Abstract
Research in tissue engineering has been focused on articular cartilage repair for more than a decade. Some pioneristic studies involved the use of hydrogels such as alginate and fibrin glue which still possess valuable potential for cartilage regeneration. One of the main issues in cartilage tissue engineering is represented by the ideal maturation of the construct, before in vivo implantation, in order to optimize matrix quality and integration. The present study was focused on the effect of in vitro culture on a fibrin glue hydrogel embedding swine chondrocytes. We performed an evaluation of the immunohistochemical and biochemical composition and of the biomechanical properties of the construct after 1 and 5 weeks of culture. We noticed that chondrocytes survived in the fibrin glue gel and enhanced their synthetic activity. In fact, DNA content remained stable, while all indices of cartilage matrix production increased (GAGs content, immunohistochemistry for collagen II and safranin-o staining). On the other hand, the biomechanical properties remained steady, indicating a gradual substitution of the hydrogel scaffold by cartilaginous matrix. This demonstrates that an optimal preculture could provide the surgeon with a better engineered cartilage for implantation. However, whether this more mature tissue will result in a more efficient regeneration of the articular surface still has to be evaluated in future investigations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Articular cartilage is characterized by unique biomechanical properties and, on the other hand, a poor intrinsic healing potential. As a matter of fact, its reparative tissue is inadequate to withstand physiologic compressive and shear forces, often leading to a further extension of the damage and ultimately to the early development of osteoarthritis [16, 18].
Several surgical techniques have been introduced in clinical practice in order to repair cartilage defects including curettage, autologous osteochondral transplantation [11], drilling through the subchondral bone [14, 19], spongialization [9] and microfractures [35]. These last three approaches share the rationale of allowing pluripotent cells from the bone marrow to invade and repair the lesion. The outcome of these procedures, unfortunately, often results in the formation of fibrocartilaginous reparative tissue, which is different in structure, composition and biomechanical properties than hyaline cartilage.
Tissue engineering has addressed the issue of articular cartilage repair with the aim of restoring the original function by the regeneration of a fully differentiated tissue. In fact, autologous chondrocytes implantation and second generation techniques based on the use of bioresorbable scaffolds preseeded with autologous chondrocytes, have been widely applied in clinical practice [4, 5, 7, 10, 13]. Nevertheless, results achieved to date are far from being satisfactory with respect to repair tissue quality, long-term follow-up and integration of the engineered tissue in the host joint [13, 21].
Several studies evaluated the potential of different hydrogels embedding cells for cartilage tissue engineering including alginate [3, 23], pluronic [15], poly(ethylene oxide) (PEO) [6, 34] and fibrin gel [12, 27, 28, 32, 33, 36]. Hydrogel scaffolds are natural or synthetic polymers that provide a supportive three-dimensional environment which allows cells to proliferate, re-differentiate and synthesize matrix [29]. In order to improve chondrogenesis, hydrogels can be implemented with biomimetic and extracellular matrix components or growth factors [2, 8]. Additionally, hydrogel can be used in the recently developed technique of organ printing [1, 8]. In all, these features make them a promising tool in tissue engineering. In particular, our group has been focusing in fibrin glue in the last years and demonstrated that fibrin gel is a suitable polymer gel for generating new cartilage matrix from articular chondrocytes [29]. Additionally, this hydrogel possesses intrinsic adhesive properties; it is biocompatible and can be prepared utilizing autologous blood; its polymerization can be finely tuned, rendering this material injectable for mini-invasive delivery.
A crucial point to be addressed is how the grade of in vitro maturation of a tissue engineered cartilage should be, prior to implantation, in order to regenerate the articular cartilage [20]. We have recently developed a culture model, in order to evaluate the degree of in vitro maturation of the engineered cartilage over time [31] and, therefore, assess the potential advantages of an in vitro preculture on the repair model of chondrocytes-fibrin glue hydrogel. The aim of this study was to assess the maturation of such a construct after 1 and 5 weeks of in vitro culture by a morphological, immunohistochemical, biochemical and biomechanical analysis.
