any statistical significance. Nevertheless, this might implicate that using local anesthetics in a joint with damaged or inflamed cartilage could result in higher chondrocytotoxicity.
The major shortcoming of this study is the in vitro setting. On one hand, this setting allows to test all the substances in a reproducible and controlled matter; on the other hand, it is not possible to easily extrapolate the results to the in vivo situation. Another shortcoming is the small sample size and high overlay between the values of the different LA in some experiments and therefore the lack of statistical significance in some experiments. However, the different protocols in this study have been able to show clear trends and significant differences between several test substances and the control group.
Conclusion
In this study, we found that local anesthetics containing Lidocaine are significantly more toxic to mature human articular chondrocytes in vitro than a saline 0.9% control group. A dose- and time-dependent effect was observed, with a higher cytotoxicity after longer exposure times and exposure to higher concentrations.
Further studies need to be done to investigate this effect on chondrocytes in vivo. In the meanwhile, we believe that the intra-articular use of Lidocaine in any concentration in clinical practice should be discouraged.
References
Alagol A, Calpur OU, Usar PS, Turan N, Pamukcu Z (2005) Intraarticular analgesia after arthroscopic knee surgery: comparison of neostigmine, clonidine, tenoxicam, morphine and bupivacaine. Knee Surg Sports Traumatol Arthrosc 13(8):658–663
Axelsson K, Gupta A, Johanzon E, Berg E, Ekback G, Rawal N, Enstrom P, Nordensson U (2008) Intraarticular administration of ketorolac, morphine, and ropivacaine combined with intraarticular patient-controlled regional analgesia for pain relief after shoulder surgery: a randomized, double-blind study. Anesth Analg 106(1):328–333
Bailie DS, Ellenbecker TS (2009) Severe chondrolysis after shoulder arthroscopy: a case series. J Shoulder Elbow Surg 18(5):742–747
Ballieul RJ, Jacobs TF, Herregods S, Van Sint Jan P, Wyler B, Vereecke H, Almqvist F, Herregods L (2009) The peri-operative use of intra-articular local anesthetics: a review. Acta Anaesthesiol Belg 60(2):101–108
Banerjee SS, Pulido P, Adelson WS, Fronek J, Hoenecke HR (2008) The efficacy of continuous bupivacaine infiltration following arthroscopic rotator cuff repair. Arthroscopy 24(4):397–402
Barber FA, Herbert MA (2002) The effectiveness of an anesthetic continuous-infusion device on postoperative pain control. Arthroscopy 18(1):76–81
Benya PD, Brown PD, Padilla SR (1988) Microfilament modification by dihydrocytochalasin B causes retinoic acid-modulated chondrocytes to reexpress the differentiated collagen phenotype without a change in shape. J Cell Biol 106(1):161–170
Berger RA, Kusuma SK, Sanders SA, Thill ES, Sporer SM (2009) The feasibility and perioperative complications of outpatient knee arthroplasty. Clin Orthop Relat Res 467(6):1443–1449
Chu CR, Coyle CH, Chu CT, Szczodry M, Seshadri V, Karpie JC, Cieslak KM, Pringle EK (2010) In vivo effects of single intra-articular injection of 0.5% bupivacaine on articular cartilage. J Bone Joint Surg Am 92(3):599–608
Chu CR, Izzo NJ, Coyle CH, Papas NE, Logar A (2008) The in vitro effects of bupivacaine on articular chondrocytes. J Bone Joint Surg Br 90(6):814–820
Chu CR, Izzo NJ, Papas NE, Fu FH (2006) In vitro exposure to 0.5% bupivacaine is cytotoxic to bovine articular chondrocytes. Arthroscopy 22(7):693–699
Decker T, Lohmann-Matthes ML (1988) A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J Immunol Methods 115(1):61–69
Dragoo JL, Korotkova T, Kanwar R, Wood B (2008) The effect of local anesthetics administered via pain pump on chondrocyte viability. Am J Sports Med 36(8):1484–1488
Dragoo JL, Korotkova T, Kim HJ, Jagadish A (2010) Chondrotoxicity of low pH, epinephrine, and preservatives found in local anesthetics containing epinephrine. Am J Sports Med 38(6):1154–1159
Gomoll AH, Kang RW, Williams JM, Bach BR, Cole BJ (2006) Chondrolysis after continuous intra-articular bupivacaine infusion: an experimental model investigating chondrotoxicity in the rabbit shoulder. Arthroscopy 22(8):813–819
