Abstract
The aims of our study were to: (1) determine if there are differences in the material properties of tendon obtained from implanted tibialis anterior, achilles, bone-patella- bone and tibialis posterior allografts; (2) determine the variability in material properties between the implanted specimens. A total of 60 specimens were collected from fresh frozen allografts implanted at ACL reconstruction. Specimens collected included 15 tibialis anterior, 15 tibialis posterior, 15 achilles and 15 bone-patella-bone tendons. Each specimen was mounted in a custom made cryogrip. The mounted specimens were loaded onto a MTS Testline servo-hydraulic testing machine in a uni-axial tensile test configuration. Specimens were subjected to a strain rate of 5% per second until the ultimate tensile stress (UTS), failure strain and high strain modulus was calculated for each specimen after being normalized for specimen dimensions. Individual material properties were tested using one way analysis of variance (ANOVA) and post hoc Tukey’s B test with a P value of <0.05 considered significant. Homogeneity of variance was assessed using the Levene’s test. As a result, no significant difference was found between all four grafts with regards to UTS, failure strain or high strain linear modulus. The UTS was plotted against the modulus demonstrating a linear relationship which is typical of soft tissues. Significant variability in the results were observed. In conclusion, there was no significant statistical difference between the material properties of the four tendon allografts tested. But significant variability in results was observed within groups and between groups, which may provide one explanation for the range of results in allograft ACL reconstruction reported in the literature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of tendon allograft in Orthopedic surgery has increased significantly over the last 15 years. Anterior cruciate ligament (ACL) reconstruction lends itself particularly to the use of tendon allograft, and it is estimated that 20% of ACL reconstructions in USA are done with allograft [6]. This equates to approximately 20,000 per year.
Traditionally indications for the use of allograft include revision surgery, multi ligament injury, insufficient or poor quality donor tissue, mild degenerative joint disease and anticipation of potential accelerated rehabilitation [5]. Currently in North America allograft is commonly used as the primary graft for ACL reconstruction except in young or high demand individuals. The popularity of allograft has increased because of decreased harvest site morbidity, easier patient rehabilitation and ease of surgical procedure [25]. Further, allografts have improved appeal because of better sterilization techniques, improved graft availability and decreased hospital costs [7]. Debate still exists as to the optimum graft choice. There has been work done on the failure strength of the main four types of allograft used in ACL reconstruction [1, 12, 27]. Recorded values include 1553 N for single strand tibialis anterior, 888 N for tibialis posterior, 1139N for bone-patella-bone (BTB), and 776 N for Achilles tendon [1].
Published data are available showing good clinical results with the use of various allograft [2, 9, 15, 22, 23]. However, there is still concern over the long term results, particularly in patients under the age of 35. A recent meta-analysis demonstrated a three times higher failure rate compared with autograft [24]. There are two major potential reasons for a reported higher failure rate with allograft. Firstly, we recognize that the biology of allograft incorporation is different to that of autograft, and it has been shown to be slower [10, 11]. Secondly, there may be greater variation in the mechanical properties of allograft tendon.
The aims of our study were to:
-
1.
Determine if there were differences in the material properties of tendon obtained from implanted tibialis anterior, Achilles, BTB and tibialis posterior allografts.
-
2.
Determine the level of variability in material properties between the implanted specimens.
Methods
The allografts used in this study were obtained from the Regional Tissue Bank, which is accredited and follows the American Association of Tissue Bank standards. Healthy females aged 15–60 and males aged 16–60 years are considered suitable donors. All grafts were fresh frozen, which is the most common preparation technique [3]. Specimens are harvested under sterile conditions and then soaked in povidone for 90 min before being rinsed. The samples are then soaked in antibiotic solution (containing bactatracin, cefalexin and Gentamin) for 15 min before being frozen while serology and culture results are obtained. These specimens can be frozen for up to 5 years. No irradiation was used.
Specimen collection
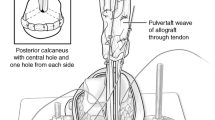
Sixty specimens were collected from fresh frozen allograft material remaining after graft preparation for ACL reconstruction. Tissue specimens collected included 15 tibialis anterior, 15 tibialis posterior, 15 Achilles and 15 BTB tendons. The BTB grafts harvested for mechanical testing included the allograft remaining after the middle third was used for reconstruction (Fig. 1).
