Abstract
This article describes second-look arthroscopic evaluation of the transplanted grafts after anatomical two-bundle ACL reconstruction, which had been performed between December 2000 and March 2004. Using two double-looped semitendinosus tendon grafts via separate femoral and tibial tunnels in the anatomical ACL footprints, 65 patients (mean age of 24 years) underwent anatomical two-bundle ACL reconstruction. The evaluation was performed for those who had undergone the procedure 5–29 months (mean 16.5) previously, with emphasis on graft tension and the presence of graft damage by meticulous probing. None of the anteromedial (AM) grafts showed rupture, while 11% of the posterolateral (PL) grafts showed substantial damage around the femoral tunnel aperture. Both the AM and PL grafts were evaluated as lax without apparent graft rupture in 9% of the knees. These results suggest that the currently performed anatomical two-bundle ACL reconstruction and postoperative regimen still remain to be improved to achieve better postoperative graft morphology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anterior cruciate ligament (ACL) reconstruction using hamstring tendon autograft has been a popular procedure because of its lower morbidity in graft harvest site [8, 10, 17]. Traditional ACL reconstructions including Rosenberg’s 1 or 2 femoral sockets (“bi-socket”) procedure [22] have been focused on restoring the anteromedial bundle by isometric graft placement [10, 15, 17, 19, 29, 33]. These reconstructions, although satisfactory outcomes have been reported [8, 10, 17, 19], may have the following potential problems, which might lead to potential graft loosening or failure: (1) insufficient restoration to normal knee kinematics [1]; (2) graft impingement against the intercondylar notch [30] or posterior cruciate ligament (PCL) [5, 28]. In reality, Toritsuka et al. [29] reported that 34% of ACL grafts showed partial tear in their anterior portion by second-look arthroscopy after Rosenberg’s ACL reconstruction.
Current improvement in instrumentation has made it possible to perform a more anatomical reconstruction to mimic the structure and function of both the anteromedial (AM) and the posterolateral (PL) bundles of the ACL [15, 18, 23, 31]. In Rosenberg’s procedure, one or two femoral sockets were created around 1 o’clock for the left or 11 o’clock for the right knee in the posterior margin of the lateral wall of the notch through a single tibial tunnel. We had shifted the position of the femoral sockets to more anatomical 2, 3 (or 9, 10) o’clock position in January 2000 (Fig. 1a), and the tibial tunnel was divided into two parallel ones in December 2000 [27] (Fig. 1b). The ACL grafts reconstructed with this technique could not only potentially perform biomechanically better [16, 31], but also have more biological advantage in graft remodeling due to increased graft-bone tunnel contact area. It was hypothesized that this anatomical reconstruction might be advantageous in preventing damage of the AM bundle, which had been observed in the isometric reconstruction [29]. Thus, the purpose of this study was to arthroscopically evaluate the transplanted grafts after the anatomical two-bundle ACL reconstruction.
Methods
Patients
Anatomical two-bundle ACL reconstruction using semitendinosus tendon autograft was performed on 283 knees between 2001 and 2003 in our institution. Of all the patients, 43 or 15% were excluded because they were lost for our follow-up evaluation; 16 or 5.6% of the patients underwent revision surgery because of repeat injury in sports activities. There were 68 or 15% of them who had consented to undergo second-look arthroscopy. As three patients were excluded from this study because they had undergone the second-look earlier than 5 months after the reconstruction, 65 knees of 65 patients (32 male and 33 female patients) with a mean age of 24.1 years, ranging from 15 to 44 years were included in this study. The remaining 156 or 55% of the patients were not included in this study because they did not undergo second-look arthroscopy.
It has been our policy to advise patients to undergo second-look arthroscopic evaluation in conjunction with hardware removal, particularly if they underwent meniscus repair. Thus, 38 patients who had undergone simultaneous meniscal repair consented to undergo the evaluation, while three were evaluated at the time of the subsequent partial meniscectomy. The remaining 24 consented to have the evaluation at the time of hardware removal, as it had caused discomfort or pain around the hardware/stitches during kneeling or Japanese sitting. None of them complained of subjective instability or giving way.
