Abstract
Our Sun and planetary system were born about 4.5 billion years ago. How did this happen, and what is the nature of our heritage from these early times? This review tries to address these questions from an astrochemical point of view. On the one hand, we have some crucial information from meteorites, comets and other small bodies of the Solar System. On the other hand, we have the results of studies on the formation process of Sun-like stars in our Galaxy. These results tell us that Sun-like stars form in dense regions of molecular clouds and that three major steps are involved before the planet-formation period. They are represented by the prestellar core, protostellar envelope and protoplanetary disk phases. Simultaneously with the evolution from one phase to the other, the chemical composition gains increasing complexity.
In this review, we first present the information on the chemical composition of meteorites, comets and other small bodies of the Solar System, which is potentially linked to the first phases of the Solar System’s formation. Then we describe the observed chemical composition in the prestellar core, protostellar envelope and protoplanetary-disk phases, including the processes that lead to them. Finally, we draw together pieces from the different objects and phases to understand whether and how much we inherited chemically from the time of the Sun’s birth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Once upon a time, there was a small cold cloud of gas and dust in an interstellar medium broken into several clumps and filaments of different masses and dimensions. Then, about 4.5 billion years ago, the small cloud became the Solar System. What happened to that primordial cloud? When, why and how did it happen? Did the Earth receive a heritage from those old eons? Can this heritage help us to understand our origins?
The answers to these questions can only come from putting together many pieces of a giant puzzle that covers different research fields: from what the Earth is made of to its evolution, from what are the most pristine meteorites from outer space that have fallen on Earth to their present composition, from which other small bodies of the Solar System, comets and asteroids, have the imprint of the first composition of the solar nebula to their origin and evolution. Last but not least, the study of other small clouds and young Sun-like stars in our Galaxy gives a wide range of possible outcomes of star and planet formation, and we would like to understand why the Solar System and the Earth chose one of them.
Each single piece of the puzzle brings precise and precious information. The problem is that sometimes the information is hidden in a scrambled code whose key is unknown. Take meteorites as an example. As explained to us by an expert colleague, assessing the composition of the Solar Nebula from the study of the meteorites is like trying to assess Napoleon’s army structure looking at the few survivors of the Russian war. How representative are those survivors? Although, evidently, they still provide very precious information, extracting the whole information from them is far from obvious. The example is to say that every single piece of the puzzle is important, even the pieces that seem to be redundant. Actually, the redundant ones are likely the most important, as they may allow to distinguish and disentangle all the various intervening effects. In this context, the study of the objects similar to the Solar System progenitor takes a particular relevance, because it can provide us with plenty of pieces to compare with the other pieces from the present Solar System. The hope is that they will provide us with the keys of the scrambled codes.
In this review, we will focus on just a subset of these pieces, those coming from the study of the chemical composition during the birth of stars and planetary systems like our Solar System. In Sect. 2, we will first give a very general overview of how we think the Solar System and stars of similar mass have formed and how this process influences the chemistry. This is based on the ensemble of observations and studies on star-forming regions and Solar System objects. Then, in Sect. 3, we will describe in detail some pieces of the puzzle which potentially connect what we observe in the objects of the Solar System nowadays and what we know about star formation in our Galaxy. The next sections will discuss star- and planet-formation studies. We will describe how the evolution of the matter from a cold cloud (Sect. 4) to a protostellar envelope (Sect. 5) and a protoplanetary disk (Sect. 6) corresponds to an increase of the molecular complexity. Section 7 will provide specific examples on the link between the present Solar System small bodies with the pre- and protostellar phase. A final section will try to draw some conclusions.
We emphasize that the present review is complementary to several reviews recently appeared in the literature on different aspects just touched upon by us and that will be cited in the appropriate sections.
2 Solar-type star formation and chemical complexity
The formation of a Sun-like star and molecular complexity proceed hand in hand. As the primordial cloud evolves into a protostellar envelope, protoplanetary disk and planetary system, the chemical composition of the gas becomes increasingly more complex. The five major phases of the process that we think have formed the Earth are sketched in Fig. 1 and here listed.
-
Phase 1:
Pre-stellar cores. These are the “small cold clouds” mentioned above. During this phase, matter slowly accumulates toward the center of the nebula. As a result, the density at the center increases while the temperature decreases. Atoms and molecules in the gas-phase freeze-out onto the cold surfaces of the sub-micron dust grains, forming the so-called icy grain mantles. Thanks to the mobility of the H atoms on the grain surfaces, hydrogenation of atoms and CO (the most abundant molecule, after H2, in cold molecular gas) takes place, forming molecules such as water (H2O), formaldehyde (H2CO), methanol (CH3OH) and other hydrogenated species.
-
Phase 2:
Protostellar envelopes. The collapse proceeds, gravitational energy is converted into radiation and the envelope around the central object, the future star, warms up. The molecules frozen in grain mantles during the previous phase acquire mobility and likely form new, more complex species. When the temperature reaches the mantle sublimation temperature, in the so-called hot corinos, the molecules in the mantles sublimate back in the gas phase, where they react and form new, more complex, molecules. Simultaneously to the collapse, a fraction of matter is violently ejected outward in the form of highly supersonic collimated jets and molecular outflows. When the outflowing material encounters the quiescent gas of the envelope and the molecular cloud, it creates shocks, where the grain mantles and refractory grains are (partially) sputtered and vaporized. Once in the gas phase, molecules can be observed via their rotational lines.
-
Phase 3:
Protoplanetary disks. The envelope dissipates with time and eventually only a circumstellar disk remains, also called protoplanetary disk. In the hot regions, close to the central object, new complex molecules are synthesized by reactions between the species formed in the protostellar phase. In the cold regions of the disk, where the vast majority of matter resides, the molecules formed in the protostellar phase freeze-out again onto the grain mantles, where part of the ice from the prestellar phase may still be present. The process of “conservation and heritage” begins.
-
Phase 4:
Planetesimal formation. The sub-micron dust grains coagulate into larger rocks, called planetesimals, the seeds of the future planets, comets and asteroids. Some of the icy grain mantles are likely preserved while the grains glue together. At least part of the previous chemical history may be conserved in the building blocks of the Solar System rocky bodies.
-
Phase 5:
Planet formation. This is the last phase of rocky planet formation, with the embryos giant impact period and the formation of the Moon and Earth. The leftovers of the process, comets and asteroids, copiously “rain” on the primitive Earth, forming the oceans and the Earth second atmosphere. The heritage conserved in the ices trapped in the planetesimals and rocks is released onto the Earth. Life emerges sometime around 2 billion years after the Earth and Moon formation.Footnote 1
Sections 4 to 6 will review and discuss in detail the chemistry in the first three phases of the process, those where the heritage is likely accumulated. Box 1 briefly explains the data and tools needed to interpret the observations and Table 1 summarizes some key proprieties of the phase 1 to phase 3 objects.
The four steps required to measure the chemical structure of an astrophysical object, as described in Box 1, including the tools needed to complete each step: (1) observations at the telescope, (2) identification of the lines and species, (3) derivation of the physical and chemical structure using radiative transfer codes, which require accurate collisional coefficients, and (4) chemical models
3 Pieces of the puzzle from the Solar System
A variety of information on the formation process of the Solar System is provided to us by the small bodies believed to be the most pristine objects of the Solar System: Kuiper Belt Objects (KBOs), comets, meteorites and particularly carbonaceous chondrites, and interplanetary dust particles (IDPs). Here we will review some properties of these objects that can shed light on the formation process when compared with what we know about other solar-type forming stars in the Galaxy. We emphasize that this summary is far from being exhaustive and the reader is invited to look at the reviews cited in the following subsections.
3.1 Where does the terrestrial water come from?
We all know how fundamental water is for the terrestrial life. It is the best solvent, allowing chemical reactions to form large biotic molecules and to break down ATP (Adenosine TriPhosphate), a process at the very base of the energy metabolism of living cells. Water had a fundamental role also on planet Earth, its history, evolution and equilibrium, for example allowing the magma to be viscous enough for tectonics to take place.
Sometimes the most obvious questions, like the one on the origin of water or why the night sky is dark, do not have obvious answers. The explanation of the dark night sky had to wait for the discovery of the expansion of the Universe, while the explanation of why Earth is so abundant in water is still hotly debated. But what are the facts? Two main facts are fundamental pieces of this puzzle. The first one is the quantity of terrestrial water, the second is its isotopic composition.
Regarding the amount of water on Earth, we can easily measure it in the Earth’s crust where it is ∼3×10−4 the Earth mass (Lécuyer et al. 2000). It is much less obvious to measure it in the mantle and core, where the vast majority of the Earth’s mass resides and where it is impossible to directly measure the volatile components. Measurements of Earth’s mantle water content are in fact based on indirect evidence, mostly using noble gases as proxies for the volatile hydrogen (Fisher 1982; Allegre et al. 1983), which implies assuming that the solar abundance ratios are maintained in the Earth mantle. The most recent estimates give a total amount of ∼2×10−3 Earth masses (Marty 2012), namely almost ten times more than in the crust. It has to be noted, though, that Earth in the Archaean was most likely more volatile-rich than in our days (e.g., Kawamoto 1996).
The second fundamental piece of the puzzle is the HDO/H2O ratio, 1.5×10−4 in the terrestrial oceans, namely about ten times larger than the elemental D/H ratio in the Solar Nebula (Geiss and Gloeckler 1998). Direct measurements of the HDO/H2O ratio in the Earth mantle are impossible, but indirect ones seem to suggest a slightly lower value than that of the oceans (Marty 2012).
The problem on the origin of the terrestrial water comes from the fact that the planetesimals that built up the Earth, if they were located at the same place where Earth is today, must have been dry. Therefore, either water came later, when Earth was mostly formed, or the planetesimals that formed the Earth were from a zone more distant than 1 AU. The first theory, also called “late veneer”, was first proposed by Delsemme (1992) and Owen and Bar-Nun (1995) and postulates that water is mostly delivered to Earth from comets, especially during the Late Heavy Bombardment (Dauphas et al. 2000; Gomes et al. 2005). For almost a decade, the theory had the problem, though, that the HDO/H2O abundance ratio in the six comets where it had been measured is about a factor of two too high (Jehin et al. 2009); see Sect. 3.4 and Fig. 4. However, new Herschel measurements are changing the situation. The measure on the 103P/Hartley2 comet gives exactly the terrestrial value (Hartogh et al. 2011), whereas measurements toward C/2009 P1 give again a larger HDO/H2O value, 2×10−4 (Bockelée-Morvan et al. 2012). The other possibility is that Earth was partly built from water-rich planetesimals from the outer zone (Morbidelli et al. 2000). Two arguments are in favour of this theory. First, the HDO/H2O ratio of carbonaceous chondrites is very similar to the terrestrial one (1.3–1.8×10−4, Robert 2003; see Sect. 3.4 and Fig. 4). Second, numerical simulations of the young Solar System from several authors predict that up to 10 % of the Earth may have been formed by planetesimals from the outer asteroid belt, providing enough water to Earth (e.g., Morbidelli et al. 2000; Raymond et al. 2009). The same simulations tend to exclude the cometary delivery as a major contribution. However, as any model, the predictions are subject to a number of uncertainties, a major one being how much water is in the outer asteroid belt planetesimals (Licandro et al. 2012).
Finally, the question on the origin of Earth’s water is somewhat linked to the question on the origin of the Earth’s atmosphere. Even though the methods are different, also for the Earth’s atmosphere it is discussed a cometary delivery versus a meteoritic origin. Likely, in this case, both sources are necessary (e.g., Dauphas 2003).
We emphasize the key role played, in both theories, by the HDO/H2O ratio in the terrestrial water, comets and asteroids. In the following sections of this review, we will see why, when and how water becomes enriched of deuterium.
3.2 Molecular species in comets and KBOs
Several molecular species have been detected in comets since decades and in KBOs since the last decade. Here we briefly summarise which species have been detected and recommend to the interested reader the reviews by Mumma and Charnley (2011) and Bockelée-Morvan (2011), and Brown (2012) on the comets and KBOs, respectively.
Comets
Two dozens of molecular species have been identified in various comets by several authors (e.g., Biver et al. 2002; Crovisier et al. 2009). More specifically:
-
(i)
H2O, CO, CO2, CH4, C2H2, C2H6, CH3OH, H2CO, NH3, HCN, HNC, CH3CN and H2S have been detected in more than 10 comets;
-
(ii)
HCOOH, HNCO, HC3N, OCS and S2 have been detected in more than one comet;
-
(iii)
HOCH2CH2OH, HCOOCH3, CH3CHO, NH2CHO, SO2, H2CS have been observed in one comet, Hale–Bopp.
Not all species are considered primary species, namely species present in the sublimated ices. Some, like HNC, are product species, namely they are the products of chemical reactions involving the primary species once ejected in the gas. Other species, like H2CO and CO, have contributions from both primary and product species. The measured abundances are summarised in Fig. 3. To this list one has to add the recent detection of glycine, the simplest of amino acids, in the 81P/Wild2 comet by the mission STARDUST (Elsila et al. 2009).
KBOs
KBOs are the objects beyond Neptune’s orbit, at an heliocentric distance between 30 and 50 AU, and are thought to hold precious information on the pristine chemical composition of the Solar Nebula at those distances. Being relatively small objects, they are difficult to study. However, in the last decade, important progress has been made. Briefly, the six large KBOs where spectroscopic observations could be obtained showed the presence in their atmosphere of H2O, CH4, N2, and CO, even though with different proportions from object to object (e.g., Barucci et al. 2005; Schaller and Brown 2007; Brown et al. 2012). In addition, ethane (C2H6), believed to be the result of CH4 photolysis processes caused by the solar wind and cosmic rays, has been detected in Makemake (Bennett et al. 2006). In other smaller KBOs, spectroscopic observations showed the presence of water, ammonia and likely methanol ices (Barucci et al. 2011; Brown et al. 2012).
From Fig. 3, it is clear that the most abundant species in comets (H2O, CO, CO2, CH4, NH3, CH3OH and C2H6) are observed also in KBOs and, in turn, the species observed in KBOs are the most abundant species in comets. Formaldehyde and hydrogen sulfide have abundances in comets comparable to CH4 and NH3. Their non detection in KBOs may, however, be due to observational effects only.
3.3 Organics in meteorites and IDPs
Carbonaceous chondrites are rich in carbon, which constitutes about 1–4 % of this kind of meteorites. Organic carbon is present in two forms, following the methods to extract the organic material: insoluble organic matter (IOM) and soluble organic matter (SOM).
IOM is mainly (≥70 %) constituted of organic compounds with a relatively complex structure (nanoglobules, venatures, …). The compounds are made of small aromatic units (with up to six rings) linked by branched aliphatic linkages shorter than seven carbon atoms (e.g., Remusat et al. 2005; Le Guillou et al. 2012). Similarly, IDPs contain about 10–12 % carbon, mostly in organic material, including aromatic and aliphatic compounds (Thomas et al. 1993; Keller et al. 2004).
SOM is principally made of carboxylic acids, aliphatic and aromatic hydrocarbons, and amino acids (e.g., Pizzarello et al. 2001). In the Murchison meteorite, they represent ∼50 %, ∼25 % and ∼10 %, respectively, of the organic soluble matter. Of particular interest, amino acids with no known terrestrial distribution have been found in meteorites. In addition, a sub-group of amino acids shows a small but significant L-enantiomeric excesses (e.g., Pizzarello et al. 2003), namely one of the two chiral forms is more abundant than the other, a characteristic of chiral biomolecules in terrestrial life.
3.4 The hydrogen and nitrogen isotopic anomalies
One direct evidence of the link between pristine small Solar System bodies like carbonaceous chondrite, IDPs and comets, and the first phases of the Sun formation (phases 1 to 3 in Fig. 1) comes from the presence of the so-called isotopic anomalies. Among the five most abundant elements in the Universe (H, He, O, C and N), three present large anomalies, namely they have isotopic values more than twice different from in the Solar Nebula: hydrogen, oxygen and nitrogen (while carbon also shows different values but to a lesser extent). Each of them brings different information. Here, we briefly review the information provided by the hydrogen and nitrogen isotopes. Oxygen isotopic anomalies are discussed in Sect. 3.5.
