Abstract
Deep venous thrombosis (DVT) is common after lower limb injury, but the effect of prophylactic treatment has not been documented in large randomised trials or meta-analyses. As a result, evidence-based recommendations have not been established. The purpose of this study was to evaluate the incidence of venous thromboembolism in patients with Achilles tendon rupture. A total of 100 consecutive patients with an acute Achilles tendon rupture were included in a prospective study and randomised to either surgical or non-surgical treatment. At 8 weeks after the initiation of treatment, 95/100 patients were screened for DVT using colour duplex sonography (CDS) with blinded interpretation by two experienced examiners and adjudication in cases of disagreement by a third person. A total of 95 patients (79 male and 16 female) with a median (range) age of 41 (24–63) years were screened for CDS at 8 weeks. Of the 95 patients, 32 had a CDS-verified thrombosis, 5 proximal and 27 distal, whereas 3 had non-fatal pulmonary embolism. Surgical treatment was performed in 49 patients, non-surgical in 46. There were no significant differences in DVT frequency between the two treatment groups. The incidence of asymptomatic and symptomatic deep venous thrombosis is high after Achilles tendon rupture and there is a need to define the possible benefit of thromboprophylaxis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Deep vein thrombosis (DVT), complicating pulmonary embolism (PE) and cardiovascular events are serious and feared complications after orthopaedic surgery. The risk of developing thromboembolism after acute Achilles tendon rupture has been demonstrated in a few studies [4, 10, 11]. However, after injury and/or surgery to the lower limbs, a clinical diagnosis of DVT is unreliable, because pain and swelling caused by trauma, surgery and immobilisation make the usual signs and symptoms of thrombosis difficult to interpret. More objective diagnostic methods are therefore required. In addition, the majority of venous thrombi are asymptomatic and the true incidence is unknown. Many clots resolve spontaneously, but in some patients PE can even complicate asymptomatic distal thrombi [3]. After hip or knee arthroplasty, an asymptomatic thrombus has been detected in some studies in half of patients suffering a pulmonary embolism [2, 16].
Although there is general agreement that patients undergoing major hip or knee surgery benefit from prophylactic anti-thrombotic therapy, there are no unanimously accepted recommendations for thromboprophylaxis in patients with isolated lower limb injury or surgery. Lassen et al. [11] demonstrated that thromboprophylaxis using LMWH (Reviparin) reduced the risk of DVT in patients treated with a below-the-knee cast for lower leg injuries in a study based on unilateral venography.
Lapidus et al. [10] performed a randomised, placebo-controlled study on 105 consecutive patients, who were treated surgically for Achilles tendon rupture. Colour duplex sonography (CDS) was used as screening and, although the patients received thromboprophylaxis with 5,000 U of dalteparin daily during a 6-week period of immobilisation, a DVT incidence of 34% was reported and no beneficial effect of thromboprophylaxis could be shown in comparison with placebo.
CDS was recently evaluated in patients with ankle fractures after 6 weeks of treatment and shown to be a valid and reliable screening method, not only in patients with proximal, symptomatic thrombi, but also in patients with distal asymptomatic thrombi [9]. CDS has improved in recent years due to technical advances; it is non-invasive and more convenient for patients than venography. However, the method requires experience to perform and interpret, a factor of importance when CDS is selected for DVT surveillance. The aim of the study was to assess the incidence of thromboembolism during the first 8 weeks after acute Achilles tendon rupture.
Methods
Patients with acute Achilles tendon rupture were screened for potential participation in a prospective study comprising three participating hospitals in Gothenburg. Computer-based sealed envelopes administered by a co-ordinator were used in the randomisation. Patients eligible for the study were randomised to either surgical or non-surgical treatment. Patients 16–65 years of age with a unilateral Achilles tendon rupture were to be included in the study if randomisation and treatment were started within 72 h from the initial injury. The exclusion criteria were diabetes, previous Achilles tendon rupture, other lower leg injuries involving functional deficit of the Achilles tendon, immunosuppressant therapy and neurovascular diseases. All patients were treated with a below-the-knee plaster cast with the foot in the equinus position for 2 weeks, followed by an adjustable lower leg brace (Don-Joy ROM-Walker) for the next 6 weeks. Range of motion training was started after 10–14 days, using the adjustable lower leg brace [14]. Full weight-bearing was started, with the brace in the neutral position. The surgical procedure was performed with the patient in the prone position under general, local or spinal anaesthesia and a tourniquet was used in some cases of general or spinal anaesthesia. An end-to-end suture, using a modified Kessler suturing technique with 1–0 PDS, was performed via a longitudinal, 5–8 cm medial skin incision. Evaluations of the Achilles tendon rupture treatment will not be presented in this study.