The tibialis anterior, posterior and Achilles allograft specimens were obtained after the minimum length required for reconstruction was calculated. The specimen was obtained from the insertion end of the tendon as this was felt to be of better quality than the muscle-tendonous junction. A minimum of 5 cm in length was required for entry into the study. The specimens were placed in sterile containers containing 0.9% normal saline and frozen at −86°C until mechanical testing. Specimens were thawed at room temperature prior to testing. Electronic vernier callipers were used to measure the exact dimensions of each tissue sample. The cross-sectional area was calculated at three locations along the tendon assuming a perfect elliptical cross-section and the mean value was used in calculations of the material properties.
The testing protocol was based on the initial work by Woo et al. [35]. Each specimen was mounted in a custom made cryogrip (Fig. 2) and the grips hand tightened. The mounted specimens were loaded onto a MTS testline servo-hydraulic mechanical testing machine (458.20 microcarasol, 498 real time controller, programmed with T-/rac software v. 2.1.1, MTS, MN, USA) in a uni-axial tensile test configuration. Liquid nitrogen was applied to the custom made cryogrips until the specimen and grip teeth measured at −10°C. The grips were then tightened to a set force by tightening the clamping bolts with a torque wrench. Temperatures were checked using a temperature probe (thermocouple, Fluke 179 multimeter) as described by Rimersa et al. [26]. The specimen gauge length (specimen length between the grips) was kept moist and prevented from freezing by irrigating with Hank’s solution. A pre-load of 1 N was applied to the specimen by adjusting the position of the actuator. The gauge length was measured with digital vernier calipers to calculate the speed for correct strain rate during testing. A digital photograph was also taken for later confirmation of the gauge length using digital image analysis using Image J (USA National Institute of Health http://www.rsb.info.nih.gov/ij/). Each specimen was pre-conditioned by running the graft through 1HZ at 1 mm displacement. Preconditioning was followed by monotonic tensile test to failure. Specimens were subjected to a set strain rate of 5% per second until failure. This rate is similar to strain rates reported in other studies [13, 31, 35]. Failure was defined as tendon rupture at the grip interface or intra-substance failure. The ultimate tensile stress (UTS), failure strain and high strain modulus were calculated for each specimen after being normalized for specimen dimensions.
The data were statistically analyzed using JMP software (SAS, Carey, NC, USA). Individual material properties were tested using one way analysis of variance (ANOVA) and post hoc Tukey’s B test with a P value of <0.05 considered significant. Homogeneity of variance was assessed using the Levene’s test.
Results
Analysis of all the data using the Kolmogorov-Smirnov(a) and the Shapiro-Wilk tests showed that it was normally distributed. No significant difference was found between all four grafts with regards to UTS (P > 0.2) or modulus (P > 0.6) (Table 1 and Fig. 3a, b). The mean UTS was highest for Achilles tendon (47.4 MPa) followed by tibialis anterior (42.4 MPa), BTB (35.20 MPa) and tibialis posterior (35.15 MPa). The high strain modulus was essentially the same for Achilles tendon and tibialis anterior, with the lowest modulus reported for BTB.
There was no significant difference in failure strain between the four graft types (P > 0.09, Table 1). The UTS was plotted against the modulus for all tendons demonstrating a linear relationship which is typical of soft tissues grafts (Fig. 4).
There was significant variation in the data points between specimens in all groups, for all parameters, as demonstrated in the box plots 3a and 3b. Analysis of varience with use of the Levene’s test showed no significant difference between the four allografts for UTS (P = 0.59), modulus (P = 0.2) or failure strain (P = 0.09).
Failure occurred at the mid-substance of the tendon in all but two cases. No graft slippage was observed at the clamps.
Discussion
There was no significant statistical difference between the material properties of the four tendon allografts tested. But significant variability in results was observed within groups and between groups, which may provide one explanation for the range of results in allograft ACL reconstruction reported in the literature.