Deuterium in comets
The first and most important isotopic anomaly, the deuterium enrichment of terrestrial water, has been already discussed in Sect. 3.1. We also briefly mentioned that comets show a D enrichment one to two times the one of the terrestrial oceans (Fig. 4). Regardless whether comets substantially contributed or not to the terrestrial water, the relatively high abundance of deuterated water can help us to understand when and where comets formed and, consequently, how the Solar System formed. So far, the HDO/H2O ratio has been observed in seven comets from the Oort Cloud, the most recent being the C/2009 P1 comet, and in one, 103P/Hartley2, from the Jupiter-family comets. In the first six comets, HDO/H2O has been measured to be ∼3×10−4 (Muñoz Caro et al. 2002; Bockelee-Morvan et al. 1998), in C/2009 P1 it is 2×10−4 (Bockelée-Morvan et al. 2012), and in 103P/Hartley2 it is 1.5×10−4 (Hartogh et al. 2011). If, on the one hand, this last measurement has brought back to life the debated late veneer theory (Sect. 3.1), it has also challenged the present view of where these comets are formed. In fact, according to the widely accepted theory, comets from the Oort Cloud and the Jupiter family were likely formed in the Uranus-Neptune zone (Dones et al. 2004), even though the Oort Cloud comets may also originate from the Jupiter–Saturn region (Brasser 2008). The HDO/H2O ratio is an almost direct measure of the temperature where the comet is formed and larger heliocentric distances are expected to correspond to colder regions. Therefore, one would expect that comets in the Oort Cloud present a similar or lower HDO/H2O ratio than the Jupiter-family comets, contrary to what is measured. Dedicated models support this simple intuitive argument (e.g., Horner et al. 2007; Kavelaars et al. 2011; Petit et al. 2012). Therefore, either comet formation theory is not correct in this aspect (for example a new theory postulates that Oort Cloud comets are captured from nearby stars; (Levison et al. 2010)), or the temperature in the Solar Nebula was not monotonically decreasing with increasing heliocentric distance. This is in principle possible during the accreting disk phase where viscosity may have created warm regions (e.g., Yang and Ciesla 2012). This will be further discussed in Sect. 7. So far for water, but deuterium enrichment is also observed in HCN, in one comet (Meier et al. 1998), and it is about 10 times larger than the water D enrichment. This difference is not necessarily a problem as it may just outline the different chemical formation pathway of these two species, as explained in Sect. 4.2.1.
Deuterium in carbonaceous chondrites and IDPs
The bulk of carbonaceous chondrites contains hydrated silicates and hydrous carbon with a D/H ratio = 1.2–2.2×10−4 (e.g., Robert 2003), very similar to that of the terrestrial oceans. However, D enrichment, similar to that measured in comets and even higher, has also been found in the so-called “hot spots”, namely micrometer-scale regions with positive isotope anomalies, in the IOM of chondrites and IDPs. These hot spots are in fact so named because of the enrichment of D and 15N, and are systematically found in small regions of organic material. The D enrichment in carbonaceous chondrites and IDPs is very variable, with regions having D/H ∼8×10−5 close to the Solar Nebula value, and others having D/H up to ∼10−2 (Alexander et al. 2007; Remusat et al. 2009). High spatial resolution measurements suggest that the largest D enrichment is associated with organic radicals (Remusat et al. 2009). Similarly, molecules in the soluble organic matter component show enhanced abundances of D species with respect to H species, at a level of D/H up to almost 10−2 (Pizzarello and Huang 2005).
15N in comets
Several measurements of the bulk of the 14N/15N in the Solar Nebula, from observations of NH3 in Jupiter (Owen et al. 2001; Fouchet et al. 2004) and the solar wind (Marty et al. 2010) give a value of ∼440, consistent with standard stellar nucleosynthesis models. In comets, though, the 14N/15N ratio measured in CN and HCN species is more than a factor 2 lower, around 150 (Arpigny et al. 2003; Manfroid et al. 2009). The origin of this 15N enrichment in comets has puzzled astrochemists for years. One possibility is that this is a direct heritage of the prestellar core phase (Sect. 4) or protoplanetary-disk phase (Sect. 6). Finally, it is also possible that 15N has been injected in the material forming the Solar System by the explosion of a nearby supernova (Sect. 3.5).
15N in chondrites and IDPs
The 14N/15N as measured in TiN in a pristine condensate Ca-Al-rich inclusion of a carbonaceous chondrites is very similar to the Solar Nebula value ∼440 (Meibom et al. 2007). However, the 14N/15N in the IOM material of carbonaceous chondrites and IDPs is low, up to ∼50 (Bonal et al. 2009, 2010; Matrajt et al. 2012), as low as in comets and significantly lower than in the Solar Nebula and the interstellar medium. Similar 15N enrichment has been reported in two amino acids (Pizzarello and Holmes 2009). Therefore, the same question on the origin of the 15N enrichment in comets applies to the organic material in carbonaceous chondrites and IDPs.
A common origin for the D and 15N enrichment in comets and the organic material in carbonaceous chondrites and IDPs?
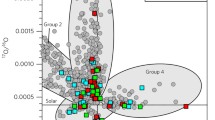
Since comets and the organic material in chondrites and IDPs are enriched in both D and 15N, the question whether the enrichment has a common origin is a natural one (e.g., Busemann et al. 2006; Aléon 2010). Against this hypothesis is that D-enriched spots in chondrites and IDPs do not coincide spatially with 15N-enriched ones (Busemann et al. 2006; Aléon 2010; Robert and Derenne 2006). Similarly, while the D enrichment differs by a factor two in 103P/Hartley2 and the other six comets, the 15N enrichment is practically the same in all comets (Fig. 4). Therefore, very likely D and 15N enrichments do not have a common origin (see also Wirström et al. 2012).
3.5 A violent start in a crowded violent environment
Short-lived nuclidesFootnote 2 present at the formation of the Solar System and now disappeared, and isotopic oxygen anomalies in meteorites tell us that the Solar System had a violent start in a violent environment. First, the young Sun irradiated the forming planetary system with a strong wind of energetic particles. Second, the Sun was likely born in a large cluster of stars where one or more massive stars exploded. All this is based on anomalies with respect to the “normal” values of the abundances of these elements, which can only be firmly known by assessing what is the normality in other forming stars and, therefore, it is an important piece of the puzzle to mention here.
A violent start
It is now well-known that young solar-type stars are bright X-rays emitters, about 103 times brighter than the present day Sun (Feigelson and Montmerle 1999; Preibisch and Feigelson 2005). It is very likely that, together with X-rays, H and He nuclei with energies larger than 10 MeV are also emitted in large quantities in the early stages of star formation (e.g., Lee et al. 1998). The Sun likely passed through a similar violent phase and irradiated the forming planetary system with energetic particles (sometimes also referred as “early solar cosmic rays”). Extinct short-lived nuclides bring traces of this violent past. Specifically, the enhanced abundances of 10Be, 7Li and 21Ne (McKeegan et al. 2000; Chaussidon and Srinivasan 2012) can only be explained by spallation reactions of solar energetic particles with O and C atoms of the Solar Nebula. Similarly, other short-lived nuclides, 36Cl, 53Mg and 41Ca, are now explained in terms of irradiation from the early Sun (e.g., Marhas et al. 2002; Gounelle and Meibom 2008).
A crowded violent environment
Several lines of evidence converge toward a picture where the Sun was born in a cluster of at least 1000 stars (see the review by Adams 2010). Likely, within this cluster, some were massive stars and some exploded a little before or during the formation of the Solar System. Since its discovery in meteorites, 26Al (Kita et al. 2000; Villeneuve et al. 2009) became one of the proofs, indeed highly debated for decades, that the Solar System was polluted with material ejected from a nearby type II supernova, whose progenitor mass is ∼25 M⊙ (Cameron and Truran 1977; Gounelle and Meibom 2008). Support to this hypothesis was added by the discovery of 60Fe (Kita et al. 1998), but the value of the 60Fe excess with respect to the Galactic one has been revised since and nowadays it is believed to be close to zero (Moynier et al. 2011). As a consequence, theories based on 60Fe have to be taken with a grain of salt (see the review by Dauphas and Chaussidon 2011). Recently, the anomalous 18O/17O in meteorites, 5.2±0.2 (see the compilation in Young et al. 2011), with respect to the Galactic one, 4.1±0.1 (Wouterloot et al. 2008) has also been taken as a proof of the injection of material from a type II supernova exploded just before the birth of the Solar System.
4 The calm before the storm: pre-stellar cores
Stars like our Sun form in slowly rotating and collapsing magnetized dense cloud cores (e.g., Goodman et al. 1993; Troland and Crutcher 2008). Dense cores not associated with stars are called “starless cores” and they represent the initial conditions in the process of star formation (Shu et al. 1987). They are the starting point of our journey. These objects have average volume densities at least one order of magnitude larger than the surrounding medium, have typical kinetic and dust temperatures of 10 K and their internal energy is dominated by thermal motions (see review by Bergin and Tafalla 2007). Not all starless cores give birth to stars, though. Some of them reach configurations close to hydrostatic equilibrium and display kinematic features consistent with oscillations (Lada et al. 2003). Others show expanding motions (Tafalla et al. 2004). This class of starless cores typically displays a relatively flat density distribution, with central densities below 105 H2 molecules cm−3. This is the critical density for gas cooling by gas-dust collisions (Goldsmith 2001) and it represents the “dividing line” for dynamical stability. Starless cores with central densities below this critical density are thermally subcritical (Keto and Caselli 2008) and they may disperse back into the interstellar medium. When the central densities of H2 molecules overcome ≃105 cm−3, starless cores become thermally supercritical and gravitational forces take over. These are the so-called prestellar cores, first identified by Ward-Thompson et al. (1994) in the sub-millimeter continuum and then chemically and kinematically labelled by Crapsi et al. (2005) using millimeter spectroscopy. It is within prestellar cores that future star and planetary systems will form.
4.1 Freeze-out, deuterium fractionation and the ionization fraction
Pre-stellar cores span a range of number densities which goes from a few times 103 cm−3 toward the outer edges, where they merge with the surrounding molecular cloud, to about 107 cm−3 within the central 1,000 AU (e.g., Evans et al. 2001; Keto and Caselli 2010), where the gas and dust temperature drops to 6–7 K (Crapsi et al. 2007; Pagani et al. 2007). These gradients in physical properties affect the chemical structure. Figure 5 schematically shows the main chemical processes in the two-zones of the prototypical prestellar core L1544, embedded in the Taurus molecular cloud. In the outer part of the core (between about 7,000 and 15,000 AU), the gas density is ≃104 cm−3 and the temperature ≃10 K. “Classical” dark-cloud chemistry is at work, with ion-molecule reactions (Herbst and Klemperer 1973) dominating the carbon chemistry, and neutral-neutral reactions which start the transformation of nitrogen atoms into N2 (e.g., Hily-Blant et al. 2010). These reactions form the “popular” species CO, N2H+ and NH3, which are widely used to study cloud structures and kinematics.
The chemical zones of the prototypical prestellar core L1544, embedded in the Taurus Molecular Cloud Complex, at a distance of 140 pc. The background color image is the 1.3 mm dust continuum emission map obtained with the IRAM-30m antenna (Ward-Thompson et al. 1999). The cyan contours show the different chemical zones, with the corresponding main chemical processes listed in the right panel. Blue labels indicate reaction partners
Freeze-out
Within the central 7,000 AU, the density increases above 105 cm−3, the temperature drops below 10 K and species heavier than He tend to disappear from the gas phase due to the process of freeze-out (the adsorption of species onto dust grain surfaces). CO freeze-out has been measured in starless and prestellar cores at a 80–90 % level (Willacy et al. 1998; Caselli et al. 1999; Bacmann et al. 2002; Redman et al. 2002). Nitrogen-bearing species have also been found to deplete from the gas phase, although not as much as CO (e.g., Bergin et al. 2002; Tafalla et al. 2006; Friesen et al. 2010). The reason for this differential freeze-out has to be found in the fact that N-bearing species, such as N2H+ and NH3, experience larger production rates when neutral species (in particular CO) start to disappear from the gas phase. The freeze-out is a natural consequence of the quiescent nature of prestellar cores: once species land on a grain surfaces, they cannot thermally evaporate (because dust temperatures are \(T_{\rm dust} \leq10\) K, typical binding energies are \(E_{\rm B} \ge 1000\) K and the thermal evaporation rate is \(\propto \exp[-E_{\rm B}/(k~T_{\rm dust})]\)) and they cannot photodesorb as interstellar photons cannot penetrate within prestellar cores (whose central regions have visual extinctions larger than 50 mag). Only a small fraction of the adsorbed species can return in the gas phase via non-thermal desorption mechanisms mainly driven by cosmic rays, such as dust impulsive heating due to cosmic-ray bombardment (e.g., Leger et al. 1985) and photodesorption due to the Far-UV (FUV) field produced by cosmic-ray impacts with H2 molecules (Prasad and Tarafdar 1983; Gredel et al. 1989; Shen et al. 2004), although molecular hydrogen formation (Willacy and Millar 1998; Roberts et al. 2007) and surface reactions involving radicals (D’Hendecourt et al. 1982) may also play a role. Desorption of mantle species by FUV photons has been included in the chemical-dynamical models of L1544, to explain the recent Herschel detection of water vapor in the center of this prototypical prestellar core (Caselli et al. 2012). Freeze-out time scales (\(t_{\rm freeze\mbox{\scriptsize-}out}\) \(\propto10^{9}/n_{\rm H}\) yr, where \(n_{\rm H}\) is the total number density of hydrogen nuclei (Jones and Williams 1985)) are significantly shorter than the dynamical (free-fall) time scale (\(t_{\rm free-fall} \propto4\times10^{7} / \sqrt{n_{\rm H}}\), Spitzer 1978), so dust grains are expected to build thick icy mantles during the prestellar phase of the star-formation process (Sect. 4.2).
Deuterium fractionation
In the cold environments of prestellar cores, another important process takes place: deuterium fractionation. The starting point is the exothermic reaction between \(\mathrm{H}_{3}^{+}\) and HD, which produces H2D+ and H2 (\(\mathrm{H}_{3}^{+}\) + HD → H2D+ + H2 + 230 K, Watson 1974). This reaction cannot proceed from right to left when the kinetic temperature is below ≃20 K and if a large fraction of H2 molecules is in para form, as expected in cold and dense cores (Flower et al. 2006; Pagani et al. 2009; Troscompt et al. 2009). Therefore, the H2D+/\(\mathrm{H}_{3}^{+}\) abundance ratio becomes significantly larger than the D elemental abundance with respect to H. When the freeze-out of neutral species (especially CO and O, which are the main destruction partners of H2D+) becomes important, deuterium fractionation is further enhanced (Dalgarno and Lepp 1984). In fact, the deuteration zone of Fig. 5 is the region where the brightest line of ortho-H2D+ has ever been detected (Caselli et al. 2003). This deuteration “jump” allows multiply deuterated forms of \(\mathrm{H}_{3}^{+}\) to thrive (Vastel et al. 2004; Parise et al. 2011) and their dissociative recombinations with electrons liberate D atoms, locally increasing the D/H ratio to values larger than 0.1 (Roberts et al. 2003). The large D/H ratio in the gas phase implies efficient deuteration of surface species (in particular CO), with the consequent production of deuterated and doubly deuterated formaldehyde as well as singly, triply and doubly deuterated methanol (e.g., Tielens 1983; Charnley et al. 1997; Caselli et al. 2002a; Taquet et al. 2012a, 2012d). HDCO, D2CO and CH2DOH have been detected in prestellar cores (Bacmann et al. 2003; Bergman et al. 2011), while doubly and triply deuterated methanol have been detected in the envelope of young stellar objects (Parise et al. 2002, 2004), see Sect. 5.
The ionization fraction
Deuterated species are the main probes of the central regions of prestellar cores, the future stellar cradles. Their observation allows us to trace the kinematics (e.g., van der Tak et al. 2005; Crapsi et al. 2007) and, together with the non-deuterated isotopologue, to measure the elusive electron number density \(n({\rm e}^{-})\), which plays a crucial role in the dynamical evolution of the cloud. In fact, electrons and ions gyrate around magnetic field lines which permeate the clouds, and decouple from the bulk motions. During the gravitational collapse, neutral species slip through magnetic field lines and collide with molecular ions in a process called ambipolar diffusion (Mouschovias 1979; Shu et al. 1987). Depending on the fraction of ions present in the gas phase, neutral-ion collisions can significantly slow down the collapse compared to free-fall. How do we measure the ionization degree? Using simple steady-state chemistry of (easy-to-observe) molecular ions, such as HCO+ and DCO+, which form from the reaction of CO with \(\mathrm{H}_{3}^{+}\) and H2D+ and are destroyed by electrons, it is easy to arrive at analytic expressions relating the observed DCO+/HCO+ abundance ratio to \(n({\rm e}^{-})\) (Guelin et al. 1977; Wootten et al. 1979). Using time dependent chemical codes (Caselli et al. 1998) and (Bergin et al. 1999) obtained values of \(x({\rm e}^{-})\) (\(\equiv n({\rm e}^{-})/n({\rm H_{2}})\)) between 10−8 and 10−6. Given that the time scale for ambipolar diffusion is \(t_{\rm AD} \simeq 2.5 \times 10^{13} x({\rm e}^{-})\) yr (Spitzer 1978), the above measurements imply values of \(t_{\rm AD} \simeq 2.5\times10^{5}\) and 2.5×107 yr, factors of 2–200 larger than \(t_{\rm free\mbox{\scriptsize -}fall}\) for prestellar cores with an average \(n_{\rm H} = 10^{5}\) cm−3.
