Abstract
There is growing evidence that cytokines such as tumor necrosis factor (TNF) α, interleukin (IL) 1β, IL-6, bone morphogenetic proteins (BMP), and nitric oxide (NO) play an important role in the pathogenesis of bone tunnel enlargement following anterior cruciate ligament (ACL) reconstruction. Furthermore, the release of these mediators has been considered a possible reason for the higher incidence of bone tunnel enlargement following hamstring tendon (HST) than following patellar tendon (PT) ACL reconstruction observed in several studies. In this investigation synovial fluid samples from 13 patients were collected immediately before (24±7 days after ACL rupture) and 7 days after ACL surgery and values of TNF-α, IL-1β, IL-6, NO, and BMP-2 were analyzed. Furthermore, the incidence of bone tunnel enlargement was assessed using radiographs 38±7 weeks after surgery. Six patients underwent autologous HST ACL reconstruction, and in seven patients an PT autograft was used. In the overall patient population there were significantly higher synovial fluid concentrations of IL-6 and BMP-2 postoperatively than preoperatively; TNF-α showed a trend towards lower postoperative levels while IL-1β and NO remained unchanged. The concentrations of NO, TNF-α, and IL-6 found in the present study were clearly higher than normal values given in the literature. Assessment of bone tunnel enlargement revealed an average increase in tibial tunnel width of 28.4±3.1% with comparable values for HST and PT ACL reconstructions. There was no significant correlation between bone tunnel enlargement and postoperative synovial fluid concentrations of TNF-α, IL-1β, IL-6, NO, and BMP-2. However, all patients with bone tunnel enlargement had higher postoperative concentrations of TNF-α, IL-6, and NO in the synovial fluid. There were no significant differences in concentrations between HST and PT groups. In conclusion, we observed an association between tibial bone tunnel enlargement and elevated synovial fluid concentrations of IL-6, TNF-α, and NO 7 days after ACL surgery indicating the potential involvement of these biological mediators in the pathogenesis of bone tunnel enlargement. However, there was no difference between HST and PT ACL reconstructions regarding synovial fluid contents of IL-6, TNF-α, IL-1β, NO, and BMP-2, suggesting a comparable biological response between these autografts following their use in ACL reconstruction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mechanism of bone tunnel enlargement following anterior cruciate ligament (ACL) reconstruction is not yet clearly understood. Among the possible causes are mechanical factors such as graft tunnel motion, stress deprivation of bone within the tunnel wall, improper graft tunnel placement, and aggressive rehabilitation [8, 12, 15, 22, 23]. Biological factors thought to contribute to tunnel enlargement include a cytokine-mediated nonspecific inflammatory response, cell necrosis due to toxic products (ethylene oxide, metal), a foreign body immune response (allografts), and heat necrosis as a response to drilling [6, 7, 12, 27, 28].
L'Insalata et al. [15] were the first to compare bone tunnel enlargement between hamstring (HST) and patellar tendon (PT) autografts, and they found significantly greater tunnel enlargement with the use of HST grafts. They proposed that the use of suspensory fixation in the HST group with fixation points far from the anatomical insertion of the ACL leads to greater graft tunnel motion [13, 15, 20, 29]. However, Clathworthy et al. [6] and Webster et al. [28] performed suspensory fixation in both HST and PT ACL reconstructions and still observed a higher incidence of bone tunnel enlargement with HST grafts. Therefore Clathworthy et al. [6] argued for a predominant biological component to bone tunnel enlargement. They proposed that proinflammatory cytokines such as tumor necrosis factor (TNF) α, interleukin (IL) 1β, and IL-6, which have been shown to be elevated in the synovial fluid after ACL disruption [5, 16], lead to osteolysis. TNF- α, IL-1β, and IL-6 in turn are known to stimulate osteoclastic activity and contribute to bone resorption [1, 2, 14]. Moreover, the free radical nitric oxide (NO) may be involved in bone tunnel enlargement following ACL surgery. It has been shown that NO has important effects on bone cell function, and that enhanced NO levels triggered by an upregulation of the inducible nitric oxide synthase (iNOS) potentiate cytokine and inflammation induced bone resorption [26]. Webster et al. [28] noted the possibility that bone tunnel enlargement is more frequent following HST than PT ACL reconstructions. They proposed that the bone block used in PT autografts acts effectively as a bone graft releasing osteoinductive bone morphogenic proteins (BMP) into the bone tunnel. Martinek et al. [17] and Morris et al. [19] have reported that BMP supplement leads to better tendon-to-bone healing in experimental animal studies.