All patients underwent routine follow-up evaluation according to the International Knee Documentation Committee (IKDC) Knee Ligament Evaluation form, including the standard physical examination, while 23 patients were excluded from the results of follow-up evaluation because of a short follow-up of less than 10 months (10 patients) or discrepancy over 2 months in timing between the follow-up evaluation and the second-look (13 patients). Thus, 42 patients were evaluated with IKDC scores. Lachman test was negative in 61 knees, or mildly positive with a firm endpoint in four knees. The pivot-shift test was negative in 60 knees, and glide in five knees. While 41 of the 42 patients (98%) were subjectively classified into normal or nearly normal, the remaining one patient, who had had articular cartilage and meniscus injuries in the lateral compartment requiring lateral meniscectomy at the time of the original ACL reconstruction, was rated abnormal because of pain. Residual anterior laxity was measured using the KT-1000 arthrometer at maximum manual forces. The mean side-to-side difference in KT measurement was 0.8 ± 1.0 mm (range 1–3 mm; Fig. 2).
Surgical technique
Graft harvesting and preparation
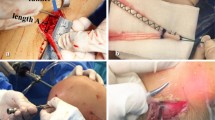
For grafting, the entire semitendinosus tendon was harvested through a 4 cm oblique longitudinal incision, medial to the tibial tubercle. The tendon was transected into half to make two double-looped grafts of 60–70 mm length and 5–6 mm diameter. An Endo-button CL (Smith and Nephew Endoscopy) was connected to the loop end, and two #3 braided polyester sutures were placed in each free end of the graft by Krackow suture [13].
ACL reconstruction procedure
The key to consistent visualization of the notch is to keep the distal femoral axis horizontally by fixing the proximal thigh using a leg holder. The entire procedure is performed arthroscopically. For the femur, two 2.4 mm guide pins were inserted at the points 5–6 mm anterior to the posterior margin of the notch at 2, 3 o’clock for the left or at 9, 10 o’clock for the right knee (Fig. 1a). The pins were overdrilled with cannulated drill bits of diameter matching with those of the grafts’ (5–6 mm). For 20 knees in the earlier series, the guide pins were inserted and overdrilled in an inside-out fashion through the far anteromedial portal established at 2–2.5 cm posterior to the standard anteromedial portal [25], with the knee flexed to 130°. For 45 knees in the latter series, the guide pins were inserted and overdrilled in an outside-in fashion using the 6 mm offset anterolateral entry femoral aimer (ref. no. 7210984, Smith and Nephew Endoscopy) [27], with the knee flexed at 70–80° by gravity. For the tibia, a guide pin was inserted from the anteromedial cortex to the center of the footprint at an angle of 45° in the frontal plane and 20–25° in the sagittal plane to the tibial axis, with the Director tibial guide (Smith and Nephew Endoscopy). The second/posterior pin and the third/anterior pin were inserted parallel to the central pin with the offset pin guide (Stepped tibial off-set guide, cat. no. 04-32C6, Smith and Nephew Endoscopy) to be at 20° to the sagittal plane or on the line from the anterior horn of the medial meniscus to the posterior horn of the lateral meniscus. After the central pin was removed, each pin was overdrilled with a drill bit of appropriate diameter (5–6 mm) (Fig. 1b). With these techniques, the two tunnels in the femur or in the tibia were never overlapped. After the grafts were fixed with two Endo-button CL on the femur, they were secured onto the tibia with two double-spike plates (DSP; MEIRA Corp., Nagoya, Aichi, Japan; US Patent No. 6117,139,21) [26] at 20° of flexion of the knee. Before final fixation at the tibial side, the length change of the grafts was measured with the isometric positioner (Smith and Nephew Endoscopy) [32]. All grafts showed elongation approaching knee extension from 120°, like the normal ACL. The average length changes of the AM and PL graft were 1.4 ± 0.6 and 2.6 ± 0.9 mm, respectively. The tensioning sutures distally connected to the two DSPs were, respectively, tied to the tensioners, mounted to a metal shell boot fixed to the tibia with a bandage (Fig. 3). An initial tension to the graft of 20–30 N was applied to each tensioning suture, based on the diameter of the graft. Finally, each graft was fixed with DSPs and two cancellous screws. Notchplasty was not required in a single case, as the anatomically placed two grafts, aligned along the long axes of the footprints and never impinged against the roof or the wall of the intercondylar notch. Thirty-two patients had associated injuries to the medial and 28 to the lateral meniscus. Among the former, five patients had undergone partial meniscectomy, 26 had meniscal repair and three had rasping only. Among those with lateral meniscus injuries, three had undergone partial meniscectomy, 23 had meniscal repair and two had rasping. No patients had had prior intra-articular or extra-articular ligament reconstructions, posterior drawer sign, varus–valgus abnormal laxity or excessive external tibial rotation.