In the surgically treated group, thromboprophylaxis with 500 ml of Macrodex®, administrated according to a study protocol, was given to ambulant patients on the day of surgery, while non-ambulant cases received a second unit within the first day postoperatively. No standard thromboprophylaxis was employed in the non-surgically treated group. Therefore, LMWH was used according to the individual surgeon’s preference. All patients followed a standardised follow-up protocol, including screening for deep venous thrombosis (DVT).
Screening for deep venous thrombosis was performed with a CDS, with a Sequoia ultrasound machine (Siemens Acuson, Mountain View, CA, USA) having a linear 6L3 ultrasound transducer. The CDS examination followed a standard procedure, similar to the one described by Lapidus et al. [9] covering the external iliac, common, deep and superficial femoral, popliteal, gastrocnemius, paired posterior tibial and peroneal veins and the proximal part of the anterior tibial and proximal junctions of the greater and lesser saphenous veins. The proximal veins were examined in the supine position with a head-up tilt of about 45°, the popliteal region in the prone position with a similar tilt and the calf veins in the sitting position with the leg extended and the foot resting on the examiner’s lap. Vein segments were assessed regarding compressibility and flow. The primary diagnostic criterion was venous incompressibility in the transverse view. Colour flow findings were used to clarify the anatomy and as supportive findings, especially in the venous segments that are less easily viewed and compressed, including the distal femoral vein at the adductor canal and the trifurcation at the proximal calf. At the time of examination, the findings related to these vein segments were recorded on a drawing of the venous system, where the extent of a possible DVT could be clarified. All examinations were also recorded on videotape, in standardised recordings usually lasting 5–10 min/examination. Examinations were performed by any of six technicians at the vascular laboratory, all with several years’ experience in venous ultrasonography. Two technicians usually collaborated for ergonomic reasons and could help each other interpret the images.
After the final inclusion, all the video recordings were reviewed by two vascular diagnostic physicians, each with more than 10 years’ experience in the method and, at the time of the review, blinded to each other’s diagnosis and to the primary diagnosis at the time of examination. The interpretations were recorded and the reviewer was required to note a decision relating to the presence of proximal DVT (in the popliteal vein or a more proximal vein) and distal DVT (in deep or muscular veins distal to the popliteal vein). In cases of disagreement between these blinded evaluations, adjudication was made by a third person, also with more than 10 years’ first-hand experience in thrombosonography.
In cases of suspected pulmonary embolism, spiral computed tomography or perfusion/ventilation lung scintigraphy was obtained and adjudicated by an experienced physician specialised in diagnostics of thromboembolism. There was no surveillance of PE in the present study.
The CDS examination was performed 8 weeks after the trauma and initiation of treatment. The follow-up included clinical examinations after 2, 8 and 12 weeks and after 6 months.
All the patients received oral and written information about the purpose and procedure of the study and written informed consent was obtained. Ethical approval was obtained from the Human Ethics Committee at the Medical Faculty, University of Gothenburg, Sweden.
Statistical analysis and end-points
The primary end-point of this study was the total incidence of VTE in patients treated for Achilles tendon rupture. Secondary end-points were distal DVT, proximal DVT and PE. All the data were analysed using SPSS statistical software 15.0 for Windows. Descriptive data are reported as median and range or mean and standard deviation (SD). A χ2 test was used in the comparison of VTE rates between the surgical and non-surgical groups. The level of significance was set at P < 0.05. All the data analyses were carried out by the investigators.
Results
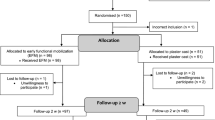
A total of 100 patients with an acute Achilles tendon rupture were randomly allocated to either surgical or non-surgical treatment in a prospective study between February 2004 and February 2007.