To determine the involvement of biological factors in the pathogenesis of bone tunnel enlargement we investigated synovial fluid concentrations of the biological mediators TNF-α, IL-1β, IL-6, NO, and BMP-2 and analyzed the incidence of bone tunnel enlargement in the course of ACL surgery, comparing HST and PT ACL reconstructions concerning these parameters.
Patients and methods
Our prospective series included 13 patients who underwent arthroscopic ACL reconstruction. Inclusion criteria were the presence of a complete ACL disruption with no concomitant collateral ligament injury, no radiographic evidence of osteoarthritis, no chondral damage greater than Outerbride grade II, and no concomitant meniscal injury requiring treatment (repair or partial meniscectomy).
Six patients received a four-stranded (doubled semitendinosus/doubled gracilis) HST autograft and seven patients a bone–patellar tendon–bone (PT) autograft. In the HST group femoral fixation was achieved with the use of a cross-pin (Transfix, Arthrex, USA) and on the tibial side bioscrew (Arthrex) fixation was utilized. In the PT group both the femoral and the tibial bone plug was secured with a bioscrew (Arthrex). The two groups followed the same accelerated rehabilitation protocol emphasizing early gain of full range of motion and muscle strength development. The two groups were similar in terms of age, sex, period between injury and surgery, and period between surgery and follow-up radiography (Table 1). All patients were informed about the nature of the experiment and gave their consent.
Synovial fluid samples
Synovial fluid samples were obtained using sterile knee puncture directly before surgery and 7 days postoperatively. The samples were immediately centrifuged at 9500 rpm for 10 min, and the supernatant was frozen at −80°C [5]. The samples were analyzed for TNF-α, IL-6 (BioSource), IL-1β, and BMP-2 (R&D Systems) using commercially available sandwich enzyme-linked immunosorbent assays according to the manufacturer's instructions. NO levels were assessed indirectly by measuring nitrite and nitrate concentrations using the Griess reaction [21, 26].
Radiographic evaluation
Anteroposterior and lateral weight-bearing radiographs were taken an average of 38±7 weeks after surgery. The incidence of femoral and tibial bone tunnel enlargement was assessed by measuring the distance between the two sclerotic margins of the bone tunnel at its widest dimension perpendicular to the longitudinal axis of the bone tunnel. The measured distance was corrected for magnification and compared with the original size of the bone tunnel. The change in tunnel size was calculated as a percentage of the diameter of the drill bit used [15, 28]. Measurements of femoral tunnel size could not be reliable performed in the PT group because of the proximity of bone block to the femoral articular surface [15].
Statistical analysis
Spearman's rank correlation was used to assess the correlation between tunnel enlargement and synovial fluid measurements. Fisher's test, paired and unpaired t test, and Wilcoxon signed rank sum test were performed to compare sample concentrations and treatment groups. Differences were considered significant at P levels of 0.05 level. Data are given as mean ±SEM.
Results
Synovial fluid samples
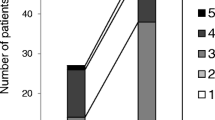
Synovial fluid concentrations of IL-6 were significantly higher after surgery (12917±2610 pg/ml) than before (1249±354 pg/ml; P<0.001) in all patients (Fig. 1), both in the HST group (P=0.015) and in the PT group (P=0.034; Fig. 2). The mean preoperative TNF-α concentration was 175±46 pg/ml, and on day 7 after surgery there was a trend towards lower values (40±5 pg/ml; Fig. 1), both in HST and in PT patients (Fig. 2). The mean IL-1β concentration remained unchanged (preoperative 8±2 pg/ml, postoperative 9±2 pg/ml; Fig. 1). NO levels showed a trend towards slightly lower concentrations after surgery (13±2 vs. 10±2 µmol/ml; Fig. 1). BMP-2 levels showed a significant increase from 80±6 to 146±16 pg/ml (P=0.003). No significant differences were observed in synovial fluid concentrations of TNF-α, IL-6, IL-1β, and NO between HST and PT groups either pre- or postoperatively (Fig. 2), nor was there a difference in BMP-2 concentrations either preoperatively (HST 76±9 pg/ml, PT 83±8 pg/ml) or postoperatively (HST 134±25 pg/ml, PT 156±21 pg/ml; Fig. 2).