In our study, there was no significant difference between all four grafts in relation to the UTS and moduli. A plot of the UTS versus the modulus for all specimens demonstrated the expected linear relationship when undertaking tensile tests of tendon. This suggests our specimens demonstrate typical mechanical behavior. Pearsall et al. [21] compared the tensile properties of doubled tibialis anterior, tibialis posterior, and peroneus longus. They had similar findings to our study. There was no significant difference between tibialis anterior and posterior with respect to UTS, although they found that tibialis anterior was a significant stiffer graft. The tendons used in that study were from individuals greater than 70 years and none of the tendons had undergone sterilization protocol.
The literature does provide data to show that the initial strength of soft tissue allografts is comparative, if not stronger than the native ACL and autograft [1, 12, 24]. Unfortunately there is confusion regarding their use in clinical practice. Studies have shown good clinical results with the use of various allograft [2, 9, 15, 22, 23]. However, there is still concern over the long term results, particularly in patients under the age of 30 [30, 32].
Singhal et al. [29] reported a 55% re-operation/failure rate in patients under 25 years with the use of tibialis anterior. Prodromos et al. [24] recently performed a meta-analysis of the use of allograft as compared to autograft. They demonstrated that allografts had significantly lower normal stability rates and a three times higher failure rate compared with autograft. Krych et al. [14] in a more recent meta-analysis of allograft BTB versus autograft BTB concluded that, ACL reconstruction with BPTB autograft was favored over BPTB allograft for graft rupture and hop test parameters. However, when irradiated and chemically processed grafts were excluded, results were not significantly different between the two grafts.
There are two main potential reasons for a reported higher failure rate with allograft. Firstly, we know that the biology of allograft incorporation is different to that of autograft. Several experimental and clinical studies have suggested that allograft tendons used to reconstruct the cruciate ligaments incorporate and remodel in a manner similar to that seen with tendon autografts [16, 28]. It is recognized that this incorporation is slower and may be one factor in the variable success of allograft. An analysis of retrieved allografts in humans found that even after 2 years, the central portion of the allografts remained acellular, and that complete attachment to bone was not present [19]. Thus failure may occur due to premature return to the activity before graft incorporation, based on the use of rehabilitation programs designed for autograft. This was probably the main cause of the 55% failure rate reported by Singhal et al. [29], where an accelerated autograft BTB program was used.
Secondly, variation in the mechanical properties of individual grafts may also contribute to the mixed results. Our results demonstrate a great deal of variability in the material properties within each tendon group. No study in the literature has focused on this observation. BTB had a mean UTS of 35.20 MPa but values ranged from 10.20 to 85.74 MPa. Thus some grafts may fail secondary to ultimate tensile strengths below the graft forces experienced during rehabilitation prior to ligamentization.
This variability suggests that other factors may be involved in optimizing tissue quality, independent of graft type. Donor age potentially plays a significant role in graft properties [20]. Given there is a wide age range of acceptable donors, there is likely to be a mismatch with the recipient. An 18 year old male may receive a graft from a 60 year old female. Variables such as leg dominence [17], sex [12, 18], life style, and activity level [28, 33] are also possible reasons for the variation in the material properties found, although there is little evidence to confirm this in the literature. Due to confidentiality and legal reasons, we do not have the demographic data available to determine this within our study group.
Sterilization with the use of low dose irradiation has been shown to have detrimental effect on the material properties of tendon [8]. We used fresh frozen grafts for this reason, as it is believed not to have a significant detrimental effect on the tendon [12]. Jones et al. [12] examined 17 BTB specimens post sterilization. While, they found no difference in the mean UTS (29 MPa) there was a greater range of results reported in the sterilized group versus the control. It is possible that this process may have contributed to specimen variation.
While, there was no statistical difference in the mean values for the material properties between the four grafts, there was a significant variation. This may suggest a beta error due to lack of specimen numbers. The variation in the modulus of the specimens, in part, is due to error introduced during testing. With soft tissue grafts it is difficult to determine the initial gage length. This introduces error when calculating both strain and subsequently modulus.