15N fractionation
On the one hand, no significant 15N fractionation (compared to the Solar Nebula value of ∼440, see Sect. 3.4) has been found in NH3 (14N/15N≃350–850, Gerin et al. 2009; 334±50, Lis et al. 2010) toward prestellar cores and protostellar envelopes, and in N2H+ (14N/15N=446±71, Bizzocchi et al. 2010) toward the prototypical prestellar core L1544. On the other hand, (Milam and Charnley 2012) and Hily-Blant et al. (submitted) found significant 15N enrichment in HCN toward prestellar cores (between 70 and 380). Similar values have been found by Adande and Ziurys (2012) in HNC observations of star-forming regions across the Galaxy. It is interesting to point out here that the 15N fractionation observed in comets (Sect. 3.4) has been measured for CN and HCN (14N/15N∼130–170, Bockelée-Morvan et al. 2008). This differential 15N fractionation for amines and nitriles has been recently reproduced in chemical models of dense clouds by Wirström et al. (2012), who suggest that the processes able to reproduce the observed differentiation could be at the origin of the poor correlation between D and 15N fractionation observed in some primitive material in our Solar System (Sect. 3.4). Thus, a further link between prestellar core chemistry and the Solar System composition has been found (see Sect. 7).
4.2 Ice formation and evolution
Interstellar dust grains are crucial for the chemical and physical evolution of interstellar clouds and for our astrochemical origins. First of all, hydrogen atoms can quickly scan their surfaces, meet and form volatile H2 molecules at rates large enough to defeat H2 photodissociation due to the interstellar radiation field (Hollenbach and Salpeter 1971; Pirronello et al. 1999; Cazaux and Tielens 2002; Cuppen and Herbst 2005). Thus, dust grains are responsible for the transition of interstellar gas in our Galaxy (as well as in external galaxies) from atomic to molecular—the first step toward chemical complexity. Secondly, they are efficient absorbers of the FUV photons, so that they act as “UV-filters”, protecting molecules within clouds from the UV destructive action. Thirdly, they catalyze the formation of important species, in particular H2O, with such high efficiency that more than 30 % of oxygen atoms are locked into water ice as soon as the visual extinction reaches values ≥3 mag (e.g., Murakawa et al. 2000; Hollenbach et al. 2009; Whittet 2010; Chiar et al. 2011). Finally, they become the main gas coolants in the central regions of prestellar cores, where the densities are above ≃105 cm−3, the temperatures fall below 10 K and species heavier than He (including important coolants such as CO) are mostly frozen onto their surfaces. In such conditions, the freeze-out rate will become even more extreme and dust grains should develop thick ice mantles. How thick? A simple estimate can be made considering that levels of CO freeze-out of about 90 % are seen within the central prestellar core regions (see Sect. 4.1). Assuming that all species heavier than helium are affected by a similar amount of freeze-out (including nitrogen, Hily-Blant et al. 2010), then in clouds with total hydrogen density of 2×106 cm−3, the total number density of heavy species frozen onto dust grains is about 1.3×103 cm−3. Further assuming that they are combined in molecules with two heavy elements on average (e.g. CO, CH3OH, CO2, H2O), the total number of solid species will be about 660 cm−3. Now, we just need to divide this number by the total number of sites on an average grain with radius 0.1 μm (≃106; Hasegawa et al. 1992) to have the number of monolayers (≃250). Considering a monolayer thickness of about 1 Å, the total mantle thickness is then 2.5×10−6 cm, or about a quarter of the grain radius. Such thick mantles boost dust coagulation (Ossenkopf and Henning 1994).
What are the main chemical processes on the surface of dust grains? Our understanding is based on (i) observations of absorption features along the line of sight of stars located behind molecular clouds or protostars embedded in dense cores (e.g., Whittet et al. 2011 and references therein), and on (ii) laboratory work (e.g., Watanabe and Kouchi 2002; Hiraoka et al. 2002; Miyauchi et al. 2008; Ioppolo et al. 2008; Fuchs et al. 2009; Dulieu et al. 2010). From these studies, we now know that surface reactions are mainly association reaction: oxygen is transformed into water via successive association reactions with hydrogen (e.g. O + H → OH; OH + H → H2O, but see Sect. 4.2.1 for more pathways to water ice); similarly, CO is transformed first into formaldehyde, H2CO, and then into methanol, CH3OH, via two and four association reactions, respectively; atomic nitrogen saturates into ammonia, NH3. Other important processes are photoprocesses and cosmic-ray bombardments. Photoprocesses are experimentally found to promote the formation of organic species more complex than CH3OH (Gerakines et al. 1996; Bennett and Kaiser 2007; Öberg et al. 2009a, 2010b) up to amino acids (e.g., Bernstein et al. 2002; Muñoz Caro et al. 2002, 2004) and allow solid species to return into the gas phase (Öberg et al. 2009b, 2009c). Cosmic rays, unlike UV photons, traverse dense cores relatively unhampered, although their flux may be reduced by a factor of a few by the mirroring effect of magnetic fields (Padovani and Galli 2011). When colliding with dust grains, they can alter mantle compositions (e.g., Palumbo et al. 2000; Ioppolo et al. 2009; Modica and Palumbo 2010; Sicilia et al. 2012; Boduch et al. 2012; Pilling et al. 2012). Cosmic rays also play a crucial role in molecular desorption, as mentioned in the previous section. Surface chemistry is one of the most challenging disciplines in astrochemistry, but in the recent years several models have been successful in reproducing the observed abundance of some simple and complex species (e.g., Aikawa et al. 2008; Garrod et al. 2009; Hollenbach et al. 2009; Cuppen et al. 2009; Cazaux et al. 2011; Taquet et al. 2012a, 2012b).
The picture that has emerged from the combination of observations, laboratory work and modeling is sketched in Fig. 6, which shows the evolution of a dust grain mantle from the outer-edge to the central regions of a prestellar core embedded in a molecular cloud bathed by the interstellar radiation field (with reference to Fig. 5 to locate the various zones). At the outer edge of the prestellar core, photoprocesses are important and the ice mantles are just beginning to form. Here, oxygen atoms are transformed into water, carbon (still not locked in CO) into methane (CH4) and nitrogen into ammonia. Water dominates the mantle composition (probably reflecting the larger cosmic abundance of oxygen relative to C and N). Moving toward the dark-cloud zone (where the prestellar core merges with the molecular cloud within which it is embedded), UV photons are absorbed by dust grains, CO becomes the second most abundance molecule (after H2) and the mantle starts to accumulate CO. CO2 also starts to form, either via cosmic-ray bombardment (Ioppolo et al. 2009)) and/or via the CO+OH reaction (Oba et al. 2010; Ioppolo et al. 2011; Noble et al. 2011; Garrod and Pauly 2011). Here, the limited amount of CO freeze-out limits the degree of deuteration to levels of ≤ a few % (as measured from the observed DCO+/HCO+ abundance ratio; e.g., Caselli et al. 2002b). Deeper into the prestellar core, CO molecules are mostly in solid form, deuteration processes are dominant and the D/H ratio reaches values above 0.1 (see Sect. 4.1). When freeze-out is dominant, the main reactive species landing on dust grain surfaces are atomic H and D. Thus, CO is not only hydrogenated into formaldehyde and methanol, but also deuterated. Large amounts of deuterated and multiply deuterated H2CO and CH3OH are produced (see Sect. 4.1).
Ice mantle evolution within a prestellar core, from the outer-edge, where the core merges with the surrounding molecular cloud, to the dark-cloud zone and deuteration zone as depicted in Fig. 5. Ice mantles become thicker and richer in complex organic molecules moving toward the center of a prestellar core, where star- and planet-formation takes place
4.2.1 The origin of water
Extra attention is given here to the production of water, because of its dominant presence in interstellar ices and its crucial role in our astrochemical origins. Recent measurements of water vapor toward a prestellar core with the Herschel Space Observatory and the use of chemical/dynamical/radiative transfer models, allowed (Caselli et al. 2012) to measure a total mass of water vapor of 0.5 Earth masses within the central 10,000 AU and predicted about 2.6 Jupiter masses of water ice (thus, plenty of ice to boost dust coagulation and the formation of giant planets via core accretion models, e.g., Pollack et al. 1996). From observations of water ices in molecular clouds (e.g., Whittet et al. 2011, and references therein), it is now well established that water ice forms on the surface of dust grains in regions of molecular clouds where the visual extinction is at least 3 mag (when the impinging radiation field is close to the average Galactic value, called the Habing field; larger extinctions are needed for stronger fields). For lower extinction values, the interstellar UV field does not allow dust grain surfaces to accumulate a significant amount of water molecules, as they are efficiently photodesorbed (Öberg et al. 2009b). Laboratory work shows that H2O can form via hydrogenation of atomic oxygen (Hiraoka et al. 1998; Dulieu et al. 2010; Jing et al. 2011), molecular oxygen (Ioppolo et al. 2008; Miyauchi et al. 2008), ozone (Mokrane et al. 2009; Romanzin et al. 2011) and via OH + H2 at 10 K (Oba et al. 2012). As the abundance of water ice in molecular clouds, within which prestellar cores form, is already large (close to 10−4 w.r.t. H2 molecules; e.g., Whittet and Duley 1991), we now generally believe that the main production of water happens before the formation of a prestellar core, as also found by chemical models (e.g., Aikawa et al. 2008; Hollenbach et al. 2009; Cazaux et al. 2010; Taquet et al. 2012b). This suggests that also the production of heavy water must be regulated by the molecular cloud characteristics. This is an important point, as the HDO/H2O ratio is well measured on Earth, comets and asteroids (Sect. 3.1), as well as in star-forming regions (Sect. 5.4). Therefore, one could use our current understanding of surface chemistry and the observed HDO/H2O abundance ratios in star-forming regions to find the link between interstellar chemistry and the Solar System.
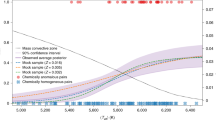
Cazaux et al. (2011) predict that significant variations in the HDO/H2O ratio can be attributed to small variations of the dust temperature at the time of ice formation. In particular, if the dust temperature is lower than ≃15 K, the HDO/H2O ratio is predicted to be ≤0.01 %, because, in these conditions, a large fraction of the dust surface is covered by H2 molecules, allowing the reaction of H2 + O to proceed despite the large barrier of 3000 K (Oba et al. 2012) did not find evidence in the laboratory that this reaction is indeed proceeding, but more laboratory work is ongoing to assess this). The HDO/H2O ratio in these conditions simply reflects the HD/H2 ratio, always close to the interstellar D/H value (≃1.5×10−5, Oliveira et al. 2003). For dust temperatures above ≃15 K, H2 molecules do not stay on the dust surface for long (as their evaporation rate becomes an increasingly large fraction of their accretion rate) and water formation will mostly happen via the reaction of oxygen with atomic hydrogen. As the gas-phase D/H ratio sharply increases above the cosmic deuterium abundance when ice formation takes place (see Fig. 1 of Cazaux et al. 2011), then the HDO/H2O ratio can be as large as a few %. In this scenario, our Solar System formed in a prestellar core embedded in a molecular cloud with dust temperature slightly above 15 K. Taquet et al. (2012b), using a multilayered formation mechanism of ice mantles (Taquet et al. 2012b), find that water is formed first on dust surfaces and that the HDO/H2O ratio depends on the (poorly constrained) ortho:para ratio of H2, on the cloud volume density and, to a lesser extent, on the dust temperature and visual extinction. However, water deuteration can also occur in the gas phase: Thi et al. (2010b) found that significant deuteration levels ([HDO]/[H2O] ≃10−3–10−2) can be produced without surface reactions and at high temperature (T>100 K), in the inner regions of protoplanetary disks (Sect. 6.2). The fractionation occurs because of the difference in activation energy between deuteration enrichment and the back reactions.
4.3 Complex organic molecules
In the freezing cold of dark clouds and prestellar cores, active gas-phase and surface chemistry produce complex organic molecules (COMs). Since the ’80s, organic molecules have been discovered in the TMC-1 dark cloud, part of the Taurus Molecular Cloud complex: methyl cyanide (CH3CN, Matthews and Sears 1983), methylcyanoacetylene (CH3C3N, Broten et al. 1984), acetaldehyde (CH3CHO Matthews et al. 1985), ketene (CH2CO; Irvine et al. 1989), methanol (CH3OH Friberg et al. 1988), methylcyanodiacetylene (CH3C5N Snyder et al. 2006), methyltriacetylene (CH3C6H Remijan et al. 2006), propylene (CH2CHCH3 Marcelino et al. 2007), methyldiacetylene (CH3C4H), cyanopolyynes (HC2n+1N, n=0,1,…,5) and C2n+1N radicals (Walmsley et al. 1984; Hirahara et al. 1992; Ohishi and Kaifu 1998; Kaifu et al. 2004) and the negative ions C6H−, C8H− (McCarthy et al. 2006; Brünken et al. 2007). Complex organics have also been found in two prestellar cores: L183 (CH3CHO, Matthews et al. 1985; HCOOH, Requena-Torres et al. 2007) and L1689B (CH3CHO, HCOOCH3, CH3OCHO, CH2CO, Bacmann et al. 2012). The chemistry of C-bearing species such as cyanopolyynes and CH3C5N can be understood if the gas phase is carbon-rich (C/O ≃ 1.2 Wakelam et al. 2006) or if polycyclic aromatic hydrocarbons (PAHs) are included in the chemistry (with a standard C/O abundance ratio of ≃0.4, Wakelam and Herbst 2008). More problematic is the explanation of complex O-bearing species, such as methanol, which require surface chemistry. Garrod et al. (2007) assumed that the energy released during the formation process could be at least partially used for the surface species to desorb upon formation, reconciling observations with theory for CH3OH and propylene (if the desorption of this species is efficient). Oxygen-bearing species more complex than methanol can also be formed on the surface of low temperature dust grains if a source of UV photons is present (Sect. 4.2). For example, in the laboratory experiments of Öberg et al. (2009a), it has been shown that the photodissociation of CH3OH produces radicals such as CH3 and CH3O (recently discovered in a dark cloud by Cernicharo et al., in press), which can then recombine to form CH3OCH3 or react with CHO (probably produced by the photodissociation of solid CH4 and H2O, see below) to form CH3CHO and HCOOCH3, respectively. Interstellar UV photons are expected to be important up to values of visual extinction of ≃3 mag (e.g., Hollenbach et al. 2009), where CO is not yet significantly frozen onto dust grains (see Fig. 5). Deeper into prestellar cores, a significantly more tenuous field of UV photons can be produced by the collisions of cosmic-rays with H2 molecules (Prasad and Tarafdar 1983; Gredel et al. 1989). It is not yet clear if this cosmic-ray induced field is able (i) to produce enough radicals, (ii) to furnish them enough energy to move on the surface, recombine and form complex molecules, and (iii) to release them into the gas phase where they are observed (see also the discussion in Taquet et al. 2012b). Consequently, it is not yet clear whether models are able to reproduce the abundances of complex molecules observed by Bacmann et al. (2012).
In summary, possible first steps toward the formation of COMs in the ice (before the switch-on of the protostar) are as follows.
(1) Production and storage of radicals. In the molecular cloud within which the prestellar core forms, at \(A_{\rm V}\) ≃3 mag, interstellar UV photons can still partially dissociate important ice components (H2O and CH4) and some of the products can be trapped within the ice, which already contains significant fractions of water (e.g., Chiar et al. 2011). Alternatively, because of the multilayered nature of icy mantles, radicals can be stored in the inner layers during mantle formation (Taquet et al. 2012b).
(2) Radical-radical reactions. As the density increases and CO starts to freeze-out onto the first water-dominated ice layers, the CO is transformed into CH3OH more and more with increasing freeze-out (given that with the freeze-out of CO and O, the H/O and H/CO abundance ratios in the gas-phase increase, as the number density of H atoms is kept about constant to 1 cm−3 by the cosmic-ray dissociation and surface re-formation of H2 molecules). The energy released during the formation of methanol is partially used by methanol itself to evaporate and partially released as heat on the icy surface, allowing some of the previously trapped radicals to move. The new radicals produced in the dissociation of CH3OH by cosmic-ray-induced UV photons (and probably some of the intermediate compounds produced during the CO→CH3OH conversion) will then participate in the formation of the observed complex organic molecules (e.g., Öberg et al. 2009a). As for the case of methanol, the energy released in the process of formation of these COMs can be partially used to return in the gas phase. The impulsive heating of dust grains due to the impact of heavy cosmic rays (Leger et al. 1985) may also temporarily enhance the mobility of the stored radicals, allowing complex molecule formation.
We emphasize that the above steps remain highly speculative and more experimental and theoretical work is necessary to better understand the grain-surface chemistry processes. Given that the observed COMs are building blocks of biologically important species, this once again underlines the importance of prestellar cores for the first steps toward our astrochemical origins.