Materials and methods
Cellular fibrin glue samples were prepared by combining fresh chondrocytes with a fibrin glue gel. Chondrocytes were isolated from articular cartilage of young pigs by collagenase digestion and resuspended in fibrinogen solution (80 × 106 cell/ml). The thrombin was added to form a fibrin glue gel composite embedding cells. Samples were placed in standard culture conditions and then retrieved after 1 and 5 weeks. After in vitro culture, samples were analyzed by standard histology with safranin-o staining; by immunohistochemistry for collagen type 2; by biochemical assays; by biomechanical testing. Experimental protocol is depicted in Fig. 1.
Articular cartilage was harvested from joints of young pigs under sterile conditions (A). Cartilage slices were digested overnight in a collagenase type 2 solution in order to isolate chondrocytes (B). Chondrocytes were then counted, evaluated for viability and re-suspended in fibrinogen solution at a concentration of 80 × 106 cell/ml (C). Thrombin was then added and the fibrin gel polymerized (D)
Articular cartilage was harvested from young pigs’ knees and shoulders under sterile conditions (Fig. 1A), avoiding the subchondral bone. Cartilage slices were digested in Ham’s medium (Celbio, Pero, MI, Italy) containing 0.1% collagenase Type 2 (DBA-Italia Srl, Segrate, MI, Italy) and 1% of the antibiotic/antimycotic solution (10,000 units penicillin, 10 mg streptomycin and 25 μg amphotericin B/ml in 0.9% sodium chloride; Sigma Chemical Co., St. Louis, MO, USA). The pieces were incubated overnight in an oscillating water bath at 37°C. Undigested tissue and debris were removed by filtering the cell suspension using a 100 μ sterile filter (BD Falcon, Bedford, MA, USA). The cell suspension obtained (Fig. 1B) was centrifuged at 1,500 rpm for 10 min.
Chondrocytes were then resuspended in a solution containing porcine fibrinogen (Fig. 1C) (Fluka Chemie GmbH, Buchs, SG, Switzerland), aprotinin (Sigma), tranexamic acid (Sigma) and adjusted to a concentration of 80 × 106 cells/ml.
Preparation of cellular fibrin glue samples
Samples were prepared in 48-multiwell plates. A volume of 300 μl of the cell/fibrinogen solution was placed in each well. A volume of 300 μl of thrombin (Chemicon International, Inc., Temecula, CA, USA) was added in order to form a fibrin glue composite with cells (Fig. 1D). The samples were left 30 min in the wells until complete polymerization was reached. Then, they were placed in culture flasks and maintained for 1 and 5 weeks. Ten samples were prepared for each group. Cell medium was Ham’s medium (Celbio) with 10% fetal bovine serum (Sigma), ascorbic acid 50 μg/ml (Sigma), 1% glutamine (EuroClone) and 1% antibiotic/antimycotic solution (Sigma). Medium was changed three times a week.
Methods of evaluation
After 1 and 5 weeks, the samples were retrieved from the flasks and analyzed macroscopically. For histological analysis, cells cultured on fibrin glue gel were fixed in 10% (v/v) phosphate-buffered formaldehyde. The samples were then dehydrated in a graded 50% (v/v), 70% (v/v), 95% (v/v) and 100% (v/v) ethanol series, embedded in paraffin and cut into 4-μm-thick sections. Finally, the sections were stained with H&E for morphological evaluation and safranin-o for the GAGs using a standard staining protocol. Other sections were used for immunohistochemical analyses of collagen type II (Chondrex staining kit, Chondrex Inc., USA) and for the identification of apoptotic nuclei using a modified TdT-mediated dUTP nick end labeling technique (DeadEnd™ Colorimetric TUNEL System, Promega, USA). Sections were counterstained with hematoxylin.
Samples for biochemical evaluation were digested by papain (Sigma) (125 μg/ml in 100 mM sodium phosphate, 10 mM sodium EDTA, 10 mM cysteine hydrochloride, 5 mM EDTA adjusted to 6.5 pH and brought to 100 ml of solution with distilled water) for 16–24 h at 60°C and then stored at −80°C until analysis. Aliquots of the papain digest were assayed separately for proteoglycan and DNA contents. The proteoglycan content was estimated by quantifying the amount of sulphated glycosaminoglycans using the 1,9-dimethylmethylene (DMB) blue dye binding assay (Polysciences Inc., Washington, PA) and a microplate reader (wavelength: 530 nm). The standard curve for the analysis was generated using bovine trachea chondroitin sulfate A (Sigma). GAGs quantity was normalized per wet weight. The DNA content was evaluated with the Quant-iT Picogreen dsDNA Assay Kit (Molecular Probes, Inc., Eugene, OR) and a fluorescence microplate reader and standard fluorescein wavelengths (excitation 485 nm, emission 538 nm, cut-off 530 nm). The standard curve for the analysis was generated using bacteriophage lambda DNA supplied with the kit.