Postoperative regimen
The knee was immobilized for a week with a brace followed by continuous passive motion exercise. Active and assisted range of motion exercise was started at 2 weeks. Partial weight bearing was allowed at 3 weeks, followed by full weight bearing at 5 weeks. After return to daily activities or work except for manual labor, jogging was allowed at 3 months. Running was allowed at 4 months, followed by return to previous sports activity at 6–9 months. All patients were hospitalized for 4–5 weeks after surgery to strictly follow the rehabilitation protocol under the supervision by a physical therapist. After leaving the hospital, they visited our hospital more than once a week to keep up our rehabilitation program up to 3 months postoperatively.
Arthroscopic evaluation of ACL graft
The grafts were evaluated based on tension and substantial damage with meticulous probing [29]. The tension of the graft was classified as taut or lax at 20–90° of knee flexion. The grafts that were as tense as normal ACL were evaluated as taut; and those with obvious loss of tension were evaluated as lax (Fig. 4). Graft damage was evaluated in each bundle throughout the whole length and classified according to whether there was a substantial rupture (Fig. 5). The site of graft rupture was also recorded. Synovial coverage over each graft was classified into the following three categories: good, fair and poor. Synovial coverage was defined to be “good” when the synovium covered the whole length of the graft, including the femoral tunnel aperture. It was “fair” when the non-synovialized area was less than 50% of the entire surface of the graft, and “poor” when more than 50% of the graft was naked without synovial coverage. The site of the non-synovialized area was also recorded (Fig. 6).
Arthroscopic classification based on the synovial coverage over the grafts. a The AM graft is covered with synovium over its entire length including femoral tunnel aperture and classified as “good.” However, the PL graft is covered with synovium only over its distal half, and classified as “fair.” b Both the AM and the PL grafts are little covered with synovium and classified as “poor.” There is a gap between the two grafts and the femoral tunnel apertures without widening of the bone tunnels (arrows)
Statistics
The χ-square test was used to compare the arthroscopic morphology between AM and PL graft (P < 0.05). Kruskal–Wallis test and one-way ANOVA were used to detect statistically significant effects of tension and the presence of rupture of the grafts on the clinical outcomes. A significance level was set at P < 0.05.
Results
Graft morphology
Arthroscopic findings on graft morphology were summarized in Table 1. As to graft damage, no apparent rupture was observed in the AM grafts, while complete or partial rupture was observed in 11% of the PL grafts at the femoral tunnel aperture. There was a significant difference in the incidence of graft damage between the AM and PL grafts (P = 0.007).
As to graft tension, both grafts were evaluated as taut in 80% of the knees. In 11% of the knees, the AM graft was evaluated as taut, while the PL graft was unclassified because of its complete or substantial rupture. Both the AM and PL grafts were evaluated as lax in 9% of the knees, and there was no significant difference in graft tension between them (P = 0.835) (Table 1).
As to the condition of synovial coverage over the AM graft, 77% of knees were evaluated as “good”, 14% as “fair” and 9% as “poor,” while over the PL graft 34% of knees were evaluated as “good”, 48% as “fair” and 16% as “poor.” Three knees (5%) were evaluated as unclassified because the PL grafts were gone or completely torn around the femoral tunnel aperture. In all cases evaluated as “fair” or “poor,” the defect in synovial coverage was seen around the femoral tunnel aperture. There was a significant difference in the condition of synovial coverage between the AM and PL grafts (P < 0.001) (Table 1). In such cases, there was a space between the graft and the femoral tunnel aperture without apparent widening of the bone tunnel (Fig. 6).