5 The cocoon phase: protostars
Once the collapse starts, the gravitational energy released at the center of the infalling envelope is converted into radiation. During the first phases of star formation, this is the main source of the protostar luminosity L ∗ and it is given by \(L_{*} = {G M_{*} \dot{M}}/{R_{*}}\), where M ∗ and R ∗ are the mass and radius of the central object, and \(\dot{M}\) is the mass accretion rate. The approximate structure of the envelope, as derived by observations of the continuum and line emission (e.g. Ceccarelli et al. 2000a; Jørgensen et al. 2002; Robitaille et al. 2006) is reported in Fig. 7. Both the density and temperature increase toward the center. Similarly, the velocity of the infalling gas increases with decreasing distance from the center with an r −1/2 power law, although part of the envelope may not be collapsing yet. The infall motion has proved difficult to disentangle from the outflow motions, but high spatial and spectral resolution observations recently obtained with ALMAFootnote 3 have succeeded to probe it unmistakably toward IRAS16293-2422 (Pineda et al. 2012). Finally, new Herschel observations provide a much more complicated picture where, at least in some sources, the cavity created by the outflowing gas is illuminated and heated by the UV photons of the central star, making the interpretation of the observed lines not straightforward (Visser et al. 2012).
Temperature and density profile of IRAS16293-2422, the prototype for chemical studies in Class 0 sources (from Crimier et al. 2010). The colored boxes represent the four chemical zones described in the text: (i) molecular cloud, (ii) CO depletion, (iii) Warm-Carbon-Chain-Chemistry (WCCC); (iv) hot corino
5.1 The chemical composition of protostellar envelopes: a powerful tool to understand the present and the past
Chemistry has been recognised to be a powerful diagnostic tool in several fields of astrophysics to understand the present and the past of the studied object. For example, at large scale, the chemical enrichment in stars throughout the Milky Way tells us about different star populations and ages, and, consequently, how the Milky Way formed (e.g., Gratton et al. 2012). Similarly, at much smaller scales, the chemical composition in protostellar envelopes tell us about their present status and past history.
Figure 7 shows the approximate and very simplified density and temperature profiles of a typical protostellar envelope. To a scale of ≥100 AU, a roughly spherical envelope heated by the internal new born star this is probably a correct description. However, at smaller scales, the envelope is not spherical, because of the presence of a circumstellar disk (Sect. 6) and the presence of multiple sources, as in the case of IRAS16293-2422 and NGC1333-IRAS4 (e.g., Wootten 1989), among the two most studied examples of solar-type protostars. Nonetheless, from a chemical point of view, four major zones can be identified (Fig. 7): (i) an outer zone, with the same chemical composition as that of the placental molecular cloud; (ii) a CO depleted zone, usually called cold envelope, with the chemistry is very similar to that of prestellar cores (Sect. 4); (iii) a CH4 ice sublimation region, where the chemistry is dominated by the warm carbon chain chemistry, called WCCC, triggered by sublimation of the methane from the grain mantles; (iv) the hot corino zone, where the chemistry is dominated by the water-matrix grain mantle sublimation and hot gas chemistry. The transition between zones (ii) to (iv) is determined by the dust temperature, which governs the sublimation of the icy mantles, whereas the CO depleted region depends on the density and age of the protostellar envelope. In the following we summarise the characteristics of the four zones.
-
Zone (i)
The chemical composition in this zone is similar to typical molecular clouds, with no particularly important freeze-out of species. Whether this zone is present or not in a protostellar envelope depends on the envelope density and age, which determines the existence of zone (ii).
-
Zone (ii)
As described in Sect. 4, if the density and age of the envelope are high enough, molecules freeze-out onto dust surfaces. Important for the various reasons again described in Sect. 4 is the region where CO freezes out, defined by a dust temperature lower than about 22 K. Jørgensen et al. (2005) found that a large fraction of Class 0 and Class I protostars have CO-depleted regions in their envelopes, typically where the density is larger than ∼105 cm−3. Models of the chemistry in young protostellar envelopes provide a theoretical interpretation to these observations (e.g., Lee et al. 2005).
-
Zone (iii)
When the dust temperature exceeds the methane sublimation temperature, ∼25 K, the chemistry is governed by the injection of methane in the gas phase, if the CH4 abundance is larger than ∼10−7. In this case, CH4 becomes a major destruction partner for C+, starting the efficient formation of C-chain molecules in the relatively warm (30–60 K) gas (Aikawa et al. 2008; Hassel et al. 2008, 2011). So far, only a few protostellar envelopes with very abundant C-chain molecules have been discovered. L1527 is the prototype of this class of sources, called Warm-Carbon-Chain-Chemistry (WCCC) sources (Sakai et al. 2008, 2010a, 2010b). Note that the abundance of methane has been indirectly inferred in those sources by modeling the observed C-chain molecules, as gaseous CH4 does not have observable rotational transitions.
-
Zone (iv)
When the dust temperature exceeds about 100 K, the grain mantles evaporate and all species trapped in them are released in the gas phase, giving rise to a rich chemistry, first discovered in high-mass protostellar envelopes and called hot core chemistry (e.g., Blake et al. 1987), and successively unveiled in low-mass protostellar envelopes (Cazaux et al. 2003). However, as will be discussed in detail in Sect. 5.2, the chemical composition of low- and high- mass cores is not identical.
The transition zones in Fig. 7 are, of course, approximate, as laboratory experiments show that ice sublimation is a complex process where molecules are released into the gas through several steps at different dust temperatures (e.g., Viti et al. 2004). Also, the outflows emanating from the central objects open up cavities which are directly illuminated by the UV photons of the new born star (e.g., van Kempen et al. 2009; Yıldız et al. 2012; Visser et al. 2012). In these cases, large Photon-Dominated-Regions (PDRs) may dominate and mask the molecular emission from the various zones, depending on the extent of the cavity.
As already mentioned, the presence of the WCCC zone (zone iii) depends on the abundance of methane in the dust mantles. Methane is formed, as the vast majority of the grain mantles, during the prestellar phase (Sect. 4). Specifically, it is believed to form by hydrogenation of neutral carbon. However, in typical molecular clouds, neutral carbon is a rare species because of the efficient formation of CO. Therefore, to have a large quantity of iced CH4, one needs particular conditions, namely a relatively high abundance of neutral carbon in the gas phase. This occurs when the transition from the diffuse cloud to molecular cloud is very fast, and a substantial fraction of carbon atoms freeze-out into the grain mantles before the CO formation is achieved (e.g., Hassel et al. 2011). Therefore, the presence of a WCCC zone may be a signature of fast collapse (Sakai et al. 2008), for example triggered by a shock from a nearby forming star or two encountering diffuse clouds. Alternatively, if the prestellar core is embedded in a relatively tenuous cloud, CO photodissociation could still play a role and led to a large amount of methane ice. Unfortunately, the limited number of observations do not allow us to go much further in the interpretation of this peculiar chemistry, and more studies are needed to fully exploit it. In the same vein, the chemical composition in the hot corino zone, as well as the observed molecular deuteration, are all largely influenced by the prestellar phase. These cases will be discussed in detail in the following paragraphs.
Last, a potentially powerful diagnostic is provided by the relative abundances of isomers of the same generic formula. Since the interstellar chemistry is dominated by kinetics, different isomers have in principle the imprint of the different chemical formation routes. Therefore, the isomer relative abundances help understanding the reactions at work and, consequently, how well we understand the interstellar chemistry. A puzzling and interesting example is provided by the isocyanic acid (HNCO) and its isomers fulminic acid (HCNO) and cyanic acid (HOCN), which have zero energies, respectively, 71 and 25 kcal/mol above HNCO. In cold gas, the HNCO/HCNO and HNCO/HOCN abundance ratios are about 50, whereas in warm gas HNCO/HCNO is about 50 and HNCO/HOCN more than 5 times larger (Marcelino et al. 2010). The difference of abundances between the different isomers is thought to be due to the different chemical routes of formation and destructions (Quan et al. 2010). However, the available gas-phase and gas-grain+gas-phase models have some difficulties in reproducing the observations and the results very much depend on the assumption made on the CH2 + NO reaction rate coefficient. Even more puzzling, these models do not explain the observed difference in the HCNO/HOCN ratio between cold and warm sources. Marcelino et al. (2010) speculate the presence of a mechanism that converts HCNO into HOCN, despite the large energy barrier necessary for the isomerisation. On the other hand, Lattelais et al. (2009) already noted that a pseudo-isomerisation seems to occur to the majority of species where different isomers have been detected. They studied 14 species and 32 isomers and found that the larger the energy difference, the larger the abundance ratio between the most stable species and its isomer, with a few exceptions. They called it the “minimum energy principle” and its origin is still unclear, as the isomerisation barriers are generally very large and different isomers are formed from different “mother” species.
Similar arguments on the diagnostic value applies for the isotopologues of a species. Nice examples are provided by the CCS and CCH studies by Sakai and collaborators (e.g., Sakai et al. 2007, 2010a). Studying the abundance ratio of 13CCS/C13CS and 13CCH/C13CH, they constrained the formation routes of CCS and CCH and demonstrated that the 12C/13C depends on the position of the carbon in the chain.
5.2 The chemical complexity in hot corinos
In the 90s, several abundant complex organic molecules (COMs) were discovered in an unbiased spectral survey of the prototype massive star-forming region, the Orion Molecular Cloud (Blake et al. 1987). Soon after, a similar rich chemistry was observed in several other massive protostellar envelopes. The proprieties of the line emission indicate that these COMs reside in compact (≤0.01 pc), dense (≥107 cm−3) and hot (≥100 K) regions, soon called “hot cores”. A simple and obvious interpretation is that the observed rich chemistry is due to the sublimation of some species from the grain mantles, called “mother” or “primary” species, and the synthesis of others, called “daughter” or “secondary” species, thanks to the high gas temperature (e.g., Charnley et al. 1992). Almost two decades later, similar results were obtained toward the envelope of the prototype low-mass protostar IRAS16293-2422 (Ceccarelli et al. 2000b; Cazaux et al. 2003), where several COMs were detected. Since then, more low-mass hot cores have been discovered and, to distinguish them from the high-mass hot cores, they were called hot corinos (Bottinelli et al. 2004a, 2004b, 2007; Lahuis et al. 2006; Jørgensen et al. 2012); see also the review by Herbst and van Dishoeck (2009). Hot corinos differ from hot cores not only for the smaller sizes, lower temperatures and densities, but also chemically. In fact, when normalized to methanol or formaldehyde, hot corinos have typically one order of magnitude more abundant COMs (such as HCOOCH3 or CH3OCH3) than hot cores (Ceccarelli et al. 2007; Bottinelli et al. 2007; Herbst and van Dishoeck 2009; Öberg et al. 2011c; Cordiner et al. 2012). The difference in the richness and COMs abundances between hot cores and hot corinos is likely due to various factors. Among them, two certainly play a major role: (i) the gas temperature, which governs the neutral-neutral reactions that often possess large activation energy barriers; (ii) the composition of the sublimated ices, governed by the past prestellar history (Sect. 4).
In addition to being weak line emitters and small objects, the study of hot corinos is also complicated by the fact that low-mass protostars are often binary or multiple systems (as in the case of high-mass protostars). The hot corino prototype IRAS16293-2422 is in fact a binary system and the two objects composing it, called A and B in the literature, show definitively a different chemistry (see for example the recent articles by Caux et al. (2011) and Jørgensen et al. (2011), and reference therein). To illustrate this aspect, Fig. 8 shows a sketch of the chemical composition of IRAS16293-2422, based on the analysis of the single-dish unbiased spectral millimeter and sub-millimeter survey carried out by Caux et al. (2011) and confirmed by the sub-millimeter interferometric unbiased survey of Jørgensen et al. (2011). Four groups of species are identified:
- Group I: :
-
Millimeter lines from simple molecules, like CN and HCO+, are dominated by the cold envelope. Also, emission from simple carbon-chains are associated with the cold envelope (see the discussion of their chemistry in Sect. 5.1).
- Group II: :
-
Source A is rich in N- and S-bearing molecules.
- Group III: :
-
Source B is rich in O-bearing COMs.
- Group IV: :
-
Molecules like CH3OH, H2CO, CH3CCH and OCS emit low-lying lines in the cold envelope and high-lying lines in the two sources A and B.
Sketch of the chemical composition of the protostellar envelope of IRAS16293-2422, a protobinary system composed of two sources, A and B, as marked. The four boxes list the species in the different components of the system: species in Group I are associated with the cold envelope surrounding A and B; species in Group II are associated with source A and in Group III with source B; species in Group IV are present in the cold envelope and the two sources
The obvious question is: why are source A and B so chemically different? They must have had a similar composition of the sublimated ices, as they belong to the same core, so that the difference is probably originating from the different evolutionary status caused by the difference in mass of the two objects (Bottinelli et al. 2004b; Caux et al. 2011; Pineda et al. 2012; Jørgensen et al. 2012). However, so far no attempt has appeared in the literature to theoretically model the two sources to understand what exactly causes the observed chemical differences.
Finally, as mentioned in Sect. 4, COMs are predicted to be formed on grain surfaces. Four fundamental steps are involved: (i) freeze-out of atoms and simple molecules (such as O and CO) on the grain surface; (ii) successive additions of H atoms to form hydrogenated species (such as CH3OH); (iii) formation and trapping of radicals, such as CH3, on the grain surfaces; (iv) combination of radicals to form COMs in the warm-up period. While laboratory experiments and quantum chemistry calculations have tested and quantified the second step, the third step is still a matter of debate. Garrod and Herbst (2006) and subsequent work from the same authors assume that the radicals are formed from the secondary UV photons emitted by the interaction of cosmic rays with H2 molecules. Specifically, it is assumed that UV photons break iced species like CH3OH into radicals like CH3 and that the broken pieces remain frozen on the grains, which may not be necessarily the case. On the other hand, Taquet et al. (2012b) showed that radicals can indeed be trapped in the grain mantles without the intervention of UV photons, just because of the intrinsic layered structure of the forming mantle.
However, it is important to emphasize that, whatever is the possible origin of the radicals, models still fail to reproduce the observed amount of COMs. For example, Fig. 9 shows the comparison between the observed and predicted methyl formate abundance normalized to the methanol one. Published models are off by at least one order of magnitude. Considering that COMs are also observed in prestellar cores (see Sect. 4) and outflows (Sect. 5.3), something basic on how COMs are formed in the ISM must still escape our understanding.
Observed (red and blue crosses: hot corinos and cores) and predicted (continuum lines) methyl formate normalized to methanol abundance as function of methanol abundance. The blue lines refer to models of hot cores (Garrod et al. 2008) and (Laas et al. 2011) and red lines to models of hot corinos (Garrod et al. 2008; Awad et al. 2010). Figure from Taquet et al. (2012b), with permission. It is probably safe to assume that the plotted values are correct within one order of magnitude
5.3 The chemical complexity in molecular outflows
The birth of a star is accompanied by a violent and substantial ejection of material simultaneous to the accretion toward the central object. The process has an enormous importance in the star-formation process because (i) it allows the infalling matter to lose angular momentum and accrete onto the central object, and (ii) the ejected material interacts with the surroundings, deeply modifying it and completely destroying, in some cases, the parental cloud (e.g., Lefloch et al. 1998; Shimajiri et al. 2008; Arce et al. 2011; López-Sepulcre et al. submitted). The ejected material creates shocks at the interface between the outflowing jet and the quiescent material. Those shocks are chemically rich sites, showing a chemical composition very similar to hot cores/corinos. In fact, in the shocks, dust grains are sputtered and vaporized releasing the mantle components and part of their refractory material into the gas phase. Moreover, shocked regions become hot enough to allow neutral-neutral reactions to take over and produce complex molecules. In the following, we will only review the studies on the chemical composition of the outflow shocks, leaving out the many and important questions on the physical structure of the shock and the acceleration mechanisms of the jet.
Although several molecular outflows have been observed and mapped in the past three decades, the study of their molecular complexity started much later. Bachiller and Perez Gutierrez (1997) were the first to show the chemical structure of L1157-B1, considered nowadays a prototype for the studies of molecular complexity in molecular outflows. Toward this source, not only relatively simple complex molecules, like methanol, have been detected (Codella et al. 2010), but also molecules considered hot cores/corinos tracers, like methyl formate (HCOOCH3), ethanol (C2H5OH), formic acid (HCOOH) and methyl cyanide (CH3CN) (Arce et al. 2008). High spatial resolution observations show that emission of these species is concentrated in a small region associated with the violent shocks at the head of the outflowing material (Codella et al. 2009). The presence of COMs in molecular outflows strongly suggests that these species were part of the sputtered icy mantles (as the time elapsed since the shock is too short for any gas-phase route to build up COMs) and provides us with another piece of the puzzle regarding their formation. The abundances normalized to methanol are at least one order of magnitude lower in molecular outflows than in hot corinos.