Biomechanical compression test was performed under unconfined geometry using an electromagnetic machine (Enduratec ELF3200, Minnetonka, MN, USA) with a load cell of 22 N, under displacement control, to evaluate the Young’s modulus (E) at 15% strain. Samples cultured for 1 week could not be tested for biomechanical integrity, as the initial degradation of fibrin glue following cell seeding rendered the specimens particularly soft. Results were compared to those of unseeded fibrin glue.
Statistical analysis
Statistical analysis was performed for the values of the biochemical and biomechanical assays. Results are presented as mean ± standard deviation. Differences between the experimental groups were evaluated by Mann–Whitney tests and considered statistically significant with P < 0.05.
Results
After 1 and 5 weeks, the samples were macroscopically analyzed and processed for histological, immunohistochemical, biochemical and biomechanical evaluation. According to our previous results [31], samples cultured for 5 weeks demonstrated higher resiliency and stiffness when probed with a pair of forceps than those cultured for 1 week.
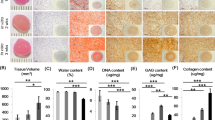
The histological evaluation (safranin-o) confirmed the presence of cartilage-like tissue maturing within the fibrin glue gel over the experimental times (Fig. 2). In particular, in the samples cultured for 1 week, a focal, pericellular GAGs deposition was present only at the periphery of the construct (Fig. 2a, b) while in the samples cultured for 5 weeks both the central and peripheral regions were safranin-o positive, with a more abundant GAGs accumulation at the periphery (Fig. 2b). The immunohistochemical assay for collagen type 2 showed a spotted staining at 1 week (Fig. 3a) and a more diffuse staining at 5 weeks, mainly at the periphery of the construct, resembling the topography of GAGs (Fig. 3b). TUNEL staining demonstrated an increase in the number of apoptotic cells over the experimental times (Fig. 4a, b).
Histological evaluation (safranin-o staining) after 1 week (a, b) and 5 weeks (c, d) in vitro. (a, original magnification 20×) After 1 week of culture, in the central part of the construct, no GAGs are present while in the periphery (b, original magnification 20×), there is some pericellular GAGs accumulation (arrows). (c, original magnification 20×) After 5 weeks, in the central region, there are some focal, pericellular safranin-o positive areas (arrows) while in the periphery (d, original magnification 10×), there is an abundant GAGs accumulation
Immunohistochemical staining for collagen type II. (a, original magnification 20×) Samples cultured for 1 week displayed a focal, pericellular staining (arrows). (b, original magnification 10×) Samples cultured for 5 weeks show a more diffuse staining, mainly located at the periphery of the construct
The biochemical evaluation was consistent with histological and immunohistochemical findings. DNA quantitation demonstrated a relevant stability of the total DNA quantity, indicating an overall cell number maintenance over the experimental times (Fig. 5a). DMB assay demonstrated a large synthesis of GAGs in the specimens cultured for 5 weeks, which was significantly higher than that of the specimens cultured for 1 week only (P < 0,05) (Fig. 5b). These results strongly suggest that chondrocytes survive and maintain a differentiated phenotype in the fibrin gel.
Biochemical analysis. A DNA quantitation shows that DNA content remains constant over the two experimental times, indicating cell survival. B GAG/wet weight shows a large increase in the total GAG quantity over the experimental times suggesting that a more mature tissue is present at 5 weeks. (The asterisk indicates a statistically significant difference between the two groups.)
Biomechanical compression test demonstrated no difference in Young’s modulus between the samples cultured for 5 weeks in vitro and the unseeded fibrin glue immediately after full polymerization (Fig. 6).
Biomechanical evaluation of samples (Young’s modulus). Samples cultured for 5 weeks maintain the compressive properties with respect to those registered for the acellular fibrin glue. As fibrin glue degrades during in vitro culture, this finding is particularly positive as this value can be almost entirely related to cells’ synthetic activity
Discussion
In this study, we demonstrated that a prolongation of the culture of a chondrocyte-fibrin glue hydrogel results in a more mature engineered cartilage, characterized by a more abundant cartilaginous matrix.