Based on the presence of graft damage and graft tension, the graft morphology could be classified into the following three groups: Group A (n = 52, 80%), both grafts were taut with no rupture; Group B (n = 7, 11%), the AM graft was taut with no graft rupture but the PL graft was ruptured or deficient at the femoral tunnel aperture; and Group C (n = 6, 9%), both grafts were lax without apparent rupture.
Correlation between graft morphology and the graft length change
The length changes of both grafts from 0° to 120° was measured intraoperatively. The average length changes of the AM and PL graft were 1.4 ± 0.6 and 2.6 ± 0.9 mm, respectively, showing a statistically significant difference (P < 0.001).
The average length changes of AM grafts were 1.4 ± 0.7 mm in Group A, 1.6 ± 0.2 mm in Group B and 1.4 ± 0.6 mm in Group C, showing no significant difference among the three groups (P = 0.83). The average length changes of PL grafts were 2.6 ± 0.9 mm in Group A, 3.1 ± 0.7 mm in Group B and 2.6 ± 0.9 mm in Group C, showing no significant difference among the three groups (P = 0.29).
Correlation between graft morphology and postoperative periods
At less than 1 year (<1-year group), 11 knees were evaluated, 34 knees at 1–2 years (1–2-year group) and 20 knees at more than 2 years (>2-year group). The correlation of graft morphology was summarized in Table 2. The numbers in the <1-year group were 8 (73%) in Group A, 2 (18%) in Group B and 1 (9%) in Group C The numbers in the 1–2-year group were 28 (83%) in Group A, 4 (11%) in Group B and 2 (6%) in Group C. The numbers in the >2-year group were 16 (80%) in Group A, 1 (5%) in Group B and 3 (15%) in Group C. There was no significant difference in graft morphology among these three postoperative evaluation periods (P = 0.682).
Correlation between graft morphology and the clinical outcomes
Subjectively, all patients were classified as ‘normal’ or ‘nearly normal’ in Group A and B. Only one patient was categorized as ‘abnormal’ in Group C because of the pain. In Group A, 26 patients were evaluated as normal and 4 as nearly normal. In Group B, 4 patients were evaluated as normal and 2 as nearly normal. In Group C, three patients were evaluated as normal, two as nearly normal and one as abnormal (Table 3.). There was no significant difference among these three groups (P = 0.083). A patient evaluated as abnormal had had chondral injury to the lateral compartment and lateral meniscal tear requiring meniscectomy at the time of ACL reconstruction.
The side-to-side difference in KT measurement was 0.6 ± 0.9 mm for patients in Group A, 1.0 ± 0.9 mm in Group B and 1.7 ± 1.0 mm in Group C, showing no significant difference among these three groups (one-way ANOVA, P = 0.054) (Table 3).
Not a single case showed positive pivot-shift test of ++ or greater in any group. Two patients showed glide in Group A and one patient each in Group B and C. There was no significant difference among these three groups (P = 0.615) (Table 3).
Discussion
Our arthroscopic evaluation has revealed that the AM graft was free from graft damage after the anatomical two-bundle ACL reconstruction, in contrast to the previous study on the Rosenberg’s bi-socket ACL reconstruction [29]. In the anatomical two-bundle ACL reconstruction, the femoral tunnels were made at a lower position than those in the Rosenberg’s reconstruction at the posterior margin of the notch, and thus more natural obliquity of the graft could be achieved [2, 27, 31]. Accordingly, the anatomically reconstructed grafts should theoretically have much less chance to suffer from impingement against the notch and/or the PCL [2, 33], and such a geographical feature could potentially contribute to the avoidance of substantial damage to the AM grafts.