It is worth noticing the presence of species not even detected in other sources, like the phosphorus nitride (PN), whose abundance is only a few times 10−10 with respect to H2 (Yamaguchi et al. 2011). In fact, molecular outflows can be considered, for some aspects, unique laboratories to understand interstellar medium chemistry. For example, hydrogen chloride (HCl) has been recently detected with the Herschel Space Observatory in L1157-B1 (Codella et al. 2012a). The measured abundance is 3–6 ×10−9, practically the same value as in high- and low-mass protostellar envelopes (e.g., Peng et al. 2010) and about 200 times lower than the Cl elemental abundance. This is a puzzling result, as chemical models predict that HCl would be the major reservoir of chlorine and observational evidence suggests that L1157-B1 is a shock site where grains are sputtered/vaporized and mantles almost entirely destroyed, as also suggested by the large fraction of silicon found in the gas phase as SiO. Therefore, the low measured HCl abundance raises the question “where is chlorine?”. It is not in the mantle, but not even in the vaporized refractory material of dust grains where silicates reside. Is then chlorine in a significantly more refractory component than silicates? Which one? All questions that will need more observations to be answered.
5.4 Water and deuterated water
Water and deuterated water are special species, because of the hints on the Earth and Solar System formation that they bring (Sect. 3) and because water plays a leading role in the thermal and chemical evolution of protostellar envelopes (Ceccarelli et al. 1996; Doty and Neufeld 1997; van Dishoeck et al. 2011). However, since water lines can only be observed from out-of-the atmosphere telescopes, the water content in the envelope of solar-type protostars has been estimated only recently.
Water abundance in hot corinos. The first estimates based on the Infrared Space Observatory (ISO) suggested that the water abundance in the hot corino region is only a few times 10−6 (e.g., Ceccarelli et al. 2000a). The more recent observations obtained with Herschel, with a much better spatial and spectral resolution, have confirmed that first claim with an increased reliability and in a larger number of sources (Kristensen et al. 2010, 2012; Visser et al. 2012; Coutens et al. 2012). If, on the one hand, these observations confirm the old theoretical predictions that water should be abundant in the innermost and warmest regions of the envelopes surrounding Class 0 protostars (Ceccarelli et al. 1996; Crimier et al. 2009), they also raise the question why the measured water abundance is much lower than that expected, ∼10−4, based on the ice measurements (Sect. 4). Finally, interferometric observations have shown that a compact \(\mathrm{H}_{2}^{18}\mathrm{O}\) emitting region is associated with the hot corinos/disk of a few Class 0 sources (Jørgensen and van Dishoeck 2010a; Persson et al. 2012).
Water abundance in molecular outflows
Again, the first estimates of the water abundance in molecular outflows were obtained with ISO and gave abundances varying from ∼10−5 to ∼10−4 (Liseau et al. 1996; Nisini et al. 2000; Benedettini et al. 2000). Water in outflows was also the target of the Submillimeter Wave Astronomy Satellite (SWAS) and Odin satellite, which were tuned on the H2O ground-state transition at 557 GHz (Franklin et al. 2008; Bjerkeli et al. 2009; Benedettini et al. 2002). More recently, the new Herschel observations are providing a mine of new information, allowing us to map the water emission along the outflow and to distinguish the water content in low to high velocity shocks. The Herschel maps show bright water emission at the shock sites of the molecular outflows (Nisini et al. 2000; Benedettini et al. 2012; Kristensen et al. 2010; Bjerkeli et al. 2011, 2012). The study of the water abundance as a function of the velocity of the shock then shows that high velocity shocks are associated with larger water abundances (Lefloch et al. 2010; Kristensen et al. 2011; Bjerkeli et al. 2011; Santangelo et al. 2012; Vasta et al. 2012; Benedettini et al. 2012), as predicted by the C-shock models (Kaufman and Neufeld 1996). These models predict that H2O is formed in the gas phase via reactions with large activation barriers (e.g. O + H2 and OH + H2; see also Hollenbach and McKee 1989). Finally, interferometric observations show that the dense shock very close to the central source produces a large quantity of water (Lefloch et al. 2011).
Deuterated water
The HDO abundance and HDO/H2O abundance ratio have been measured toward a handful of hot corinos, with different techniques. From single-dish telescopes (IRAM 30m and ISO first, then Herschel) the HDO/H2O has been estimated to be 3 % in IRAS16293-2422 (Parise et al. 2005; Coutens et al. 2012) and ≥1 % in NGC1333-IRAS2A (Liu et al. 2011). Estimates obtained with interferometric observations of HDO and \(\mathrm{H}_{2}^{18}\mathrm{O}\) lines give ≤0.06 % in NGC1333- IRAS4B (Jørgensen and van Dishoeck 2010a), and 14 % and 22 % toward NGC1333- IRAS2A and NGC1333- IRAS4A, respectively (Taquet et al. 2012c). Note that the interferometric observations provide a direct, almost model-independent, estimate of the HDO/H2O abundance ratio as they do measure the extent of the emission and use the rare \(\mathrm{H}_{2}^{18}\mathrm{O}\) isotopologue reducing the problem of line opacity. In summary, the HDO/H2O ratio has been measured toward four hot corinos: in three of them it is larger than a few percent, whereas in NGC1333- IRAS4B it is at least one order of magnitude lower. Herschel observations have also allowed, for the first time, to estimate the HDO/H2O in a molecular outflow shock, L1157-B1 (0.4–2×10−3, Codella et al. 2012b), a likely direct measure of the deuteration in the ice. The situation is summarised in Fig. 10. The differences in the HDO/H2O abundance ratio probably reflect the different conditions, density and temperature, when the ice was formed (see Sects. 4 and 7.2).
HDO/H2O abundance ration in the envelope of Class 0 protostars and the L1157-B1 outflow shock. References: IRAS16293-2422 (Coutens et al. 2012), NGC1333-IRAS2A (Taquet et al. 2012c), NGC1333-IRAS4B (Jørgensen and van Dishoeck 2010b), NGC1333-IRAS4A (Taquet et al. 2012c), L1157-B1 (Codella et al. 2012b). The differences in the HDO/H2O abundance ratio probably reflect the different conditions, density and temperature, at the time when ice mantles formed (see Sects. 4 and 7.2)
Doubly deuterated water
Although it has a very low abundance, D2O has an important diagnostic power as it sets very tight constraints to models of water formation. So far, thanks to Herschel, D2O/H2O has been measured only toward the cold envelope of IRAS16293-2422, with the observations of both the para and ortho forms of D2O (Butner et al. 2007; Vastel et al. 2010). The D2O/H2O abundance ratio as a result is found to be 1–4×10−3 (Coutens et al. 2012). Similarly, the para-D2O/H2O toward the hot corino is ∼5×10−5 (Butner et al. 2007). Assuming an ortho-to-para ratio equal to 2 gives D2O/H2O∼10−4. For example, comparison with the model by Taquet et al. (2012d) indicates that the bulk of water was formed on grains when the cloud/envelope temperature was 10 K and the density between 104 and 105 cm−3. In other words, when the density at the center of the IRAS16293-2422 prestellar cloud reached 106 cm−3, the oxygen not locked into CO was almost entirely already converted into water.
5.5 Deuteration of other species
As water, several molecules present large deuteration factors in low-mass protostellar envelopes and molecular outflows (e.g., Parise et al. 2006 and Codella et al. 2012b, respectively). Figure 11 presents a graphic summary of the observations of species with detected doubly or triply deuterated isotopologues. The deuterated ratios are extremely high, with enhancements of the D/H of up to 13 orders of magnitude with respect to the elemental D/H abundance ratio. Given the conditions in the envelopes of the protostars (Sect. 5.1 and Fig. 7), the observed deuteration is mostly an inherited product of the prestellar phase (Sect. 4). Furthermore, for the typical physical condition where the deuterated molecules have been detected, the measured deuteration ratios likely reflect the deuteration on the grain mantles (e.g., Charnley et al. 1997).
Measured deuteration ratios of singly, doubly and triply deuterated isotopologues. Based on the modeling of the formation of H2O, H2CO and CH3OH (Cazaux et al. 2011; Taquet et al. 2012d) we speculate that the increasing deuteration reflects the formation time of the species on the ices. References: H2O: Liu et al. (2011), Coutens et al. (2012), Taquet et al. (2012c), Butner et al. (2007), Vastel et al. (2010); H2S: Vastel et al. (2003); NH3: Loinard et al. (2001), van der Tak et al. (2002); H2CS: Marcelino et al. (2005); H2CO: Ceccarelli et al. (1998), Parise et al. (2006); CH3OH: Parise et al. (2002, 2004, 2006)
We emphasize here that the deuteration ratio is not the same for all species. As mentioned in Sect. 4, the lower deuteration ratio of water with respect to formaldehyde and methanol probably reflects the different epoch in which the bulk of the iced species has been formed (during the prestellar phase). Specifically, water is (mostly) formed before formaldehyde, and methanol is the last in the sequence (Cazaux et al. 2011; Taquet et al. 2012a, 2012d). Even though not specific modeling has been published for all observed deuterated species, we speculate that the sequence in the figure represents a temporal sequence of the species formation.
Finally, the comparison between the singly and doubly deuterated isotopologues provides some interesting additional information. First, if the deuterium atoms were purely statistically distributed, namely just proportional to the D/H ratio, then it would hold: D species/D2 species = 4 (D species/H species)−1. As shown in Fig. 12, this is not the case for the measured deuteration of H2O, NH3, H2CS and H2CO. As also noted by Butner et al. (2007), this points to a change of the atomic D/H ratio during the formation of those species or to an origin from gas-phase reactions. On the contrary, the statistical relation is roughly valid for H2S and CH3OH. This suggests that these two species have been formed on the grain surfaces in a very short time, when the atomic D/H ratio can be considered roughly constant.
Measured ratios of singly to doubly deuterated isotopologues of the species marked in the plot. References as in Fig. 11
In summary, the observed deuteration ratios tell us that H2O, NH3, H2CS and H2CO were formed at various stages during the star-formation process, with different values of the atomic gas D/H ratio. On the other hand, H2S and CH3OH were formed in a shorter time range. This behaviour roughly agrees with the models of the formation of H2O, H2CO and CH3OH on grains, that predict that methanol is only formed at very late time, whereas water and formaldehyde are formed over a larger period of time (Cazaux et al. 2011; Taquet et al. 2012a, 2012b). It is, however, possible that the species not close to the statistical value are, at least in part, gas-phase products.
As a final remark, it is important to emphasize that low- and high-mass protostellar envelopes present important differences in the molecular deuteration. A clear example is provided by the CH2DOH/CH3OD abundance ratio, which is at least one order of magnitude larger in low-mass than in high-mass protostellar envelopes (Ratajczak et al. 2011; Peng et al. 2012).
6 Toward planet formation: protoplanetary disks
Starless and prestellar cores present evidence of overall (slow) rotation (Arquilla and Goldsmith 1986; Goodman et al. 1993; Caselli et al. 2002), thus they possess an initial angular momentum. As a natural consequence of angular momentum conservation, the collapse of prestellar cores produces flattened structures which harbor the future protoplanetary disks. Even non-rotating collapsing cores are expected to produce flattened structures in the presence of magnetic fields, as explained in the following. As ionized particles within the core are linked to the magnetic field lines, while neutrals only feel the gravitational field, a drag between ions and neutrals is established during the collapse phase (see Sect. 4). Galli and Shu (1993a, 1993b) found that during the collapse of a singular isothermal sphere (i.e. an unstable spherical cloud with a density profile proportional to r −2, thus with a singularity in the center, Shu 1977), the magnetic field, dragged by the flow, deflects the infalling gas toward the midplane, forming a large (≃2000 AU) “pseudodisk”. The magnetic field lines, initially parallel, are shaped as an hourglass, consistent with observations of polarization maps of the dust continuum emission toward young stellar objects (e.g., Girart et al. 2006; Frau et al. 2011). The twisting of magnetic field lines in the pseudodisk acts as a “magnetic break”, in the sense that it slows down the rotation by transferring angular momentum from the inner regions (which tend to rotate faster for angular momentum conservation) of the pseudodisk toward its outer parts (Basu and Mouschovias 1994). Indeed, magnetic breaking is so efficient, that disks cannot form at all in ideal magneto-hydrodynamic (IMHD)Footnote 4 simulations of collapsing cores (e.g., Allen et al. 2003; Mellon and Li 2008; Hennebelle and Fromang 2008). More recently, the inclusion of non-ideal MHD effects, in particular the Hall effectFootnote 5 (Braiding and Wardle 2012; Krasnopolsky et al. 2011), has helped to avoid this so-called magnetic breaking catastrophe, allowing disks of about 100 AU to form (even without initial rotation of the collapsing cloud Braiding and Wardle 2012). This has also been shown in simulations by Machida et al. (2011), who found rapid growth to ≥100 AU of the circumstellar disk when depletion of the infalling envelope is taken into account, and by Joos et al. (2012), who explored the case of magnetic fields non-aligned with the rotation axis and found less efficient angular momentum transport, allowing the formation of ≃100–200 AU disks, with masses as large as 10 % the original core mass. These characteristics are similar to the young self-gravitating protoplanetary disks (we refer to them as “embedded disks”) which can become gravitationally unstable (e.g., Laughlin and Bodenheimer 1994; Boss 1997; Durisen et al. 2007 and references therein; Boley and Durisen 2008; Vorobyov 2011) and which represent the starting point of our final journey toward the formation of a planetary system. Here we will focus on the chemical evolution (see Armitage (2011) and Williams and Cieza (2011) for comprehensive reviews on the physical characteristics and evolution of protoplanetary disks).
6.1 Embedded disks: chemistry at the dawn of planet formation
Young disks are embedded within the thick and massive envelopes of Class 0 sources (see Sect. 5). Therefore, they are not easy to study and it is hard to put constraints on theoretical predictions. Indirect evidence of young disks in Class 0 sources is given by the presence of collimated outflows, observed with millimeter and sub-millimeter telescopes (Sect. 5). ALMA will of course revolutionize this field. After the pioneer work by, e.g., Chandler et al. (1995), Brown et al. (2000) and Looney et al. (2000), further steps toward the characterization of these embedded disks have been made by Jørgensen et al. (2007, 2009), and Enoch et al. (2011). With the help of interferometric observations (able to filter out the surrounding envelopes), these authors found evidence of compact embedded disks in Class 0 sources, with masses ranging from 0.04 to 1.7 M⊙. Choi et al. (2007) observed NH3 with the Very Large Array (VLA) and found a 130 AU circumstellar disk around NGC1333 IRAS4A2. With the IRAM Plateau de Bure Interferometer (PdBI), Jørgensen and van Dishoeck (2010b) measured water vapor (\(\mathrm{H}_{2}^{18}\mathrm{O}\)) in the inner 25 AU of the NGC1333 IRAS4B disk, suggesting the presence of a thin warm layer containing about 25 Earth masses of material. Toward the same object, Jørgensen and van Dishoeck (2010a) also set a stringent upper limit on the HDO/H2O abundance ratio to 6×10−4 (Sect. 5). Pineda et al. (2012) observed methyl formate with ALMA toward IRAS16293-2422, a binary Class 0 source in Ophiuchus (Sect. 5), and found the first evidence of infall toward source B and evidence of rotation toward source A, consistent with an almost edge-on disk (see also Rodríguez et al. 2005). If confirmed, this could be the first chemically and kinematically characterized embedded disk (discovered with a complex organic molecule!).
What are the chemical model predictions of these embedded disks? Visser et al. (2009, 2011) have been the first to self-consistently follow the chemistry in a two-dimensional axisymmetric model of a collapsing (initially) spherical and slowly rotating cloud, on its way toward the formation of a protoplanetary disk. The material infalling in the equatorial plane, within the centrifugal radius,Footnote 6 forms the disk, whose evolution is also considered assuming no mixing. The disk-envelope boundary and the outflow cavities are well defined. Detailed predictions are given about the ice and gas-phase composition of the cloud-disk system at different evolutionary phases. At the end of the collapse phase, they find that disks can be divided in zones with different chemical history, which will ultimately affect the composition of comets formed in different zones. Different results are found by Ilee et al. (2011), who used the hydrodynamic simulations of a young and relatively massive (0.39 M⊙) disk by Boley (2009) as input in their gas-phase and simple surface chemistry network. Boley’s disk resembles in mass and size the embedded disk mentioned above, it is non-axysimmetric and present complex spiral and physical structure, with shocks moving with the spirals arms (see Fig. 13, middle panel in the left, which reports the gas column density map). No accretion of material from the envelope and no outflow is considered. Despite these assumptions, the disk structure is complex and its physical characteristics are continuously stirred by the rotating spiral arms. Because of this continuous mixing, Ilee et al. (2011) found no separated chemical zones as in the case of Visser et al. (2011), but they identified species able to trace the inner regions of the disk (such as H2O, HNO and NH3) and those tracing the spiral arms (e.g. H2CO and HCO+). Examples of these column density maps are given in Fig. 13 (bottom right panels), which also summarizes the various physical mechanisms to be considered for a comprehensive study of the earliest stages of star formation: the collapsing envelope of a Class 0 source (red semicircle) under the influence of magnetic fields (yellow lines and curves in the figure), the pseudodisk (blue), the central embedded disk (violet) and the outflow (orange) driven by the central protostar (red semicircle). Furuya et al. (2012) studied the chemical evolution of a molecular core toward the formation of the first hydrostatic core (protostellar precursor) using three-dimensional radiation hydrodynamic simulations. They show that after a first destruction of molecules, simple species such as CO, H2O and N2 reform and more complex molecules (CH3OH and HCOOCH3) can trace the first hydrostatic core, on its way to becoming a protostar. ALMA observations are needed to disentangle the various phenomena at work during the earliest stages of star formation, to test model predictions of collapsing magnetized prestellar cores and to unveil the physical and chemical structure of the embedded disks, precursors to the protoplanetary disks which will be reviewed in the next sections.