As a matter of fact, a crucial question in cartilage and osteochondral tissue engineering is the definition of the level of maturation an engineered graft should have, at the time of implantation, to support an optimal repair [17, 20]. A stiffer construct would be easier to be handled and implanted by the surgeon, but it is not clear how the development of the construct may interfere with its integration to the native tissue of the host joint. As a matter of fact, integration of immature constructs involves cell proliferation and the progressive formation of cartilaginous tissue, while integration of more mature constructs involves only the secretion of extracellular matrix components [22, 30]. On the other hand, it has been demonstrated that preculturing the composites in a medium containing chondrogenic growth factors enhances the production of cartilaginous matrix and improves the equilibrium modulus of the newly formed tissue produced by human articular chondrocytes seeded onto esterified hyaluronic acid nonwoven meshes following ectopic implantation [20]. Moreover, this is also consistent with the trend of using precultured matrices in the cell-based therapy for cartilage repair [5, 7, 21].
We evaluated the maturation of our engineered composite after 1 and 5 weeks of standard in vitro culture. In all, the histological, immunohistochemical and biochemical analysis demonstrated an improvement of the cartilaginous quality at the longer experimental time, with higher quantity of collagen type 2 at the immunohistochemical staining and of GAGs at the biochemical evaluation. Interestingly, the quantity of DNA remained stable over the experimental times demonstrating that fibrin glue offers a suitable environment for cells survival and proliferation. Consequently, the large increase in the total GAGs quantity was due to an increase in the synthetic activity per cell at 5 weeks, while this activity at 1 week was negligible. This finding suggests that in this model it is required more than 1 week for determining significant chondrogenesis at the protein level. Results obtained from the biomechanical analysis confirmed those of the biochemical evaluation. As a matter of fact, there was no difference in the compression properties between the engineered constructs and the control specimens made of the mere fibrin glue, suggesting that a newly formed matrix replaced the fibrin glue while it degrades during in vitro culture, as showed by the histological images of this and of previous studies [24–28, 31]. However, the neotissue was mainly located at the periphery of the construct, suggesting that in the central region the synthetic activity of the cells is reduced. This depends on the limitation to nutrient diffusion and waste removal within the construct when cultured in standard static conditions. Regarding cell survival and proliferation, the fact that overall amount of DNA remains stable, while the number of apoptotic cells increases, suggests that chondrocytes proliferate and maintain a stable number within the fibrin glue hydrogel, proliferating in the outer part of the specimen. The limitations of this study include the use of chondrocytes harvested from young pigs and the fact that the cells utilized here were fresh, nonexpanded chondrocytes, while those employed in a potential similar clinical model would include expanded cells. Further studies would be therefore needed to test the effect of the monolayer culture to the chondrocytes in this model.
A fine modulation of fibrin glue degradation, achievable by regulating fibrinogen and thrombin concentration and by adding proteinase inhibitors such as aprotinin or tranexamic acid [29, 32] is desirable, in order to maintain chondrocytes in a stable three-dimensional environment, while they switch to the differentiated phenotype and start chondrogenesis. From a clinical standpoint, the maintenance of volume and shape is a crucial issue. Our data suggest that the hydrogel scaffold should be stable for at least few weeks, as after 1 week only the proteoglycan synthesis is negligible. Additionally, these results were achieved by culturing the samples in vitro in standard conditions, with neither growth factors nor biomechanical stimuli. We believe that these results could be improved, in terms of reduction of the time needed for chondrogenesis, volume stability and total quantity of GAGs, by adding growth factors [20] or biomechanical stimuli in vitro, or in vivo in a physiologic environment.
Conclusion
Taken together, these results suggest that culturing in vitro an engineered composite, based on cellular fibrin glue, improves the overall quality of the construct. This could enhance the rate of graft survival after implantation [17]. Further in vitro studies with expanded cells are required in order to duplicate the clinical conditions for testing the potential use of this model. Additionally, in vivo studies, in nude mice and eventually in a large animal model, are needed to evaluate the effect of the level of maturation of the engineered tissue on the capacity of its additional development and integration in a physiologic environment.