All the patients were randomised and treated within 72 h after the injury. Surgical treatment was performed in 51 patients and 49 patients were treated non-surgically. Anaesthetic technique used was spinal, local or general anaesthesia, in 26 (27.4%), 19 (20%) and 4 (4.2%) patients, respectively. Tourniquet was used in 26 (27.4%) patients.
The main results are presented in Table 1. Of the 100 patients, a total of 95 patients (79 male and 16 female), with a median (range) age of 41 (24–63) years, underwent screening using CDS. Three patients did not comply with the study design and changed treatment groups; two preferred surgical treatment and one patient was not operated on for other reasons. They were therefore excluded from the outcome analysis. Two patients sustained re-ruptures and had undergone re-operation at the time of the planned screening examination and were consequently excluded from the examination with CDS. A total of 41 patients in the surgically treated group had prophylactic treatment with Macrodex® and an additional 7 patients were given thromboprophylaxis with LMWH, 2 in the non-surgically treated group and 6 in the surgically treated group, because of perceived risk factors.
We found 32 patients (27 males and 5 females) with a CDS-verified DVT, 5 proximal and 27 distal, and three non-fatal PE. The median (range) age was 43 (30–63) years in patients with DVT. Ten patients complained of symptoms when the clinical examination was performed 2 weeks after the initial treatment was started, at the time when the cast was replaced with the adjustable brace. One patient with symptoms underwent an acute CDS, whereas five patients with symptoms underwent both an acute CDS and screening after 8 weeks. In three patients, CDS and a venography were performed on the same day.
Of the 32 patients, 3 had subjective symptoms of PE including dyspnoea; all of them were verified with either CT or ventilation/perfusion scintigraphy. Among the PE patients, two had distal CDS-verified thrombosis and the third had a proximal thrombosis.
DVT was found in 18/46 patients receiving non-surgical treatment and in 14/49 receiving surgical treatment. There were no significant differences (P = 0.217) in DVT frequency between the two groups. Of the 95 patients included in the analysis, 7 had prophylaxis with 5,000 U of dalteparin (Fragmin, Pfizer) administered subcutaneously once a day for 10 days. Of these patients, the CDS was normal in six, while one developed a distal thrombus. Verified VTE was treated according to the routine at the local hospital.
Discussion
The principal finding in the present study was a VTE rate of 34%, with 5 proximal and 27 distal DVTs, making a total of 32 cases, 22 asymptomatic and 10 symptomatic. Of the 95 patients, 3 developed symptoms of PE, all of them from the non-surgically treated group, and the diagnosis was verified in all these cases. There was no fatal PE in this trial.
Three hospitals were included in the study, and all patients were assessed with CDS at one hospital. The diagnostic screening procedure used in the present study was CDS performed 8 weeks after trauma and the initiation of treatment. A recent study has demonstrated that CDS is a reliable method of screening for DVT 6 weeks after ankle fracture surgery [9]. Previous studies have evaluated the accuracy of CDS, but with heterogeneous results [1, 5, 6, 12, 13, 15]. Technical advances in image resolution and transducer technology, as well as the addition of colour Doppler, have improved the accuracy of CDS, although it is still dependent on the examiner’s experience.
The use of CDS to detect asymptomatic distal DVT, especially directly after trauma or surgery, has been challenged [12, 15]. In the present study, CDS was performed 8 weeks after the injury and, as a result, oedema, haematoma and swelling will most probably involve fewer technical problems and the interpretation of the CDS findings might be more unambiguous.
Since CDS is a non-invasive method, it is less uncomfortable for the patient as compared with venography. CDS is also less expensive and the examination can usually be performed more rapidly. It is, however, still too early to claim that the method can replace venography when it comes to the screening of asymptomatic patients.
Validation of CDS 8 weeks after Achilles tendon rupture was not performed previously; however, the method was validated in a comparable clinical setting, 6 weeks after lower limb injury, and a high specificity and sensitivity were demonstrated [9].
The results of the examinations were interpreted by two experienced physicians blinded to the treatment group. To minimise the potential for bias, all the results with a verified deep venous thrombosis were re-evaluated after the study had been completed. This second assessment was made in a blinded fashion, i.e. without any knowledge of the primary outcome. After this blinded evaluation was performed, an adjudication in cases of disagreement was made by a third person, also with long (more than 10 years) experience of CDS for the diagnosis of thrombosis.