The patella tendon had the largest variation in modulus. This in part may be due to the use of tendon from the periphery of the allograft. The peripheral 1/3 of the patella tendon has been shown to be less stiff than the middle 1/3 [6]. The patella tendon may also have been further influenced by the strain rate used in the study. Other authors have used testing strain rates of 100% per second, because this rate is thought to produce soft tissue disruption before bony avulsion in the clinical setting [26, 34]. This may have lead to different values for ultimate failure load and stiffness, although with the use of the cryogrip this effect should be minimized. Allograft patellar tendon may also be more suceptable to alteration, as authors have reported increased laxity with these grafts in the clinical situation [4].
A further criticism of this paper could be that we did not use complete specimens. We wanted to use “implant ready” allograft which had undergone our local sterilization procedures. This would best represent the properties of the grafts that we were using in clinical practice. This is also why we investigated the material properties rather than the mechanical behavior of complete grafts. Given the cost and a limited graft supply we did not feel that we could justify the sacrifice of 60 implantable specimens. Using the insertion end of the grafts this provided a better representation of the implanted grafts.
The results of this study imply the tendon from various allograft types available in our tissue bank have similar material properties. There is significant variation of UTS and moduli between specimens, which may provide one reason for inconsistency of the results in the current literature. Further work is needed to determine what and how, patient and processing factors effect graft material/mechanical properties. This will then enable optimal graft selection and potentially greater consistency in reported outcomes.
Conclusions
There was no significant statistical difference between the material properties of the four tendon allografts tested. But significant variability in results was observed within groups and between groups, which may provide one explanation for the range of results in allograft ACL reconstruction reported in the literature.
References
Almqvist KF, Jan H, Vercruysse C, Verbeeck R et al (2007) The tibialis tendon as a valuable anterior cruciate ligament allograft substitute: biomechanical properties. Knee Surg Sports Traumatol Arthrosc 15(11):1326–1330
Bach BR Jr, Aadalen KJ, Dennis MG (2005) Primary anterior curciate ligament reconstruction using fresh frozen, non-irradiated patellar tendon allograft: minimum 2 year follow-up. Am J Sports Med 33:284–292
Barbour SA, King W (2006) The safe and effective use of allograft tissue. In: Scott WN (ed) Insall and scott surgery of the knee, vol 1, 4th edn. Elsevier, Philadelphia, pp 686–692
Barrett G, Stokes D, White M (2005) Anterior cruciate reconstruction in patients older than 40 years: allograft versus autograft patellar tendon. Am J Sports Med 33:1505–1512
Busam M, Rue JPH, Bach BR (2007) Fresh frozen allograft anterior cruciate reconstruction. Clin Sports Med 26:607–623
Cohen SB, Sekiya JK (2007) Allograft safety in anterior cruciate ligament reconstruction. Clin Sports Med 26:597–605
Cole DW, Ginn TA, Chen GJ et al (2005) Cost comparison of ACL reconstruction: autograft versus allograft. Arthroscopy 21(7):786–790
Gibbons MJ, Butler DL, Grood ES et al (1988) Effects of gamma irradiation on the initial mechanical and material properties of goat bone-patellar tendon-bone allografts. J Orthop Res 6:95–102
Indelli PF, Dillingham MF, Fanton GS et al (2004) Anterior cruciate ligament reconstruction using cryopreserved allograft. Clin Orthop Related Res 420:268–275
Jackson DW, Corsetti J, Simon TM (1996) Biologic incorporation of allograft anterior cruciate ligament replacements. Clin Orthop 324:126–133
Jackson DW, Grood ES, Arnoczky SP et al (1987) Freeze-dried anterior cruciate ligament allografts. Preliminary studies in a goat model. Am J Sports Med 15:295–303
Jones DB, Huddleston PM, Zobitz ME et al (2007) Mechanical properties of patellar tendon allografts subjected to chemical sterilization. Arthroscopy 23(4):400–405