References
Ahmed TA, Dare EV, Hincke M (2008) Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev 14:199–215
Becker JC, Domschke W, Pohle T (2004) Biological in vitro effects of fibrin glue: fibroblast proliferation, expression and binding of growth factors. Scand J Gastroenterol 39:927–932
Bonaventure J, Kadhom N, Cohen-Solal L, Ng KH, Bourguignon J, Lasselin C, Freisinger P (1994) Reexpression of cartilage-specific genes by dedifferentiated human articular chondrocytes cultured in alginate beads. Exp Cell Res 212:97–104
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331:889–895
Cherubino P, Grassi FA, Bulgheroni P, Ronga M (2003) Autologous chondrocyte implantation using a bilayer collagen membrane: a preliminary report. J Orthop Surg (Hong Kong) 11:10–15
Elisseeff J, Anseth K, Sims D, McIntosh W, Randolph M, Yaremchuk M, Langer R (1999) Transdermal photopolymerization of poly(ethylene oxide)-based injectable hydrogels for tissue-engineered cartilage. Plast Reconstr Surg 104:1014–1022
Erggelet C, Sittinger M, Lahm A (2003) The arthroscopic implantation of autologous chondrocytes for the treatment of full-thickness cartilage defects of the knee joint. Arthroscopy 19:108–110
Fedorovich NE, Alblas J, de Wijn JR, Hennink WE, Verbout AJ, Dhert WJ (2007) Hydrogels as extracellular matrices for skeletal tissue engineering: state-of-the-art and novel application in organ printing. Tissue Eng 13:1905–1925
Ficat RP, Ficat C, Gedeon P, Toussaint JB (1979) Spongialization: a new treatment for diseased patellae. Clin Orthop Relat Res 144:74–83
Grigolo B, Roseti L, De Franceschi L, Piacentini A, Cattini L, Manfredini M, Faccini R, Facchini A (2005) Molecular and immunohistological characterization of human cartilage two years following autologous cell transplantation. J Bone Joint Surg Am 87:46–57
Hangody L, Kish G, Karpati Z, Udvarhelyi I, Szigeti I, Bely M (1998) Mosaicplasty for the treatment of articular cartilage defects: application in clinical practice. Orthopedics 21:751–756
Homminga GN, Buma P, Koot HW, van der Kraan PM, van den Berg WB (1993) Chondrocyte behavior in fibrin glue in vitro. Acta Orthop Scand 64:441–445
Hunziker EB (2002) Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthr Cartil 10:432–463
Levy AS, Lohnes J, Sculley S, LeCroy M, Garrett W (1996) Chondral delamination of the knee in soccer players. Am J Sports Med 24:634–639
Liu Y, Chen F, Liu W, Cui L, Shang Q, Xia W, Wang J, Cui Y, Yang G, Liu D, Wu J, Xu R, Buonocore SD, Cao Y (2002) Repairing large porcine full-thickness defects of articular cartilage using autologous chondrocyte-engineered cartilage. Tissue Eng 8:709–721
Mankin HJ (1982) The response of articular cartilage to mechanical injury. J Bone Joint Surg 64A:460–466
Martin I, Miot S, Barbero A, Jakob M, Wendt D (2007) Osteochondral tissue engineering. J Biomech 40:750–765
Meachim G, Roberts C (1971) Repair of the joint surface from subarticular tissue in the rabbit knee. J Anat 109:317–327
Mitchell N, Shepard N (1976) The resurfacing of adult rabbit articular cartilage by multiple perforations through the subchondral bone. J Bone Joint Surg Am 58:230–233
Moretti M, Wendt D, Dickinson SC, Sims TJ, Hollander AP, Kelly DJ, Prendergast PJ, Heberer M, Martin I (2005) Effects of in vitro preculture on in vivo development of human engineered cartilage in an ectopic model. Tissue Eng 11:1421–1428
Niemeyer P, Pestka JM, Kreuz PC, Erggelet C, Schmal H, Suedkamp NP, Steinwachs M (2008) Characteristic complications after autologous chondrocyte implantation for cartilage defects of the knee joint. Am J Sports Med 36:2091–2099