On the other hand, the present study showed that 11% of PL grafts were partially or completely damaged at the femoral tunnel aperture. Even in patients without apparent graft rupture, we observed poor synovial coverage over the graft around the femoral tunnel aperture. Particularly, synovial coverage of the PL grafts was poorer than that of the AM grafts. In such cases, there was a space between the graft and the femoral tunnel aperture without apparent widening of the bone tunnel (Fig. 6), suggesting insufficient graft-tunnel incorporation. Therefore, the incidence of PL graft damage may increase with longer follow-up than described in the present results.
It has been suggested that the PL graft is exposed to excessive stress from the femoral tunnel by the windshield wiper and/or bungee cord effect [11, 24]. In a normal knee, Bach et al. [3] and Zavras et al. [33] indicated that the PL bundle strain was significantly higher than the AM bundle strain throughout the range of motion. Sakane et al. [23] reported that the PL bundle of the normal ACL carries the majority of the load during anterior tibial loading between full extension and 15° of flexion. Yonetani et al. [32] measured the length changes of grafts intraoperatively in anatomical two-bundle reconstruction and reported that the length change of the PL graft was larger than that of the AM graft throughout the range of motion. Thus, the PL graft could be subject to an excessive stress near extension after the anatomical two-bundle reconstruction and such biomechanical environment might be responsible for the PL graft damage or poor synovial coverage around the tunnel aperture of the PL graft. Accordingly, the initial graft tension as well as knee flexion angle at the time of graft fixation might be reconsidered to avoid over-stress exerted onto the PL graft.
Theoretically tendon-bone healing requires at least 8–12 weeks [21]. However, current postoperative rehabilitation protocols emphasize early mobilization to prevent joint contracture or arthrofibrosis, and thus resulting in the exertion of excessive stress on the graft tissue before sufficient graft-bone tunnel incorporation [11, 21]. Therefore, biological and/or biomechanical manipulation to promote tendon-to-bone healing would be required in the future to overcome the problem [4, 9, 14]. While we tried to create the tunnels to closely mimic the two anatomical ACL bundles as described, we could not state that their positions were strictly or biomechanically optimized, based on some particular theories such as the concept of isometry, which had been prevailing in the last century. Therefore, the position we chose for the tunnel for the PL graft could be one of the potential factors responsible for its tear or damage.
It should be noted that, in spite of certain impairment of the PL graft in Group B, there was no statistically significant difference in clinical outcome between Group A and B. Recently, the importance of the PL bundle in rotational control has been suggested by biomechanical studies [6, 31] and there was a concern that the PL graft damage may lead to increased rotational instability of the knee. However, there was no significant correlation between the PL graft damage and the residual pivot-shift. The pivot-shift test is known to be the most conventional method to assess the rotational instability of knees [7]. However, it has shown a limitation of sensitivity [12, 20] or inter-observer variability. Ristanis et al. [20] analyzed functional dynamic knee stability in vivo during pivoting after descending stairs with a six-camera opto-electronic system and reported that significant increase in tibial rotation was found in those with negative pivot-shift test. Therefore, a more precise measurement method than the pivot shift test would be necessary to precisely interpret the results. Moreover, although PL graft damage was not correlated to clinical outcomes in the present study, the knees with partially damaged grafts might potentially lead to graft failure in the future due to stress concentration of the remaining AM graft.
As to the tension of the grafts, 9% of knees were evaluated as “lax” in both AM and PL grafts and classified into Group C, while no statistically significant difference was found in clinical outcome among Group C and the other groups. If the KT value closely reflects graft tension, these results seem unreasonable. The potential explanations for this discrepancy are as follows: the first would be the small sample size of this study. KT value in Group C tended to be inferior to that in the other groups, although not significant. The significance might become positive with a larger sample size. The second explanation could be the difference between graft laxity and joint laxity. Arthroscopic evaluation could only address graft laxity, while KT measurement reflects joint laxity that implies not only graft laxity but also integrity of the other restraints (capsule, meniscus, collateral ligament, etc.). Therefore, our results suggest that second-look arthroscopy is still desirable for thorough evaluation of the actual condition of the transplanted graft. Surgeons should have a chance to perform second-look arthroscopic evaluation as frequently as practical to objectively evaluate the procedure they had performed. Even if there was no significant correlation between graft morphology and clinical outcome, loss of tension and/or partial graft rupture may potentially lead to total graft failure in the future. Further advancement in operative procedure and postoperative rehabilitation would be required to improve the results after the anatomical multi-bundle ACL reconstruction using hamstrings tendon autograft.