Radiographic evaluation
The evaluation of bone tunnel enlargement in the overall patient population revealed an average increase in tibial tunnel width of 28.4±3.1%. The comparison between HST (mean 26.7±2.9%) and PT (mean 29.9±5.5%) ACL reconstructions showed no significant difference (Table 2). There was no significant correlation between bone tunnel enlargement and postoperative synovial fluid concentrations of TNF-α, IL-1β, IL-6, NO, and BMP-2.
Discussion
It has been proposed that biological factors play an important role in the pathogenesis of bone tunnel enlargement following ACL reconstruction. Cameron et al. [5] showed that the concentrations of proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 are elevated in the synovial fluid both immediately and several weeks after ACL injury. These cytokines are known to stimulate osteoclastic activity and contribute to bone resorption [1, 2, 14]. Several authors have proposed that after ACL surgery synovial fluid leaks into the bone tunnel and exposes the bone to the proinflammatory cytokines ("synovial bathing effect") [8, 10, 12, 15]. This theory is supported by the findings of Berg et al. [4]. The authors investigated interarticular bone tunnel healing in a rabbit animal model and found that tunnel healing was slower and less complete in the articular part of the tunnel than in the tunnel parts which were farther from the synovial environment.
The present study investigated the synovial fluid concentrations of the proinflammatory cytokines TNF-α, IL-1β, and IL-6 immediately before ACL surgery (24±7 days after ACL rupture) and 7 days after the operation. IL-6 levels increased significantly from the pre- to the postoperative measurements, while TNF-α concentrations showed a trend towards lower concentrations postoperatively. IL-1β concentrations remained unchanged throughout the course of ACL surgery. These data suggest that ACL surgery has a greater impact on IL-6 than on TNF-α or IL-1β synovial fluid levels in the time period assessed.
As a limitation of this study, we did not collect synovial fluid samples from healthy knees for ethical reasons. However, there are data available concerning cytokine concentrations in the normal human synovial fluid: IL-6: 0.8±0.4 pg/ml; IL-1β: 10±4.3 pg/ml, and TNF-α: less than 4.4 pg/ml [5]. The comparison of cytokine concentrations between different studies must be with cautious of course; however, the same means of sample collection and preparation and the same assay technique was used in both studies. Thereafter, the synovial fluid concentrations of IL-6 (12917±2610 pg/ml) and TNF-α (40±5 pg/ml) found 7 days after ACL surgery in the present study can be considered as clearly higher than normal levels of these cytokines, while IL-1β levels (9±2 pg/ml) seem to be in the range of healthy knees.
The present study assessed NO production in the synovial fluid following ACL rupture for the first time. NO is known to be involved in cytokine and inflammation induced bone loss which occurs in diseases such as rheumatoid arthritis [9, 21, 26]. NO levels were assessed indirectly by measuring nitrite/nitrate using the Griess reaction [21, 26]. At a mean of 24±7 days after ACL rupture we found an average nitrite/nitrate concentration of 13±2 µmol/l, which did not change significantly 7 days after ACL reconstruction (10±2 µmol/l). These NO values are lower than those in patients with rheumatoid arthritis (30.68±2.94 µmol/l) [21]; however, even low concentrations of NO are considered to contribute to bone resorption [26].
The present study analyzed BMP-2 levels since it is thought that BMPs may be involved in the process of bone tunnel enlargement following ACL surgery [28]. We found significantly higher BMP-2 concentrations 7 days after ACL surgery than preoperatively, which may be an effect of the drilling of the bone tunnels. However, there was no significant difference between HST and PT autografts concerning BMP-2 levels.
The present study also assessed bone tunnel enlargement using follow-up radiographs and observed an average increase in tibial tunnel width of 28.4±3.1%, with comparable values for HST (26.7±2.9%) and PT (29.9±5.5%) ACL reconstructions. These data conflict with the results of other studies [6, 15, 28] which report greater tunnel enlargement with the use of HST grafts. These studies [6, 15, 28] found similar tibial tunnel enlargement with HST grafts (23.1–30.3%) as our study (26.7±2.9%). Concerning PT autografts the tibial tunnel enlargement in our study and that in the studies by Clatworthy et al. [6] and Webster et al. [28] cannot be compared reliably because the latter two used proximal tibial interference screw fixation. However, L'Insalata et al. [15] used similar interference screw fixation for PT ACL reconstruction and reported an average tibial tunnel enlargement of 14.4±16.1%, compared to 29.9±5.5% in the present study. The reason for this difference is not clear. A possible explanation is that L'Insalata et al. [15] used metallic interference screws while we used bioabsorbable interference screw fixation. However, McGuire et al. [18] compared metallic and absorbable interference screws in PT ACL reconstruction and found no radiographic or clinical differences. A review of the literature revealed conformity with the results of our study. Using metallic interference screws for PT graft fixation, Fink et al. [11] reported an increase of 30.6% in tibial tunnel diameter and Barber et al. [3] found that 90% of their patients had 20% or more tibial tunnel enlargement with the use of absorbable interference screws in PT ACL reconstruction.