The earliest stages of a protoplanetary disk. Magnetic fields (yellow curves) and the initial rotation of the prestellar core lead to the formation of a flattened structure (the “pseudodisk”, size 2000 AU) surrounding the accreting protostar. In the central few hundred AU, the embedded disk can be self-gravitating, develop spiral structure and experience fragmentation. Molecules such as H2O and H2CO are good tracers of these central regions of young embedded disk (Ilee et al. 2011)
6.2 “Naked” protoplanetary disks
The embedded phase of disks does not last long. After about 0.5 Myr since the birth of the protostar/disk/outflow system, the parent core envelope quickly disperses and the disk enters a new phase which lasts several Myr (Williams and Cieza 2011). This is the T Tauri (or Class II) phase. The disk mass is now only a few % the stellar mass (Williams and Cieza 2011) and the motions are expected to be Keplerian. Despite being “naked” disks, thus easier to observe than during the earlier embedded phase, the physical and chemical processes at work are complex and more (interferometric) data are sorely needed to fully understand them. Figure 14 shows a schematic picture of a T Tauri disk, compiled from a combination of figures found in Öberg et al. (2011b), Dullemond and Monnier (2010); Semenov (2011), Dullemond et al. (2007b), and Bergin et al. (2007): (1) within the central 1 AU from the star, a pure gas disk and the dust inner rim are present. This zone is mainly probed by Br-γ lines (e.g. Muzerolle et al. 2003; Malbet et al. 2007; Tatulli et al. 2007; Goto et al. 2012), H2 (Bergin et al. 2004; France et al. 2012), as well as near-infrared lines of CO, H2O, OH (Salyk et al. 2008; Carr and Najita 2008; Pontoppidan et al. 2010a) and simple organic molecules (Mandell et al. 2012). (2) Moving away from the central star, one finds the “puffed-up” inner dust wall (Natta et al. 2001), clearly seen in the near-infared continuum, where the higher temperature affects its vertical scale height, which is set by hydrostatic equilibrium. Within the central few AU, mid-infrared emission of H2O, CO and the organic molecules HCN and C2H2 have been measured (Lahuis et al. 2006; Carr and Najita 2008). Carr and Najita (2008) note that the HCN/H2O abundance ratio is largest in the most massive disks and speculate that this may be indication of the sequestration of H2O in the outer disk during the process of planetesimal formation. It is interesting to note that toward the disks surrounding the intermediate-mass (≃2<M/M⊙<8) Herbig Ae/Be stars, no organic molecules have been detected (Pontoppidan et al. 2010b; Salyk et al. 2011) and water is only seen in the far-infared at larger radii (≃15–20 AU; Fedele et al. 2012), probably due to the larger UV fluxes compared to T Tauri stars. Beyond the “wall”, the disk is thought to have a layered structure. (3) A photon-dominated region (PDR) is present all around the disk, which is exposed to the stellar and interstellar UV field, as well as the stellar X-rays. Here, forbidden line emission from the well-known PDR coolants, [CII]158 μm, [OI]63 μm and 145 μm, are observed (Sturm et al. 2010; Podio et al. 2012), although the [CII]158 μm and the [OI]145 μm are not always detected (Mathews et al. 2010; Thi et al. 2010a). (4) A warm molecular layer. Just below the PDR zone, molecules survive, although photochemistry is still playing an important role (Henning et al. 2010; Aresu et al. 2012). The gas and dust are warm and radical and ions dominate the gas composition (Semenov 2011). (5) A dark-cloud chemistry zone, where the temperature drops below 20 K, molecular freeze-out becomes important and simple species typically found in dark clouds are detected: CO isotopologues with evidence of depletion (Dutrey et al. 1996, 2007a; Qi et al. 2004), CN, HCN, HNC, CS, HCO+, C2H and H2CO (Dutrey et al. 1997; van Zadelhoff et al. 2001; Thi et al. 2004; Chapillon et al. 2012b), N2H+ (Dutrey et al. 2007b), SO (Fuente et al. 2010), CS (Dutrey et al. 2011), DCO+ (van Dishoeck et al. 2003), H2D+ (Ceccarelli et al. 2004), HDO (Ceccarelli et al. 2005), but see Guilloteau et al. (2006), HC3N (Chapillon et al. 2012a). Qi et al. (2008) spatially resolved the emission of DCO+ and measured the deuterium fraction across the disk of TW Hydrae, finding a range between 0.01 and 0.1, with a peak around 70 AU. They also measured the DCN/HCN abundance ratio, ≃0.02, similar to that measured in the jets of material coming from the nucleus of comet Hale–Bopp (Meier et al. 1998). Öberg et al. (2010a) used the Sub-Millimeter Array (SMA) to image disks of six Taurus sources with spectral type from M1 to A4, finding similar intensities of CN and HCN lines in T Tauri and Herbig Ae stars, but a significantly different chemical richness: deuterated molecules, N2H+ and H2CO were only detected toward T Tauri star disks, implying a lack of long-lived cold regions in the disks of the more massive Herbig Ae stars (see also Öberg et al. 2011a). Water vapor in the cold outer disk has been detected toward TW Hydrae by Hogerheijde et al. (2011) with Herschel, revealing a hidden large reservoir of water ice at large radii (between 100 and 200 AU). Indeed, ice features have been detected in the direction of edge-on protoplanetary disks by Terada et al. (2007) and Honda et al. (2009). More recently, Aikawa et al. (2012) measured with the AKARI satellite several ice features in edge-on Class II disks, including a faint HDO feature, which allowed them to measure a solid HDO/H2O abundance ratio between 2 % and 22 % (significantly larger than the HDO/H2O ratio measured in comets and in star-forming regions; see Sects. 3 and 5). (6) The midplane, characterized by cold and dense regions, with large amounts of molecular freeze-out, where only light species can survive (Öberg et al. 2011b), in analogy with the central ≃1000 AU of prestellar cores (Sect. 4).
Schematic structure of a “naked” protoplanetary disk, adapted from Öberg et al. (2011b), Dullemond and Monnier (2010), Semenov (2011), Dullemond et al. (2007b), and Bergin et al. (2007). The various regions are labeled. The black dots with various sizes represent the coagulated dust in the disk midplane. See text for details
Several chemical models of this protoplanetary-disk phase, with various degrees of complexity, have been developed: X-ray chemistry (Glassgold et al. 1997; Meijerink et al. 2008; Stäuber et al. 2005), surface chemistry (e.g., Willacy and Langer 2000), accretion flows (Aikawa et al. 1999; Ilgner et al. 2004), thermal balance (Gorti and Hollenbach 2004), grain growth (Aikawa and Nomura 2006; Vasyunin et al. 2011), UV continuum and Lyα radiation (Bergin et al. 2003; Fogel et al. 2011), turbulence-driven diffusion (Xie et al. 1995; Willacy et al. 2006), viscous accretion, turbulence mixing and disk winds (Hersant et al. 2009; Heinzeller et al. 2011), photochemistry and wavelength-dependent reaction cross sections (Walsh et al. 2012), comprehensive physical, chemical and radiative transfer modeling (Gorti and Hollenbach 2008; Woitke et al. 2010; Kamp et al. 2011). Despite the advances in chemical complexity, large uncertainties are still present on several reaction rates (Vasyunin et al. 2008) and collisional coefficients, so that laboratory studies and theoretical investigations are still sorely needed to improve the reliability of modern astrochemical models. Moreover, the large uncertainties in the process of dust evolution and coagulation in disks are also shaking our understanding of the disk chemical structure. Laboratory experiments (e.g., Güttler et al. 2010; Schräpler et al. 2012), numerical simulations (e.g., Zsom et al. 2011) and theoretical work (e.g., Dominik et al. 2007; Windmark et al. 2012) are fundamental to progress in this field and an effort has to be made to link dust coagulation models with astrochemistry.
As schematically shown in Fig. 14, in the midplane the dust settles and coagulates with its thick icy mantles and larger grains tend to settle first (e.g., Dullemond et al. 2007a). The differential dust settling and the presence of some degree of turbulence mixing, maintains a population of small dust grains in the upper layers of the disk (see also D’Alessio et al. 1999). This includes polycyclic aromatic hydrocarbons (PAHs), ubiquitous in active star-forming regions (Tielens 2005) and also present in protoplanetary disks, especially around the intermediate-mass Herbig Ae/Be stars (e.g., Habart et al. 2004b; Acke and van den Ancker 2004; Keller et al. 2008; see also Kamp 2011 for a recent review of PAH in disks). PAH features have been detected in only 8 % of the less massive T Tauri stars (Geers et al. 2006). PAHs are not only important from an organic and pre-biotic chemistry point of view, but also for the physical structure of disks, as they can be photoionized, releasing energetic photons which heat the gas, thus maintaining flared disk structures (Kamp 2011). Moreover, PAHs boost the formation of H2 molecules (Habart et al. 2004a), thus the atomic-to-molecular transition in the upper disk atmospheres. Habart et al. (2006) spatially resolved the 3.3 μm PAH feature toward Herbig Ae/Be stars, finding that the emission originates from within 30 AU of the star. In T Tauri stars, the less intense stellar UV field makes the detection of PAH features more difficult (as PAH features are excited by photons). Visser et al. (2007) predict that PAHs in T Tauri disks can survive much closer to the star (down to about 0.01 AU for a 50-carbon atoms PAH) compared to the Herbig disks (down to 5 AU for PAHs with 96 carbon atoms). However, Siebenmorgen and Krügel (2010) include the effects of extreme UV and X-ray components in their models and find very efficient PAH destruction also in T Tauri stars; by taking into account typical X-ray luminosities, Siebenmorgen and Heymann (2012) are able to reproduce the different PAH detection probabilities observed in T Tauri and Herbig Ae disks. Fedele et al. (2008) found PAH emission co-spatial with the [OI]63 μm line, i.e. in the photon-dominated zone of the disk of a Herbig star. As UV photons can break the weaker C-H bonds in PAHs and their carbon skeleton can also brake above a certain threshold of energy intake (Guhathakurta and Draine 1989), the presence of PAHs in the upper atmosphere of disks hints at some replenishing mechanism, possibly vertical mixing (Siebenmorgen and Heymann 2012), which maintains a population of small grains mixed with the gas (Dullemond et al. 2007a). Habart et al. (2004b) suggest that the observed PAHs are evaporated from the icy grain mantles within the disk, while others consider them as the result of fragmentation of larger grains (Rafikov 2006). The mixing of PAHs within the icy mantles of dust grains, could provide an interesting starting point for the formation of more complex molecules, once dust grains start to coagulate and form larger bodies (Bouwman et al. 2011b, 2011a).
6.3 From debris to icy worlds
The transition between protoplanetary disks and planetary system is far from being understood. As Williams and Cieza (2011) pointed out, “exactly how and when protoplanetary disks evolve into planetary debris disks remains an open question”. In protoplanetary disks, there is plenty of evidence of dust grain growth (Beckwith et al. 1990; D’Alessio et al. 2001; Wood et al. 2002; Testi et al. 2003; Wilner et al. 2005; Andrews and Williams 2007; Isella et al. 2006; Cortes et al. 2009; Lommen et al. 2010; Lee et al. 2011), dust settling (Duchêne et al. 2003; Calvet et al. 2005a; D’Alessio et al. 2006; Furlan et al. 2006), dust processing e.g. presence of crystalline silicates (Kessler-Silacci et al. 2005; Natta et al. 2007; Sargent et al. 2009; Merín et al. 2007; Olofsson et al. 2012; Riaz et al. 2012), inner holes (probably carved by a planet or by photoevaporation, Calvet et al. 2005b; Hughes et al. 2009; Andrews et al. 2009; Andrews et al. 2011; Cieza et al. 2012). Debris disks are also observed, with their poor gas content and with evidence of large grains and/or planets (Wyatt 2008; Hughes et al. 2011; Ricci et al. 2012). Despite all these measurements, the story behind grain growth and planetesimal formation remains obscure (see also previous subsection). For example, one of the biggest challenges for planet-formation theories is the so-called “meter-size barrier”, where models show destructive collisions and rapid inward migration of meter-sized solids (Weidenschilling 1977; Williams and Cieza 2011). Nevertheless, the presence of large grains in protoplanetary disks and the structure of our Solar System tell us that dust grains coagulate and evolve toward rocks, comets, asteroids, planetesimals, planets and moons. There are connections between the petrology observed in protoplanetary disks and that in our Solar System bodies. In fact, crystalline grains detected in comets (Wooden et al. 1999, 2004; Zolensky et al. 2008), who also suggest aqueous alterations in the comet P81/Wild2, are mostly made out of Mg-rich olivine grains, consistent with observations of gas-rich T Tauri disks. Fe-rich grains have been observed in several interplanetary dust particles (IDPs, e.g., Brunetto et al. 2011) and recently in warm debris disks (Olofsson et al. 2012). Such Fe-rich grains may be due to a secondary alteration of the disk mineralogy (see also Nguyen et al. 2007), probably originated within large differentiated bodies (as in the case of the S-type asteroid recently studied with the Hayabusa re-entry module; Nakamura et al. 2011). In this scenario, planetesimals form with internal temperatures large enough (from the decay of short-lived radionuclides) to allow the melting and gravitational segregation of silica and metals. Destructive collisions among these planetesimals would then contribute to the production of the Fe-rich particles found in IDPs and in warm debris disks and to the replenishment of small dust grains in our Solar System as well as in exo-zodiacal belts.
Let us now retrace the history of a dust grain during the process of star and planet formation. The starting point has to be found within dense cores, where dust grains have thick icy mantles (see Sect. 4 and Fig. 6) and show some evidence of coagulation (e.g., Keto and Caselli 2010; Pagani et al. 2010), also found soon after protostellar birth, in Class 0 objects (Jørgensen et al. 2007; Kwon et al. 2009; Chiang et al. 2012). As we have seen in previous sections, these dust mantles are rich in water and simple organic material and the chemical complexity in ices appears to increase with dynamical evolution. Figure 15 show a schematic possible scenario of the formation of a debris in the late stages of evolution of protoplanetary disks. (1) Soon after the formation of the protoplanetary disk, dust grains coagulate and become fluffy aggregates of the original icy-dust grains. They may go through shocks during the early “stirring” of the embedded self-gravitating disks (Fig. 13). (2) During the “naked” T Tauri phase, some vertical and radial mixing may expose the fluffy aggregate to stellar and interstellar UV photons and stellar X-rays, so that icy material on the surface can partially be photodesorbed and partially reprocessed, with the production of radicals and formation of an organic residue on the surface (the yellow layer in the figure) and formation of complex organic molecules in the ice trapped within the aggregate. (3) Further processing and coagulation (including some crystalline dust reformed in the inner parts of the disk) could then lead to a cometary-like body, where “dirty ice” (i.e. ice mixed with complex organic molecules) is a major component.
Sketch of a debris disk and a speculative history of a debris: 1. Dust grains with their icy mantles (blue rings) coagulate and form a fluffy structure, on top of which more ice can adsorb/form; 2. Exposure to UV photons and X-rays changes the inner structure of the grain and allows the surface icy mantle to be reprocesses and form a refractory carbonaceous material; 3. Further coagulation will lead to small rocks, composed by a mixture of the fluffy grains in 1 & 2 with their refractory organic material and “dirty” ice, glued together by a mixture of amorphous and crystalline dust material
We are now ready to attempt assembling some pieces of the puzzle.
7 Putting together some pieces of the puzzle
7.1 Molecules in comets and solar-type protostars
All molecules detected in comets are also observed in star-forming regions. However, the measured abundances in comets and Sun-like star-formation regions are not the same. This is clearly shown in Fig. 16, which reports the abundances, normalized to the methanol abundance, of species detected in various comets (see the reviews Mumma and Charnley 2011 and Bockelée-Morvan 2011, and Sect. 3.2) and those in the hot corino and cold envelope of IRAS16293-2422 (see Sect. 5.1). A similar plot is obtained also if the normalisation is done with respect to water rather than methanol. In general, species are more abundant with respect to methanol (and water) in IRAS16293-2422, both in the cold envelope and the hot corino, than in comets by more than a factor of ten. In other words, the chemistry in comets seems to be less rich than in both the cold envelope and the hot corino of IRAS16293. It is, therefore, probably fortuitous the rough correlation found in the abundance of a fewer molecules in comets and hot cores (e.g., Bockelée-Morvan 2011).