Obradovic B, Martin I, Padera RF, Treppo S, Freed LE, Vunjak-Novakovic G (2001) Integration of engineered cartilage. J Orthop Res 19:1089–1097
Paige KT, Cima LG, Yaremchuk MJ, Schloo BL, Vacanti JP, Vacanti CA (1996) De novo cartilage generation using calcium alginate-chondrocyte constructs. Plast Reconstr Surg 97:168–178
Peretti GM, Buragas MS, Scotti C, Mangiavini L, Sosio C, Di Giancamillo D, Domeneghini C, Fraschini G (2006) An in vitro tissue engineered model for osteochondral repair. Sport Sci Health 1:153–157
Peretti GM, Bonassar LJ, Caruso EM, Randolph MA, Trahan CA, Zaleske DJ (1999) Biomechanical analysis of a chondrocyte-based repair model of articular cartilage. Tissue Eng 5:317–326
Peretti GM, Randolph MA, Caruso EM, Rossetti F, Zaleske DJ (1998) Bonding of cartilage matrices with cultured chondrocytes: an experimental model. J Orthop Res 16:89–95
Peretti GM, Zaporojan V, Spangenberg KM, Randolph MA, Fellers J, Bonassar LJ (2003) Cell-based bonding of articular cartilage: an extended study. J Biomed Mater Res A 64:517–524
Peretti GM, Randolph MA, Villa MT, Buragas MS, Yaremchuk MJ (2000) Cell-based tissue-engineered allogeneic implant for cartilage repair. Tissue Eng 6:567–576
Peretti GM, Xu JW, Bonassar LJ, Kirchhoff CH, Yaremchuk MJ, Randolph MA (2006) Review of injectable cartilage engineering using fibrin gel in mice and swine models. Tissue Eng 12:1151–1168
Schaefer D, Martin I, Jundt G, Seidel J, Heberer M, Grodzinsky A, Bergin I, Vunjak-Novakovic G, Freed LE (2002) Tissue-engineered composites for the repair of large osteochondral defects. Arthritis Rheum 46:2524–2534
Scotti C, Buragas MS, Mangiavini L, Sosio C, Di Giancamillo A, Domeneghini C, Fraschini G, Peretti GM (2007) A tissue engineered osteochondral plug: an in vitro morphological evaluation. Knee Surg Sports Traumatol Arthrosc 15:1363–1369
Silverman RP, Passaretti D, Huang W, Randolph MA, Yaremchuk MJ (1999) Injectable tissue-engineered cartilage using a fibrin glue polymer. Plast Reconstr Surg 103:1809–1818
Sims CD, Butler PE, Cao YL, Casanova R, Randolph MA, Black A, Vacanti CA, Yaremchuk MJ (1998) Tissue engineered neocartilage using plasma derived polymer substrates and chondrocytes. Plast Reconstr Surg 101:1580–1585
Sims CD, Butler PE, Casanova R, Lee BT, Randolph MA, Lee WP, Vacanti CA, Yaremchuk MJ (1996) Injectable cartilage using polyethylene oxide polymer substrates. Plast Reconstr Surg 98:843–850
Steadman JR, Rodkey WG, Briggs KK, Rodrigo JJ (1999) The microfracture technic in the management of complete cartilage defects in the knee joint. Orthopade 28:26–32
Xu JW, Zaporojan V, Peretti GM, Roses RE, Morse KB, Roy AK, Mesa JM, Randolph MA, Bonassar LJ, Yaremchuk MJ (2004) Injectable tissue-engineered cartilage with different chondrocyte sources. Plast Reconstr Surg 113:1361–1371
Acknowledgments
This work was done at the Stem Cell Research Institute, directed by Professor Giulio Cossu. The authors gratefully acknowledge Dr. Corrado Sosio, Dr. Alessandro Pozzi, Dr. Alessia Di Giancamillo and Dr. Daniela Deponti for their assistance in sample preparation and analysis. A special thanks is given to Mr. Paolo Stortini for his precious help in histological analysis and to the Spaccio Agricolo Agripig for their assistance in animal management. This work was funded by the Fondazione CARIPLO.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scotti, C., Mangiavini, L., Boschetti, F. et al. Effect of in vitro culture on a chondrocyte-fibrin glue hydrogel for cartilage repair. Knee Surg Sports Traumatol Arthrosc 18, 1400–1406 (2010). https://doi.org/10.1007/s00167-009-1014-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-009-1014-7