Thromboprophylaxis for lower leg injuries have been evaluated in several previous studies. In patients immobilised with a plaster cast due to lower leg injury, Kujath et al. [7] demonstrated a reduction in the incidence of DVT when prophylactic treatment was used. In this randomised trial, CDS screening was performed to detect DVT and to compare LMWH treatment and placebo. The DVT rates in the two treatment groups were 5 and 16%, respectively.
In a randomised, controlled trial, using venographic screening, Lassen et al. [11] reported a total DVT incidence of 14% in 371 patients treated with either LMWH (reviparin 1750 anti-Xa units) or placebo after ankle fracture or Achilles tendon rupture. The DVT rates were 9% in the treatment group and 19% in the control group respectively. On the other hand, in a randomised, controlled study in a mixed group of lower leg injuries, Jorgensen et al. [4] showed that LMWH (tinzaparin 3500 anti-Xa IU) was not sufficient to prevent DVT in patients treated with a lower leg cast. DVT was diagnosed in 10% (10/99) and 17% (18/106) of the patients in the treatment and control groups, respectively. In that study, 20% of the patients were treated due to Achilles tendon rupture. In those patients, DVT was found in 2/20 (10%) and 6/21 (29%) in the treatment and placebo groups, respectively. In a recently published randomised, controlled study, Lapidus et al. [10] used CDS screening and demonstrated a total DVT rate of 34–36% in patients treated with either dalteparin (5,000 IU) or placebo, concluding that thromboprophylaxis did not affect the incidence of DVT during immobilisation after Achilles tendon rupture.
The same authors presented data from a registry comprising 30,816 consecutive patients, 668 of whom were treated due to Achilles tendon rupture, and symptomatic DVT was verified in 47 (7%) within 6 weeks [8]. No thromboprophylaxis was given and no screening procedures were performed [8].
Evaluations of risk factors were not feasible in the present study due to the limited size of the cohort. The data in the present study were derived from a study comparing re-rupture rates between surgically and non-surgically treated patients with acute Achilles tendon ruptures, which also was the basis for the sample size calculation. CDS was primarily done to describe the natural history of thromboembolism in this group of patients who did not receive VTE prophylaxis.
However, in the present study, there were no significant differences in DVT rates between the treatment groups. All three patients with verified PE in our study were found in the non-surgically treated group, which might be a coincidence. However, the surgical group was given prophylactic treatment with Macrodex®, which might be of importance as it has a documented effect of preventing PE.
The development of thromboprophylactic measures has reduced the frequency of venous thromboembolism (VTE) and the use of thromboprophylaxis has become routine after major orthopaedic surgery. However, the question of whether prophylaxis after minor surgery and lower limb injury is necessary still remains an issue for debate. In the absence of guidelines regarding prophylactic treatment in patients with Achilles tendon rupture and, in the light of contradictory results from literature, no standard procedure for thromboprophylaxis was used in our study.
A reliable screening procedure could potentially increase the safety of patients, since the detection of asymptomatic clots could limit the risk of the further propagation and development of pulmonary embolism, but no positive cost benefit of such a routine has been demonstrated.
Conclusion
Venous thromboembolism was demonstrated in 34% of the patients within 2 months after acute total Achilles tendon rupture. Five patients had DVT in the popliteal, thigh and/or pelvic region and three patients developed PE. We found no significant difference in the incidence of DVT between surgical or non-surgical treatment. There is a need for further research to clarify the role of thromboprophylactic treatment in this group of patients.