Kennedy J, Hawkins R, Willis R et al (2005) Tension studies of human knee ligaments. J Bone Joint Surg Am 58:350–355
Krych AJ, Jackson JD, Hoskin TL (2008) A meta-analysis of patellar tendon autograft versus patellar tendon allograft in anterior cruciate ligament reconstruction. Arthroscopy 24(3):292–298
Kuechle DK, Pearson SE, Beach WR et al (2002) Allograft anterior cruciate reconstruction in patients over 40 years. Arthroscopy 18:845–853
Kustos T, Balint L, Than P, Bardos T (2004) Comparative study of autograft or allograft in primary anterior cruciate ligament reconstruction. Int Orthop 28:290–293
Louis-Ugbo J, Leeson B, Hutton WC (2004) Tensile properties of fresh human calcaneal (Achilles) tendons. Clin Anat 17(1):30–35
Magnusson SP, Hansen M, Langberg H et al (2007) The adaptability of tendon to loading differs in men and women. Int J Exp Pathol 88(4):237–240
Malinin TI, Levitt RL, Bashore C et al (2002) Study of retrieved allografts used to replace anterior cruciate ligaments. Arthroscopy 18:163–170
Nakagaki WR, Biancalana A, Benevides GP et al (2007) Biomechanical and biochemical properties of chicken calcaneal tendon under effect of age and nonforced active exercise. Connect Tissue Res 48(5):219–228
Pearsall AW, Hollis JM, Russell GV (2003) A biomechanical comparison of three lower extremitytendons for ligamentous reconstruction about the knee. Arthroscopy 19(10):1091–1096
Peterson RK, Shelton WR, Bomboy AL (2001) Allograft versus autograft patella tendon anterior cruciate reconstruction: a 5 year follow-up. Arthroscopy 17:9–13
Poehling GG, Curl WW, Lee CA et al (2005) Analysis of outcomes of anterior cruciate repair with 5 year follow-up: allograft versus autograft. Arthroscopy 21(7):774–785
Prodromos C, Joyce B, Shi K (2007) A meta-analysis of stability of autografts compared to allografts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 15(7):851–856
Rihn JA, Irrgang JJ, Chhabra A et al (2006) Does irradiation affect the clinical outcome of patellar tendon allograft ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 14:885–896
Rimersa DJ, Schamardt HC (1982) The cryo-jaw, a clamp designed for rheology studies of horse digital flexor tendons. J Biomechanics 15(8):619–620
Schimizzi A, Wedemeyer M, Odell T (2007) Effects of a novel sterilization process on soft tissue mechanical properties for anterior cruciate ligament allografts. Am J Sports Med 35(4):612–616
Shino K, Kawasaki T, Hirose H et al (1984) Replacement of the anterior cruciate ligament by an allogeneic tendon graft. An experimental study in the dog. J Bone Joint Surg Br 66:672–681
Singhal MC, Gardiner JR, Johnson DL (2007) Failure of primary anterior cruciate ligament surgery using anterior tibialis allograft. Arthroscopy 23(5):469–475
Stringham DR, Pelmas CJ, Burks RT et al (1996) Comparison of anterior cruciate ligament reconstructions using patellar tendon autograft or allograft. Arthroscopy 12:414–421
Trent P, Walker P, Wolf B (1976) Ligament length patterns, strength, and rotational axes of the knee joint. Clin Orthop Relat Res 117:263–270
Victor J, Bellemans J, Witvrouw E et al (1997) Graft selection in anterior cruciate ligament reconstruction: prospective analysis of patellar tendon autografts compared with allografts. Int Orthop 21:93–97
Westh E, Kongsgaard M, Bojsen-Moller J et al (2007) Effect of habitual exercise on the structural and mechanical properties of human tendon, in vivo, in men and women. Scand J Med Sci Sports 18(1):23–30
Wilson T, Zafuta M, Zobitz M (1999) A biomechanical analysis of matched bone-patellar tendon-bone and double-looped semitendinosus and gracilis tendon grafts. Am J Sports Med 27:202–207
Woo SL, Hollis JM, Adams DJ et al (1991) Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am J Sports Med 19(3):217–225
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Penn, D., Willet, T.L., Glazebrook, M. et al. Is there significant variation in the material properties of four different allografts implanted for ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 17, 260–265 (2009). https://doi.org/10.1007/s00167-008-0678-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-008-0678-8