Conclusion
None of the anteromedial grafts showed rupture, while 11% of the posterolateral grafts showed substantial damage around the femoral tunnel aperture in the clinically satisfactory knees after the anatomical two-bundle ACL reconstruction.
References
Almekinders LC, Pandarinath R Rahusen FT (2004) Knee stability following anterior cruciate ligament rupture and surgery. The contribution of irreducible tibial subluxation. J Bone Joint Surg Am 86-A:983–987
Arnold MP, Kooloos J, van Kampen A (2001) Single-incision technique misses the anatomical femoral anterior cruciate ligament insertion: a cadaver study. Knee Surg Sports Traumatol Arthrosc 9:194–199
Bach JM, Hull ML (1998) Strain inhomogeneity in the anterior cruciate ligament under application of external and muscular loads. J Biomech Eng 120:497–503
Demirag B, Sarisozen B, Ozer O, Kaplan T, Ozturk C (2005) Enhancement of tendon-bone healing of anterior cruciate ligament grafts by blockage of matrix metalloproteinases. J Bone Joint Surg Am 87:2401–2410
Fujimoto E, Sumen Y, Deie M, Yasumoto M, Kobayashi K, Ochi M (2004) Anterior cruciate ligament graft impingement against the posterior cruciate ligament: diagnosis using MRI plus three-dimensional reconstruction software. Magn Reson Imaging 22:1125–1129
Gabriel MT, Wong EK, Woo SL, Yagi M, Debski RE (2004) Distribution of in situ forces in the anterior cruciate ligament in response to rotatory loads. J Orthop Res 22:85–89
Galway HR, MacIntosh DL (1980) The lateral pivot shift: a symptom and sign of anterior cruciate ligament insufficiency. Clin Orthop Relat Res 147:45–50
Giron F, Aglietti P, Cuomo P, Mondanelli N, Ciardullo A (2004) Anterior cruciate ligament reconstruction with double-looped semitendinosus and gracilis tendon graft directly fixed to cortical bone: 5-year results. Knee Surg Sports Traumatol Arthrosc 13(2):81–91
Grassman SR, McDonald DB, Thornton GM, Shrive NG, Frank CB (2002) Early healing processes of free tendon grafts within bone tunnels is bone-specific: a morphological study in a rabbit model. Knee 9:21–26
Hamada M, Shino K, Horibe S, Mitsuoka T, Miyama T, Shiozaki Y, Mae T (2001) Single- versus bi-socket anterior cruciate ligament reconstruction using autogenous multiple-stranded hamstring tendons with endobutton femoral fixation: a prospective study. Arthroscopy 17:801–807
Hoher J, Withrow J, Livesay G (1998) Early stress causes graft-tunnel motion in hamstring grafts. Trans Orthop Res 23:44
Hole RL, Lintner DM, Kamaric E, Moseley JB (1996) Increased tibial translation after partial sectioning of the anterior cruciate ligament. Am J Sports Med 24:556–560
Krackow KA, Thomas SC, Jones LC (1988) Ligament-tendon fixation: analysis of a new stitch and comparison with standard techniques. Orthopedics 11(6):909–917
Kyung HS, Kim SY, Oh CW, Kim SJ (2003) Tendon-to-bone tunnel healing in a rabbit model: the effect of periosteum augmentation at the tendon-to-bone interface. Knee Surg Sports Traumatol Arthrosc 11:9–15
Mae T, Shino K, Miyama T, Shinjo H, Ochi T, Yoshikawa H, Fujie H (2001) Single- versus two-femoral socket anterior cruciate ligament reconstruction technique: biomechanical analysis using a robotic simulator. Arthroscopy 17:708–716
Mae T, Shino K, Matsumoto N, Nakata K, Nakamura N, Iwahashi T (2006) Force sharing between two grafts in the anatomical two-bundle anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 14:505–509
Maeda A, Shino K, Horibe S, Nakata K, Buccafusca G (1996) Anterior cruciate ligament reconstruction with multistranded autogenous semitendinosus tendon. Am J Sports Med 24:504–509