The present study found no significant correlation between bone tunnel enlargement and the postoperative synovial fluid concentrations of TNF-α, IL-1β, IL-6, NO, and BMP-2. However, it is known that the biological activity of cytokines may depend not on their absolute level [25], and that there is great interindividual variability in the cytokine response between patients [5, 6, 12]. In the present study we observed that every patient presenting bone tunnel enlargement on postoperative radiographs had elevated levels of the proinflammatory mediators TNF-α, IL-6, and NO 7 days after ACL surgery in the synovial fluid. Since bone tunnel enlargement is considered to be most pronounced in the first weeks after surgery [10, 24], we propose that TNF-α, IL-6, and NO are involved in this process.
As noted above, Clatworthy et al. [6] and Webster et al. [28] explain the observed differences in bone tunnel enlargement between HST and PT ACL reconstructions in terms of cytokines such as TNF-α, IL-6 or BMP-2. However, in our study there was no significant difference between HST and PT autografts regarding any of the quantitatively analyzed biological mediators. These findings are in accordance with the similar tibial tunnel enlargement observed and indicate a comparable biological response between HST and PT ACL reconstructions.
In conclusion, we observed an association between tibial bone tunnel enlargement and elevated synovial fluid concentrations of IL-6, TNF-α, and NO 7 days after ACL surgery, indicating the potential involvement of these biological mediators in the pathogenesis of bone tunnel enlargement. However, there was no difference between HST and PT ACL reconstructions regarding the incidence of tibial tunnel enlargement and synovial fluid contents of IL-6, TNF-α, IL-1β, NO, and BMP-2. These data suggest that there is a comparable biological response concerning HST and PT autografts following their use in ACL reconstruction.
References
Al-Saffar N, Revell PA (1999) Pathology of the bone-implant interface. J Long Term Eff Med Implants 9:319–347
Barber FA, Spruill B, Sheluga M (2003) The effect of outlet fixation on tunnel widening. Arthroscopy 19:485–492
Bauer TW, Schils J (1999) The pathology of total joint arthroplasty. II. Mechanisms of implant failure. Skeletal Radiol 28:483–497
Berg EE, Pollard ME, Kang Q (2001) Interarticular bone tunnel healing. Arthroscopy 17:189–195
Cameron M, Buchgraber A, Passler H, Vogt M, Thonar E, Fu F, Evans CH (1997) The natural history of the anterior cruciate ligament-deficient knee. Changes in synovial fluid cytokine and keratan sulfate concentrations. Am J Sports Med 25:751–754
Clathworthy MG, Annear P, Bulow JU, Bartlett RJ (1999) Tunnel widening in anterior cruciate ligament reconstruction: a prospective evaluation of hamstring and patella tendon grafts. Knee Surg Sports Traumatol Arthrosc 7:138–145
Dyer CR, Elrod BF (1995) Tibial and femoral bone tunnel enlargement following allograft replacement of the anterior cruciate ligament. Arthroscopy 11:352–354
Fahey M, Indelicato PA (1994) Bone tunnel enlargement after anterior cruciate ligament replacement. Am J Sports Med 22:410–414
Farrell AJ, Blake DR, Palmer RM, Moncada S (1992) Increased concentrations of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic diseases. Ann Rheum Dis 51:1219–1222
Fink C, Hackl C, Hoser C, Zapp M, Benedetto KP (2000) Bone tunnel widening following ACL reconstruction—a point of concern or curiosity. ACL study group meeting, Rhodes, Greece
Fink C, Zapp M, Benedetto KP, Hackl W, Hoser C, Rieger M (2001) Tibial tunnel enlargement following anterior cruciate ligament reconstruction with patellar tendon autograft. Arthroscopy 17:138–143