Abundances (with respect to CH3OH) of molecules detected in comets (blue) and in the hot corino (red) and cold envelope (green) of IRAS16293-2422. References: for comets (Mumma and Charnley 2011) and (Bockelée-Morvan 2011); for IRAS16293-2422 (Ceccarelli et al. 2000b; Schöier et al. 2002) and (Cazaux et al. 2003)
Where does this difference come from? The molecules in the cold envelope of IRAS16293-2422 are likely the product of gas-phase chemistry (but see the comments in Sect. 4) in cold gas, where CO is largely frozen into the grain mantles. Therefore, the systematic difference between the molecular abundances in comets and the cold envelope may point to different physical conditions, likely warmer, at the time of the comet formation. Similarly, the molecules in the hot corino are thought to mostly reflect the composition of the grain mantles during the pre-collapse phase, so that the difference in this case also suggests warmer conditions of the material when the cometary ices were formed. There are, however, also other possibilities. It is possible that the cometary ices have undergone a massive reprocessing of the molecular composition due to the long irradiation from cosmic rays and solar wind particles and UV irradiation. Or it is possible that our Sun’s progenitor, in fact, did not resemble the IRAS16293-2422 protostar, which is rather isolated, whereas the proto-Sun likely was born in a crowded and much harsher environment (Sect. 3.5). Our census of the molecular composition in comets and in protostellar objects thought to be similar to the proto-Sun is still too poor to have a definitive answer.
7.2 Origin of deuterated molecules in comets and chondrites
For a long period it has been though that there is a link between the chemistry in comets, chondrites and interstellar medium, especially because of the enhanced abundance of deuterated molecules (Fig. 17). It is possible that the link is not direct, meaning that it may not be due to the passage of the molecular deuteration from one phase to the next, during the formation of the Solar System. However, the link certainly exists because the chemistry regulating the molecular deuteration is common to all phases and it has to do with the low temperatures occurring during the star and planet formation.
Two key parameters play a major role in the molecular deuteration, regardless of the details which depend on the specific molecule: the ratios of H2D+/\(\mathrm{H}^{+}_{3}\) and of the atomic D/H in the gas (Sects. 4.1 and 5.5). Figure 18 shows how the H2D+/\(\mathrm{H}^{+}_{3}\) ratio depends on the gas temperature. Another important parameter for this ratio is the abundance of gaseous CO, as CO is a major destroyer of molecular ions, being the most abundant heavy-atom-bearing neutral molecule. In cold and dense regions, CO may freeze-out onto the grain mantles and disappear, therefore, from the gas phase (Sect. 4.1). Figure 18 also shows the dependence of the H2D+/\(\mathrm{H}^{+}_{3}\) and the other isotopologues of \(\mathrm{H}^{+}_{3}\) as a function of the CO depletion, namely how much the CO abundance is reduced with respect to the standard molecular cloud value. In general, molecular deuteration exceeding 10 % requires not only cold gas but also a substantial drop of the CO abundance in the gas phase.
Theoretical abundances of the \(\mathrm{H}^{+}_{3}\) isotopologues in the gas. The left panel (a) reports the plot of the ratio \(\mathrm{H}_{2}\mathrm{D}^{+}/\mathrm{H}^{+}_{3}\) as function of the gas temperature, for gas with no CO depletion. The right panel (b) shows the abundance ratio of all the \(\mathrm{H}^{+}_{3}\) isotopologues as a function of CO depletion, for a gas with temperature 10 K. In both cases, the gas density is assumed 105 cm−3 (adapted from Ceccarelli et al. 2005)
Similarly, Fig. 19 shows how the gaseous D/H ratio, which governs the molecular deuteration of grain-surface product molecules (Sect. 4.2), varies with the CO depletion in different situations. Also in this case, large (≥10 %) molecular deuteration can only be achieved in cold gas deprived of CO.
Theoretical abundances of the HDO/H2O (blue curves) and D/H (orange curves) as a function of the CO depletion, for three different densities and incident visual extinction. Dotted lines: 104 cm−3 and 2 mag; dashed lines: 104 cm−3 and 4 mag; solid lines: 105 cm−3 and 10 mag. Courtesy of Taquet et al. (2012b)
Therefore, the relatively low water deuteration measured on Earth, in comets and in chondrites with respect to the values measured in the prestellar and protostellar phases suggest substantial remixing of water ices since their first formation. How much of the first ices remains in the terrestrial, cometary and chondritic water is difficult to say but cannot be substantial. On the contrary, the extremely large deuteration found in the chondritic organic material, both soluble and insoluble, testifies either the preservation of molecular species since the very first stages (where not only the temperature was very low but also the gas was deprived of CO, Sect. 4.1), or the presence of similar conditions in some zones of the Solar System up to a late stage of the protoplanetary-disk phase.
7.3 The 15N enrichment in comets and chondrites
In our Solar System, the 15N enhancement spans a large range of values. As seen in Sect. 3.4, 14N/15N is around 150 in comets, <300 in “primitive” material (such as IDPs and carbonaceous chondrites), <100 in interplanetary dust particles (IDPs) and carbonaceous chondrites “hotspots”, where the largest D- and 15N fractions have been measured (e.g., Messenger 2000), ∼450 in Jupiter’s atmosphere (representative of the protosolar value), 272 on Earth. In prestellar cores (Sect. 4), current data show a differential 15N fractionation between amine- and nitrile-bearing species, with the largest 15N enhancement found in the latter (between 70 and 380 in HCN and HNC, Bonal et al. 2012), while no significant enhancement is found in NH3 and N2H+, both highly D fractionated. The situation is summarised in Fig. 17. Thus, D and 15N fractionations do not go hand in hand in all species within prestellar cores, resembling the mixed level of correlation between D and 15N enrichments in primitive material of our Solar System. Moreover, the cometary 15N enhancement has been measured in HCN and CN, again suggesting a prestellar core origin. It will be interesting to find out if amines will ever experience significant 15N at all during the pre- and/or proto- stellar phase. Wirström et al. (2012) predict large 15N fractionation in NH3 at late times (>a few million years), so maybe only relatively long-lived dense cores will have the chance to have both amines and nitriles highly 15N fractionated. However, this has all to be tested with observations, which should be extended also to the starless cores found in massive star-forming regions (e.g., Wienen et al. 2012) to check if environmental conditions affect the fractionation process. As 15N enhancement has been measured in IOMs and amino acids trapped in carbonaceous chondrites (Sect. 3.4), amines in prestellar cores should also be able to experience significant enhancement, if a link between these two extreme stages has to be found (Bonal et al. 2012). However, we do not know if further processes within icy mantles or within the “rocks” made out of coagulated icy-dust grains, during the proto-Sun stage, would affect the 15N fractionation in IOM and amino acids found in carbonaceous chondrites. Experiments are needed here.
8 Concluding remarks
Our chemical heritage is hidden in the large amount of information obtained by observations of Solar System bodies and star and planet-forming regions, and it needs to be deciphered. In this review, glimpses of links between the present star and planet formation in our Galaxy and the remote past of our Solar System have been given. A summary of these glimpses is given here, together with comments/open questions and suggestions for future developments:
-
Water on Earth. The total amount of water on Earth is ≥2×10−3 Earth masses, a small fraction of the total amount of water vapor measured in a prestellar core (≃800 Earth masses) and in a protoplanetary disk (≃1.5 Earth masses), and a negligible factor of the deduced water ice mass in the prestellar core and protoplanetary disk (at least three orders of magnitude larger than the water vapor mass). Thus, a large water reservoir was originally available to seed a large number of Solar System bodies, as in fact observed in moons, comets, KBOs and asteroids. Tracing the formation, storage and delivery of water will require more observations as well as a better understanding of the icy-dust coagulation process during the prestellar and protoplanetary-disk phases.
-
Complex organic molecules, COMs. Organic material in meteorites and IDPs is organized in aromatic and aliphatic compounds, carboxylic acids and amino acids, including those found in all living beings on Earth. PAHs, hydrocarbons and complex organic molecules observed in star-forming regions are a simpler version (building blocks) of the organic material found in Solar System bodies. Thus, interstellar COMs may have contributed to the formation of organic matter during the processing of coagulated icy-dust particles once the proto-Sun was born. More experiments are needed to confirm this statement. Moreover, COMs observed in comets have abundances relative to methanol more than a factor of ten lower than those measured in hot corinos, possibly suggesting that the hot corino conditions and chemical history may not be representative of our Solar System. It is also possible that the difference arises because of a substantial reprocessing of the protostellar material during the protoplanetary disk phase. Finally, the chemical composition of the envelopes of solar-type protostars in crowded environments populated by massive stars, as suggested in the case of the proto-Sun, has not so far been studied, due to the sensitivity of the available instrumentation. The advent of ALMA should clarify this aspect in the near future.
-
D fractionation in water. The HDO/H2O abundance ratio measured in comets is between 1 and 2 times that measured in our oceans (1.5×10−4), whereas in cold envelopes and hot corinos a larger spread of this ratio has been found: from ≤6×10−4 to 0.2. Is the D fraction in water set at the beginning of the star-formation process or is it modified during the various phases of star and planet formation? Or does the water D enrichment observed in hot corinos probe only the outer layers of the ices, those that sublimate first, while the bulk of the ice, less deuterated as probably inherited in the previous phases, remains frozen and hidden? Is this the ice that we observe in comets? And which process forms water in comets if it is not inherited? Is it surface chemistry, like in prestellar objects, or gas-phase chemistry? In order to answer all these questions, we will need to measure the HDO/H2O in different stages of the protostellar evolution and use observations to constrain detailed chemical models, where gas-phase and surface processes are linked, spanning a broad range of physical conditions.
-
D fractionation in other molecules. The D fractionation is an active process in the cold gas of molecular clouds and becomes one of the dominant chemical processes within prestellar cores, in regions where abundant neutral species (mainly CO and O) freeze-out onto dust grains. Here, the increase of the D/H elemental ratio in the gas phase is thought to be responsible for the efficient deuteration of methanol, which happens on the surface of dust grains (as no gas-phase routes are available). Organic molecules such as methanol and formaldehyde observed in star-forming regions (in particular toward low-mass protostellar envelopes) display a D fraction orders of magnitude higher than that measured in water. This differential D fractionation of water and organics is also measured in comets, where DCN/HCN ∼10 times HDO/H2O. Similarly, in the hot spots of carbonaceous chondrites and IDPs, the D/H associated with organic radicals reaches values as high as 1 %, suggesting, in this case, a direct link with the pre- and protostellar phases of the Solar System’s formation.
-
15N fractionation. 15N enrichments in HCN and HNC are measured in comets, with values similar to those observed in prestellar cores and Galactic star-forming regions. No significant 15N fractionation is measured in NH3 and N2H+, which are, on the other hand, highly deuterated during the prestellar core phase. Thus, no significant correlation is expected between D- and 15N-fractionated material in Solar System bodies, as in fact measured. More observations of 15N isotopologues of NH3 and N2H+ in a larger sample of prestellar cores (also including massive prestellar cores) are needed to look for possible fractionations of these species and to understand if different environmental conditions affect the fractionation process.
To further advance in this field, different communities need to join the effort and work together. In particular, the star-formation and Solar System communities should continuously exchange new results and information on new measurements, experiments and theoretical developments. At the same time, laboratory work on molecular spectroscopy to identify the observed lines, on rate coefficients to understand chemical pathways, on surface chemistry and dust coagulation to understand ice formation and ice/dust evolution, as well as calculations of collisional coefficients required for radiative transfer studies are all necessary for a correct interpretation of observations. Finally, theoretical and observational astrophysicists and astrochemists should work together to make sure that, on the one hand, the best physical and dynamical model is used as input for astrochemical modeling and, on the other hand, the best physical parameters derived from the combination of observations, astrochemistry and radiative transfer, are used as input in the physical/dynamical models of star and planet-forming regions. To understand our origins, we cannot work alone!
Notes
The famous fossils of cyanobacteria of Australia and for long considered as the first traces of life dated 3.5 Myr (Schopf et al. 2002), are interpreted as inorganic condensations (Skrzypczak et al. 2003; García et al. 2002) and still source of intense debate (Marshall et al. 2011). Conversely, there is consensus on the rise of life about 2 Gyr after the Earth formation, as testified by the rise in the O2 abundance in the atmosphere (Czaja 2010).
Short-lived nuclides are the radionuclides with half-lives shorter than about 10 Myr.
The Atacama Large Millimeter/sub-millimeter Array.
IMHD assumes that the mass to magnetic-flux ratio is constant, which implies that magnetic field lines follow the gas motions, i.e. the magnetic field is “frozen” into the neutral medium.
The Hall effect mainly operates at volume densities between 108 and 1011 cm−3 (Wardle 2004), where the more massive charged particles (ions and charged dust grains) decouple from the magnetic field and collisionally couple with the neutral gas.
The radius at which the gravitational force is balanced by the centrifugal force.