References
Davidson BL, Elliott CG, Lensing AW (1992) Low accuracy of color Doppler ultrasound in the detection of proximal leg vein thrombosis in asymptomatic high-risk patients: the RD Heparin Arthroplasty Group. Ann Intern Med 117:735–738
Eriksson BI, Kälebo P, Anthymyr BA, Wadenvik H, Tengborn L, Risberg B (1991) Prevention of deep-vein thrombosis and pulmonary embolism after total hip replacement: comparison of low-molecular-weight heparin and unfractionated heparin. J Bone Joint Surg Am 73:484–493
Havig O (1977) Deep vein thrombosis and pulmonary embolism: an autopsy study with multiple regression analysis of possible risk factors. Acta Chir Scand Suppl 478:1–120
Jorgensen PS, Warming T, Hansen K, Paltved C, Vibeke Berg H, Jensen R, Kirchhoff-Jensen R, Kjaer L, Kerbouche N, Leth-Espensen P, Narvestad E, Rasmussen SW, Sloth C, Torholm C, Wille-Jorgensen P (2002) Low molecular weight heparin (Innohep) as thromboprophylaxis in outpatients with a plaster cast: a venographic controlled study. Thromb Res 105:477–480
Kassai B, Boissel JP, Cucherat M, Sonie S, Shah NR, Leizorovicz A (2004) A systematic review of the accuracy of ultrasound in the diagnosis of deep venous thrombosis in asymptomatic patients. Thromb Haemost 91:655–666
Kearon C (2001) Noninvasive diagnosis of deep vein thrombosis in postoperative patients. Semin Thromb Hemost 27:3–8
Kujath P, Spannagel U, Habscheid W (1993) Incidence and prophylaxis of deep venous thrombosis in outpatients with injury of the lower limb. Haemostasis 23(1):20–26
Lapidus, L (2007) Venous thromboembolism and mortality following orthopaedic surgery: a prospective 6 week follow-up of more than 30,000 consecutive patients surgically treated between years 1996 and 2003. Thesis for doctoral degree (Ph.D), Karolinska Institutet at Stockholm Söder Hospital, Stockholm, Sweden, ISBN 978–91–7357–111–1
Lapidus L, de Bri E, Ponzer S, Elvin A, Noren A, Rosfors S (2006) High sensitivity with color duplex sonography in thrombosis screening after ankle fracture surgery. J Thromb Haemost 4:807–812
Lapidus LJ, Rosfors S, Ponzer S, Levander C, Elvin A, Larfars G, de Bri E (2007) Prolonged thromboprophylaxis with dalteparin after surgical treatment of achilles tendon rupture: a randomized, placebo-controlled study. J Orthop Trauma 21:52–57
Lassen MR, Borris LC, Nakov RL (2002) Use of the low-molecular-weight heparin reviparin to prevent deep-vein thrombosis after leg injury requiring immobilization. N Engl J Med 347:726–730
Magnusson M, Eriksson BI, Kälebo P, Sivertsson R (1996) Is colour Doppler ultrasound a sensitive screening method in diagnosing deep vein thrombosis after hip surgery? Thromb Haemost 75:242–245
Mattos MA, Melendres G, Sumner DS, Hood DB, Barkmeier LD, Hodgson KJ, Ramsey DE (1996) Prevalence and distribution of calf vein thrombosis in patients with symptomatic deep venous thrombosis: a color-flow duplex study. J Vasc Surg 24:738–744
Möller M, Movin T, Granhed H, Lind K, Faxen E, Karlsson J (2001) Acute rupture of tendon Achilles. A prospective randomised study of comparison between surgical and non-surgical treatment. J Bone Joint Surg Br 83:843–848
Schellong SM, Beyer J, Kakkar AK, Halbritter K, Eriksson BI, Turpie AG, Misselwitz F, Kälebo P (2007) Ultrasound screening for asymptomatic deep vein thrombosis after major orthopaedic surgery: the VENUS study. J Thromb Haemost 5:1431–1437
White RH, Romano PS, Zhou H, Rodrigo J, Bargar W (1998) Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med 158:1525–1531
Acknowledgments
The authors thank Gunnel Sandgren and Marie Magnusson for their help in interpreting the CDS results, Henry Eriksson for interpreting the diagnostic results of pulmonary embolism and the ultrasound technicians at the Department of Clinical Physiology for performing the ultrasound examinations. We also thank the Swedish National Centre for Research in Sports (CIF) and the local Research and Development Council of Halland, Sweden, for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nilsson-Helander, K., Thurin, A., Karlsson, J. et al. High incidence of deep venous thrombosis after Achilles tendon rupture: a prospective study. Knee Surg Sports Traumatol Arthrosc 17, 1234–1238 (2009). https://doi.org/10.1007/s00167-009-0727-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-009-0727-y