Green WT Jr (1971) Behavior of articular chondrocytes in cell culture. Clin Orthop Relat Res 75:248–260
Grishko V, Xu M, Wilson G, AWt Pearsall (2010) Apoptosis and mitochondrial dysfunction in human chondrocytes following exposure to lidocaine, bupivacaine, and ropivacaine. J Bone Joint Surg Am 92(3):609–618
Guerne PA, Carson DA, Lotz M (1990) IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J Immunol 144(2):499–505
Guo JF, Jourdian GW, MacCallum DK (1989) Culture and growth characteristics of chondrocytes encapsulated in alginate beads. Connect Tissue Res 19(2–4):277–297
Hansen BP, Beck CL, Beck EP, Townsley RW (2007) Postarthroscopic glenohumeral chondrolysis. Am J Sports Med 35(10):1628–1634
Henrotin YE, De Groote DD, Labasse AH, Gaspar SE, Zheng SX, Geenen VG, Reginster JY (1996) Effects of exogenous IL-1 beta, TNF alpha, IL-6, IL-8 and LIF on cytokine production by human articular chondrocytes. Osteoarthritis Cartilage 4(3):163–173
Karpie JC, Chu CR (2007) Lidocaine exhibits dose- and time-dependent cytotoxic effects on bovine articular chondrocytes in vitro. Am J Sports Med 35(10):1621–1627
Legrand C, Bour JM, Jacob C, Capiaumont J, Martial A, Marc A, Wudtke M, Kretzmer G, Demangel C, Duval D et al (1992) Lactate dehydrogenase (LDH) activity of the cultured eukaryotic cells as marker of the number of dead cells in the medium [corrected]. J Biotechnol 25(3):231–243
Levy JC, Frankle M (2008) Bilateral shoulder chondrolysis following arthroscopy. A report of two cases. J Bone Joint Surg Am 90(11):2546–2547 author reply 2547–2548
Levy JC, Virani NA, Frankle MA, Cuff D, Pupello DR, Hamelin JA (2008) Young patients with shoulder chondrolysis following arthroscopic shoulder surgery treated with total shoulder arthroplasty. J Shoulder Elbow Surg 17(3):380–388
Lo IK, Sciore P, Chung M, Liang S, Boorman RB, Thornton GM, Rattner JB, Muldrew K (2009) Local anesthetics induce chondrocyte death in bovine articular cartilage disks in a dose- and duration-dependent manner. Arthroscopy 25(7):707–715
Masuda K, Sah RL, Hejna MJ, Thonar EJ (2003) A novel two-step method for the formation of tissue-engineered cartilage by mature bovine chondrocytes: the alginate-recovered-chondrocyte (ARC) method. J Orthop Res 21(1):139–148
Pelissier P, Svartz L, Rakotondriamihary S, Nouette-Gaulin K, Dousset V (2008) Postoperative analgesia with continuous intra-articular infusion of ropivacaine following trapeziectomy. Chir Main 27(5):222–226
Piper SL, Kim HT (2008) Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg Am 90(5):986–991
Saltzman M, Mercer D, Bertelsen A, Warme W, Matsen F (2009) Postsurgical chondrolysis of the shoulder. Orthopedics 32(3):215
Seshadri V, Coyle CH, Chu CR (2009) Lidocaine potentiates the chondrotoxicity of methylprednisolone. Arthroscopy 25(4):337–347
Verbruggen G, Veys EM, Wieme N, Malfait AM, Gijselbrecht L, Nimmegeers J, Almquist KF, Broddelez C (1990) The synthesis and immobilisation of cartilage-specific proteoglycan by human chondrocytes in different concentrations of agarose. Clin Exp Rheumatol 8(4):371–378
Verbruggen G, Wang J, Wang L, Elewaut D, Veys EM (2004) Analysis of chondrocyte functional markers and pericellular matrix components by flow cytometry. Methods Mol Med 100:183–208
Wang J, Verdonk P, Elewaut D, Veys EM, Verbruggen G (2003) Homeostasis of the extracellular matrix of normal and osteoarthritic human articular cartilage chondrocytes in vitro. Osteoarthritis Cartilage 11(11):801–809
Acknowledgments
Study was completed with the support from the Research Grant Program of the Society for Anesthesia and Resuscitation of Belgium. Special thanks to the department of Rheumatology for lending us the necessary equipment and infrastructure.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dr. T. F. Jacobs and Dr. P. S. Vansintjan have contributed equally to this article.
Rights and permissions
About this article
Cite this article
Jacobs, T.F., Vansintjan, P.S., Roels, N. et al. The effect of Lidocaine on the viability of cultivated mature human cartilage cells: an in vitro study. Knee Surg Sports Traumatol Arthrosc 19, 1206–1213 (2011). https://doi.org/10.1007/s00167-011-1420-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-011-1420-5