Höher J, Möller HD, Fu FH (1998) Bone tunnel enlargement after anterior cruciate ligament reconstruction: fact or fiction. Knee Surg Sports Traumatol Arthrosc 6:231–240
Ishibashi Y, Rudi TW, Livesay GA, Stone JD, Fu FH, Woo SL (1997) The effect of anterior cruciate ligament graft fixation site at the tibia on the knee stability: using a robotic testing system. Arthroscopy 13:177–182
Jacobs JJ, Roebuck KA, Archibeck M, Hallab NJ, Glant TT (2001) Osteolysis: basic science. Clin Orthop 393:71–77
L'Insalata JC, Klatt B, Fu FH, Harner CD (1997) Tunnel expansion following anterior cruciate ligament reconstruction: a comparison of hamstring and patellar tendon autografts. Knee Surg Sports Traumatol Arthrosc 5:234–238
Marks P, Cameron M (2000) Inflammatory cytokine profiles correlate with the degree of chondrosis in the chronic anterior cruciate ligament deficient knee. Presented at the ACL Study Group Meeting, Rhodes, Greece
Martinek V, Lattermann C, Usas A, Abramovitch S, Woo SL, Fu FH, Huard J (2002) Enhancement of tendon-bone integration of anterior cruciate ligament grafts with bone morphogenetic protein-2 gene transfer: a histological and biomechanical study. J Bone Joint Surg Am 84:1123–1131
McGuire DA, Barber FA, Elrod BF, Paulos LE (1999) Bioabsorbable interference screws for graft fixation in anterior cruciate ligament reconstruction. Arthroscopy 15:463–473
Morris H, Nicklin S, Harrison J, Yu Y, Walsh WR (2000) Augmentation of tendon-bone healing with exogenous OP-1 in an ovine ACL reconstruction. Presented at the ACL Study Group Meeting, Rhodes, Greece
Nebelung W, Becker R, Merkel M, Röpke M (1998) Bone tunnel enlargement after anterior cruciate ligament reconstruction with Semitendinosus tendon using Endobutton fixation on the femoral side. Arthroscopy 14:810–881
Novaes GS, de Mello SB, Laurindo IM, Palacios FA, Cossermelli W (1997) Intra-articular nitric oxide levels in patients with rheumatoid arthritis. Rev Hosp Clin Fac Med Sao Paulo 52:55–59
Peyrache MD, Dijan P, Christel P, Witvoet J (1996) Tibial tunnel enlargement after anteriorcruciate ligament reconstruction by autogenous bone-patellar tendon-bone graft. Knee Surg Sports Traumatol Arthrosc 4:2–8
Segawa H, Omori G, Tomita S, Koga Y (2001) Bone tunnel enlargement after anterior cruciate ligament reconstruction using hamstring tendons. Knee Surg Sports Traumatol Arthrosc 9:206–210
Simonian PT, Erickson MS, Larson RV, O'kane JW (2000) Tunnel expansion after hamstring anterior cruciate ligament reconstruction with 1-incision EndoButton femoral fixation. Arthroscopy 16:707–714
Van den Berg WB (1999) The role of cytokines and growth factors in cartilage destruction in osteoarthritis and rheumatoid arthritis. Z Rheumatol 58:136–141
Van't Hof RJ, Ralston SH (2001) Nitric oxide and bone. Immunology 103:255–261
Vergis A, Gilquist J (1995) Graft failure in intra-articular anterior cruciate ligament reconstruction: a review of the literature. Arthroscopy 11:312–321
Webster KE, Feller JA, Hameister KA (2001) Bone tunnel enlargement following anterior cruciate ligament reconstruction: a randomised comparison of hamstring and patellar tendon grafts with 2-year follow-up. Knee Surg Sports Traumatol Arthrosc 9:86–91
Zysk SP, Krüger A, Baur A, Veihelmann A, Refior HJ (2000) Tripled semitendinosus anterior cruciate ligament reconstruction with Endobutton fixation. Acta Orthop Scand 71:381–386
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zysk, S.P., Fraunberger, P., Veihelmann, A. et al. Tunnel enlargement and changes in synovial fluid cytokine profile following anterior cruciate ligament reconstruction with patellar tendon and hamstring tendon autografts. Knee Surg Sports Traumatol Arthrosc 12, 98–103 (2004). https://doi.org/10.1007/s00167-003-0426-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-003-0426-z