Muneta T, Sekiya I, Yagishita K, Ogiuchi T, Yamamoto H, Shinomiya K (1999) Two-bundle reconstruction of the anterior cruciate ligament using semitendinosus tendon with endobuttons: operative technique and preliminary results. Arthroscopy 15:618–624
Natsu-ume T, Shino K, Nakata K, Nakamura N, Toritsuka Y, Mae T (2001) Endoscopic reconstruction of the anterior cruciate ligament with quadrupled hamstring tendons: a correlation between MRI changes and restored stability of the knee. J Bone Joint Surg Br 83:834–837
Ristanis S, Stergiou N, Patras K, Vasiliadis HS, Giakas G, Georgoulis AD (2005) Excessive tibial rotation during high-demand activities is not restored by anterior cruciate ligament reconstruction. Arthroscopy 21:1323–1329
Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF (1993) Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am 75:1795–1803
Rosenberg TD (1993) Technique for endoscopic rnethod of ACL reconstruction. Technical Bulletin, Mansfield, MA, Acufex Microsurgical
Sakane M, Fox RJ, Woo SL, Livesay GA, Li G, Fu FH (1997) In situ forces in the anterior cruciate ligament and its bundles in response to anterior tibial loads. J Orthop Res 15:285–293
Segawa H, Koga Y, Omori G, Sakamoto M, Hara T (2005) Contact pressure in anterior cruciate ligament bone tunnels: comparison of endoscopic and two-incision technique. Arthroscopy 21:439–444
Shino K, Horibe S, Hamada M (2002) Allograft anterior cruciate ligament reconstruction. Tech Knee Surg 1:78–85
Shino K, Mae T, Maeda A, Miyama T, Shinjo H, Kawakami H (2002) Graft fixation with predetermined tension using a new device, the double spike plate. Arthroscopy 18:908–911
Shino K, Nakata K, Nakamura N, Mae T, Otsubo H, Iwahashi T, Nakagawa S (2005) Anatomic ACL reconstruction using two double-looped hamstring tendon grafts via twin femoral and triple tibial tunnels. Oper Tech Orthop 15:130–134
Simmons R, Howell SM, Hull ML (2003) Effect of the angle of the femoral and tibial tunnels in the coronal plane and incremental excision of the posterior cruciate ligament on tension of an anterior cruciate ligament graft: an in vitro study. J Bone Joint Surg Am 85-A:1018–1029
Toritsuka Y, Shino K, Horibe S, Mitsuoka T,Hamada M,Nakata K, Nakamura N, Yoshikawa H (2004) Second-look arthroscopy of anterior cruciate ligament grafts with multistranded hamstring tendons. Arthroscopy 20:287–293
Tsuchiya A (1995) Biomechanical study on the optimal running route for anterior cruciate ligament reconstruction. Nippon Seikeigeka Gakkai Zasshi 69:1113–1125
Yagi M, Wong EK, Kanamori A, Debski RE, Fu FH, Woo SL (2002) Biomechanical analysis of an anatomic anterior cruciate ligament reconstruction. Am J Sports Med 30:660–666
Yonetani Y, Toritsuka Y, Yamada Y, Iwahashi T, Yoshikawa H, Shino K (2005) Graft length changes in the bi-socket anterior cruciate ligament reconstruction: comparison between isometric and anatomic femoral tunnel placement. Arthroscopy 21:1317–1322
Zavras TD, Race A, Bull AM, Amis AA (2001) A comparative study of ‘isometric’ points for anterior cruciate ligament graft attachment. Knee Surg Sports Traumatol Arthrosc 9:28–33
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Otsubo, H., Shino, K., Nakamura, N. et al. Arthroscopic evaluation of ACL grafts reconstructed with the anatomical two-bundle technique using hamstring tendon autograft. Knee Surg Sports Traumatol Arthrosc 15, 720–728 (2007). https://doi.org/10.1007/s00167-006-0274-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-006-0274-8