References
Acke B, van den Ancker ME (2004) ISO spectroscopy of disks around Herbig Ae/Be stars. Astron Astrophys 426:151–170. doi:10.1051/0004-6361:20040400
Adams FC (2010) The birth environment of the solar system. Annu Rev Astron Astrophys 48:47–85. doi:10.1146/annurev-astro-081309-130830
Adande GR, Ziurys LM (2012) Millimeter-wave observations of CN and HNC and their 15N isotopologues: a new evaluation of the 14N/15N ratio across the galaxy. Astrophys J 744:194. doi:10.1088/0004-637X/744/2/194
Aikawa Y, Nomura H (2006) Physical and chemical structure of protoplanetary disks with grain growth. Astrophys J 642:1152–1162. doi:10.1086/501114
Aikawa Y, Umebayashi T, Nakano T, Miyama SM (1999) Evolution of molecular abundances in protoplanetary disks with accretion flow. Astrophys J 519:705–725. doi:10.1086/307400
Aikawa Y, Wakelam V, Garrod RT, Herbst E (2008) Molecular evolution and star formation: from prestellar cores to protostellar cores. Astrophys J 674:984–996. doi:10.1086/524096
Aikawa Y, Kamuro D, Sakon I, Itoh Y, Terada H, Noble JA, Pontoppidan KM, Fraser HJ, Tamura M, Kandori R, Kawamura A, Ueno M (2012) AKARI observations of ice absorption bands towards edge-on young stellar objects. Astron Astrophys 538:A57. doi:10.1051/0004-6361/201015999
Aléon J (2010) Multiple origins of nitrogen isotopic anomalies in meteorites and comets. Astrophys J 722:1342–1351. doi:10.1088/0004-637X/722/2/1342
Alexander CMO, Fogel M, Yabuta H, Cody GD (2007) The origin and evolution of chondrites recorded in the elemental and isotopic compositions of their macromolecular organic matter. Geochim Cosmochim Acta 71:4380–4403. doi:10.1016/j.gca.2007.06.052
Allegre CJ, Staudacher T, Sarda P, Kurz M (1983) Constraints on evolution of earth’s mantle from rare gas systematics. Nature 303:762–766. doi:10.1038/303762a0
Allen A, Li ZY, Shu FH (2003) Collapse of magnetized singular isothermal toroids. II. Rotation and magnetic braking. Astrophys J 599:363–379. doi:10.1086/379243
Andrews SM, Williams JP (2007) High-Resolution submillimeter constraints on circumstellar disk structure. Astrophys J 659:705–728. doi:10.1086/511741
Andrews SM, Wilner DJ, Hughes AM, Qi C, Dullemond CP (2009) Protoplanetary disk structures in Ophiuchus. Astrophys J 700:1502–1523. doi:10.1088/0004-637X/700/2/1502
Andrews SM, Wilner DJ, Espaillat C, Hughes AM, Dullemond CP, McClure MK, Qi C, Brown JM (2011) Resolved images of large cavities in protoplanetary transition disks. Astrophys J 732:42. doi:10.1088/0004-637X/732/1/42
Arce HG, Santiago-García J, Jørgensen JK, Tafalla M, Bachiller R (2008) Complex molecules in the L1157 molecular outflow. Astrophys J Lett 681:L21–L24. doi:10.1086/590110
Arce HG, Borkin MA, Goodman AA, Pineda JE, Beaumont CN (2011) A bubbling nearby molecular cloud: COMPLETE shells in Perseus. Astrophys J 742:105. doi:10.1088/0004-637X/742/2/105
Aresu G, Meijerink R, Kamp I, Spaans M, Thi WF, Woitke P (2012) FUV and X-ray irradiated protoplanetary disks: a grid of models II—gas diagnostic line emission. ArXiv e-prints
Armitage PJ (2011) Dynamics of protoplanetary disks. Annu Rev Astron Astrophys 49:195–236. doi:10.1146/annurev-astro-081710-102521
Arpigny C, Jehin E, Manfroid J, Hutsemékers D, Schulz R, Stüwe JA, Zucconi JM, Ilyin I (2003) Anomalous nitrogen isotope ratio in comets. Science 301:1522–1525. doi:10.1126/science.1086711
Arquilla R, Goldsmith PF (1986) A detailed examination of the kinematics of rotating dark clouds. Astrophys J 303:356–374. doi:10.1086/164082
Awad Z, Viti S, Collings MP, Williams DA (2010) Warm cores around regions of low-mass star formation. Mon Not R Astron Soc 407:2511–2518. doi:10.1111/j.1365-2966.2010.17077.x
Bachiller R, Perez Gutierrez M (1997) Shock chemistry in the young bipolar outflow L1157. Astrophys J Lett 487:L93. doi:10.1086/310877
Bacmann A, Lefloch B, Ceccarelli C, Castets A, Steinacker J, Loinard L (2002) The degree of CO depletion in pre-stellar cores. Astron Astrophys 389:L6–L10. doi:10.1051/0004-6361:20020652
Bacmann A, Lefloch B, Ceccarelli C, Steinacker J, Castets A, Loinard L (2003) CO depletion and deuterium fractionation in prestellar cores. Astrophys J Lett 585:L55–L58. doi:10.1086/374263
Bacmann A, Taquet V, Faure A, Kahane C, Ceccarelli C (2012) Detection of complex organic molecules in a prestellar core: a new challenge for astrochemical models. Astron Astrophys 541:L12. doi:10.1051/0004-6361/201219207
Barucci MA, Belskaya IN, Fulchignoni M, Birlan M (2005) Taxonomy of centaurs and Trans-Neptunian objects. Astron J 130:1291–1298. doi:10.1086/431957
Barucci MA, Dotto E, Levasseur-Regourd AC (2011) Space missions to small bodies: asteroids and cometary nuclei. Astron Astrophys Rev 19:48. doi:10.1007/s00159-011-0048-2
Basu S, Mouschovias TC (1994) Magnetic braking, ambipolar diffusion, and the formation of cloud cores and protostars. 1. Axisymmetric solutions. Astrophys J 432:720–741. doi:10.1086/174611
Beckwith SVW, Sargent AI, Chini RS, Guesten R (1990) A survey for circumstellar disks around young stellar objects. Astron J 99:924–945. doi:10.1086/115385
Benedettini M, Giannini T, Nisini B, Tommasi E, Lorenzetti D, Di Giorgio AM, Saraceno P, Smith HA, White GJ (2000) The ISO spectroscopic view of the HH 24-26 region. Astron Astrophys 359:148–158
Benedettini M, Viti S, Giannini T, Nisini B, Goldsmith PF, Saraceno P (2002) Comparing SWAS and ISO observations of water in outflows. Astron Astrophys 395:657–662. doi:10.1051/0004-6361:20021303
Benedettini M, Busquet G, Lefloch B, Codella C, Cabrit S, Ceccarelli C, Giannini T, Nisini B, Vasta M, Cernicharo J, Lorenzani A, di Giorgio AM (2012) The CHESS survey of the L1157-B1 shock: the dissociative jet shock as revealed by Herschel-PACS. Astron Astrophys 539:L3. doi:10.1051/0004-6361/201118732
Bennett CJ, Kaiser RI (2007) On the formation of glycolaldehyde (HCOCH2OH) and methyl formate (HCOOCH3) in interstellar ice analogs. Astrophys J 661:899–909. doi:10.1086/516745
Bennett CJ, Jamieson CS, Osamura Y, Kaiser RI (2006) Laboratory studies on the irradiation of methane in interstellar, cometary, and solar system ices. Astrophys J 653:792–811. doi:10.1086/508561
Bergin EA, Tafalla M (2007) Cold dark clouds: the initial conditions for star formation. Annu Rev Astron Astrophys 45:339–396. doi:10.1146/annurev.astro.45.071206.100404
Bergin EA, Plume R, Williams JP, Myers PC (1999) The ionization fraction in dense molecular gas. II. Massive cores. Astrophys J 512:724–739. doi:10.1086/306791
Bergin EA, Alves J, Huard T, Lada CJ (2002) N2H+ and C18O depletion in a cold dark cloud. Astrophys J Lett 570:L101–L104. doi:10.1086/340950
Bergin E, Calvet N, D’Alessio P, Herczeg GJ (2003) The effects of UV continuum and Lyα radiation on the chemical equilibrium of T Tauri disks. Astrophys J Lett 591:L159–L162. doi:10.1086/377148
Bergin E, Calvet N, Sitko ML, Abgrall H, D’Alessio P, Herczeg GJ, Roueff E, Qi C, Lynch DK, Russell RW, Brafford SM, Perry RB (2004) A new probe of the planet-forming region in T Tauri disks. Astrophys J Lett 614:L133–L136. doi:10.1086/425865
Bergin EA, Aikawa Y, Blake GA, van Dishoeck EF (2007) The chemical evolution of protoplanetary disks. In: Protostars and planets V, pp 751–766
Bergman P, Parise B, Liseau R, Larsson B (2011) Deuterated formaldehyde in ρ Ophiuchi A. Astron Astrophys 527:A39. doi:10.1051/0004-6361/201015012
Bernstein MP, Dworkin JP, Sandford SA, Cooper GW, Allamandola LJ (2002) Racemic amino acids from the ultraviolet photolysis of interstellar ice analogues. Nature 416:401–403
Biver N, Bockelée-Morvan D, Crovisier J, Colom P, Henry F, Moreno R, Paubert G, Despois D, Lis DC (2002) Chemical composition diversity among 24 comets observed at radio wavelengths. Earth Moon Planets 90:323–333
Bizzocchi L, Caselli P, Dore L (2010) Detection of N15NH+ in L1544. Astron Astrophys 510:L5. doi:10.1051/0004-6361/200913835
Bjerkeli P, Liseau R, Olberg M, Falgarone E, Frisk U, Hjalmarson Å, Klotz A, Larsson B, Olofsson AOH, Olofsson G, Ristorcelli I, Sandqvist A (2009) Odin observations of water in molecular outflows and shocks. Astron Astrophys 507:1455–1466. doi:10.1051/0004-6361/200912064
Bjerkeli P, Liseau R, Nisini B, Tafalla M, Benedettini M, Bergman P, Dionatos O, Giannini T, Herczeg G, Justtanont K, Larsson B, McOey C, Olberg M, Olofsson AOH (2011) Herschel observations of the Herbig–Haro objects HH 52-54. Astron Astrophys 533:A80. doi:10.1051/0004-6361/201116846
Bjerkeli P, Liseau R, Larsson B, Rydbeck G, Nisini B, Tafalla M, Antoniucci S, Benedettini M, Bergman P, Cabrit S, Giannini T, Melnick G, Neufeld D, Santangelo G, van Dishoeck EF (2012) H2O line mapping at high spatial and spectral resolution. Herschel observations of the VLA 1623 outflow. Astron Astrophys 546:A29. doi:10.1051/0004-6361/201219776
Blake GA, Sutton EC, Masson CR, Phillips TG (1987) Molecular abundances in OMC-1—the chemical composition of interstellar molecular clouds and the influence of massive star formation. Astrophys J 315:621–645. doi:10.1086/165165
Bockelée-Morvan D (2011) An overview of comet composition. In: IAU symposium, vol 280, pp 261–274. doi:10.1017/S1743921311025038
Bockelee-Morvan D, Gautier D, Lis DC, Young K, Keene J, Phillips T, Owen T, Crovisier J, Goldsmith PF, Bergin EA, Despois D, Wootten A (1998) Deuterated water in comet C/1996 B2 (Hyakutake) and its implications for the origin of comets. Icarus 133:147–162. doi:10.1006/icar.1998.5916
Bockelée-Morvan D, Biver N, Jehin E, Cochran AL, Wiesemeyer H, Manfroid J, Hutsemékers D, Arpigny C, Boissier J, Cochran W, Colom P, Crovisier J, Milutinovic N, Moreno R, Prochaska JX, Ramirez I, Schulz R, Zucconi JM (2008) Large excess of heavy nitrogen in both hydrogen cyanide and cyanogen from comet 17P/Holmes. Astrophys J Lett 679:L49–L52. doi:10.1086/588781
Bockelée-Morvan D, Biver N, de Swinyard B, Val-Borro M, Crovisier J, Hartogh P, Lis DC, Moreno R, Szutowicz S, Lellouch E, Emprechtinger M, Blake GA, Courtin R, Jarchow C, Kidger M, Küppers M, Rengel M, Davis GR, Fulton T, Naylor D, Sidher S (2012) Herschel measurements of the D/H and 16O/18O ratios in water in the Oort-cloud comet C/2009 P1 (Garrard). Astron Astrophys 544:L15. doi:10.1051/0004-6361/201219744
Boduch P, Domaracka A, Fulvio D, Langlinay T, Lv XY, Palumbo ME, Rothard H, Strazzulla G (2012) Chemistry induced by energetic ions in water ice mixed with molecular nitrogen and oxygen. Astron Astrophys 544:A30. doi:10.1051/0004-6361/201219365
Boley AC (2009) The two modes of gas giant planet formation. Astrophys J Lett 695:L53–L57. doi:10.1088/0004-637X/695/1/L53
Boley AC, Durisen RH (2008) Gravitational instabilities, chondrule formation, and the FU Orionis phenomenon. Astrophys J 685:1193–1209. doi:10.1086/591013
Bonal L, Huss GR, Nagashima K, Krot AN (2009) Hydrogen isotopic composition of 15N-rich clasts in the CB/CH-like chondrite Isheyevo. Meteorit Planet Sci 72:5178
Bonal L, Huss GR, Krot AN, Nagashima K, Ishii HA, Bradley JP (2010) Highly 15N-enriched chondritic clasts in the CB/CH-like meteorite Isheyevo. Geochim Cosmochim Acta 74:6590–6609. doi:10.1016/j.gca.2010.08.017
Bonal L, Hily-Blant P, Faure A, Quirico E (2012) Highly variable 15N-Enrichments in solar system reflect different routes of interstellar N isotopic fractionation. Meteorit Planet Sci 75:5226
Boss AP (1997) Giant planet formation by gravitational instability. Science 276:1836–1839. doi:10.1126/science.276.5320.1836
Bottinelli S, Ceccarelli C, Lefloch B, Williams JP, Castets A, Caux E, Cazaux S, Maret S, Parise B, Tielens AGGM (2004a) Complex molecules in the hot core of the Low-Mass protostar NGC 1333 IRAS 4A. Astrophys J 615:354–358. doi:10.1086/423952
Bottinelli S, Ceccarelli C, Neri R, Williams JP, Caux E, Cazaux S, Lefloch B, Maret S, Tielens AGGM (2004b) Near-Arcsecond resolution observations of the hot corino of the solar-type protostar IRAS 16293-2422. Astrophys J Lett 617:L69–L72. doi:10.1086/426964
Bottinelli S, Ceccarelli C, Williams JP, Lefloch B (2007) Hot corinos in NGC 1333-IRAS4B and IRAS2A. Astron Astrophys 463:601–610. doi:10.1051/0004-6361:20066242
Bouwman J, Cuppen HM, Steglich M, Allamandola LJ, Linnartz H (2011a) Photochemistry of polycyclic aromatic hydrocarbons in cosmic water ice. II. Near UV/VIS spectroscopy and ionization rates. Astron Astrophys 529:A46. doi:10.1051/0004-6361/201015762
Bouwman J, Mattioda AL, Linnartz H, Allamandola LJ (2011b) Photochemistry of polycyclic aromatic hydrocarbons in cosmic water ice. I. Mid-IR spectroscopy and photoproducts. Astron Astrophys 525:A93. doi:10.1051/0004-6361/201015059
Braiding CR, Wardle M (2012) The hall effect in star formation. Mon Not R Astron Soc 422:261–281. doi:10.1111/j.1365-2966.2012.20601.x
Brasser R (2008) A two-stage formation process for the Oort comet cloud and its implications. Astron Astrophys 492:251–255. doi:10.1051/0004-6361:200810452
Broten NW, MacLeod JM, Avery LW, Irvine WM, Hoglund B, Friberg P, Hjalmarson A (1984) The detection of interstellar methylcyanoacetylene. Astrophys J Lett 276:L25–L29. doi:10.1086/184181
Brown ME (2012) The compositions of Kuiper Belt objects. Annu Rev Earth Planet Sci 40:467–494. doi:10.1146/annurev-earth-042711-105352
Brown DW, Chandler CJ, Carlstrom JE, Hills RE, Lay OP, Matthews BC, Richer JS, Wilson CD (2000) A submillimetre survey for protostellar accretion discs using the JCMT-CSO interferometer. Mon Not R Astron Soc 319:154–162. doi:10.1046/j.1365-8711.2000.03805.x
Brown ME, Schaller EL, Fraser WC (2012) Water ice in the Kuiper Belt. Astron J 143:146. doi:10.1088/0004-6256/143/6/146
Brunetto R, Borg J, Dartois E, Rietmeijer FJM, Grossemy F, Sandt C, Le Sergeant D’Hendecourt L, Rotundi A, Dumas P, Djouadi Z, Jamme F (2011) Mid-IR, Far-IR, Raman micro-spectroscopy, and FESEM-EDX study of IDP L2021C5: clues to its origin. Icarus 212:896–910. doi:10.1016/j.icarus.2011.01.038
Brünken S, Gupta H, Gottlieb CA, McCarthy MC, Thaddeus P (2007) Detection of the carbon chain negative ion C8H− in TMC-1. Astrophys J Lett 664:L43–L46. doi:10.1086/520703
Busemann H, Alexander CMO, Nittler LR, Zega TJ, Stroud RM, Bajt S, Cody GD, Yabuta H (2006) Correlated analyses of D- and 15N-rich carbon grains from CR2 chondrite EET 92042. In: Proceedings of 69th annual meeting of the meteoritical society, Zurich, Switzerland, August 6–11, 2006. Meteoritics & planetary science, vol 41, p 5327. abs/2006M%26PSA..41.5327B
Butner HM, Charnley SB, Ceccarelli C, Rodgers SD, Pardo JR, Parise B, Cernicharo J, Davis GR (2007) Discovery of interstellar heavy water. Astrophys J Lett 659:L137–L140. doi:10.1086/517883
Calvet N, Briceño C, Hernández J, Hoyer S, Hartmann L, Sicilia-Aguilar A, Megeath ST, D’Alessio P (2005a) Disk evolution in the orion OB1 association. Astron J 129:935–946. doi:10.1086/426910
Calvet N, D’Alessio P, Watson DM, Franco-Hernández R, Furlan E, Green J, Sutter PM, Forrest WJ, Hartmann L, Uchida KI, Keller LD, Sargent B, Najita J, Herter TL, Barry DJ, Hall P (2005b) Disks in transition in the Taurus population: Spitzer IRS spectra of GM Aurigae and DM Tauri. Astrophys J Lett 630:L185–L188. doi:10.1086/491652
Cameron AGW, Truran JW (1977) The supernova trigger for formation of the solar system. Icarus 30:447–461. doi:10.1016/0019-1035(77)90101-4
Carr JS, Najita JR (2008) Organic molecules and water in the planet formation region of young circumstellar disks. Science 319:1504. doi:10.1126/science.1153807
Caselli P, Walmsley CM, Terzieva R, Herbst E (1998) The ionization fraction in dense cloud cores. Astrophys J 499:234. doi:10.1086/305624
Caselli P, Walmsley CM, Tafalla M, Dore L, Myers PC (1999) CO depletion in the starless cloud core L1544. Astrophys J Lett 523:L165–L169. doi:10.1086/312280
Caselli P, Benson PJ, Myers PC, Tafalla M (2002) Dense cores in dark clouds. XIV. N2H+ (1-0) maps of dense cloud cores. Astrophys J 572:238–263. doi:10.1086/340195
Caselli P, Stantcheva T, Shalabiea O, Shematovich VI, Herbst E (2002a) Deuterium fractionation on interstellar grains studied with modified rate equations and a Monte Carlo approach. Planet Space Sci 50:1257–1266. doi:10.1016/S0032-0633(02)00092-2
Caselli P, Walmsley CM, Zucconi A, Tafalla M, Dore L, Myers PC (2002b) Molecular ions in L1544. II. The ionization degree. Astrophys J 565:344–358. doi:10.1086/324302
Caselli P, van der Tak FFS, Ceccarelli C, Bacmann A (2003) Abundant H2D+ in the pre-stellar core L1544. Astron Astrophys 403:L37–L41. doi:10.1051/0004-6361:20030526
Caselli P, Keto E, Bergin EA, Tafalla M, Aikawa Y, Douglas T, Pagani L, Yildiz UA, van der Tak FFS, Walmsley CM, Codella C, Nisini B, Kristensen LE, van Dishoeck EF (2012) First detection of water vapor in a pre-stellar core. ArXiv e-prints
Caux E, Kahane C, Castets A, Coutens A, Ceccarelli C, Bacmann A, Bisschop S, Bottinelli S, Comito C, Helmich FP, Lefloch B, Parise B, Schilke P, Tielens AGGM, van Dishoeck E, Vastel C, Wakelam V, Walters A (2011) TIMASSS: the IRAS 16293-2422 millimeter and submillimeter spectral survey. I. Observations, calibration, and analysis of the line kinematics. Astron Astrophys 532:A23. doi:10.1051/0004-6361/201015399
Cazaux S, Tielens AGGM (2002) Molecular hydrogen formation in the interstellar medium. Astrophys J Lett 575:L29–L32. doi:10.1086/342607
Cazaux S, Tielens AGGM, Ceccarelli C, Castets A, Wakelam V, Caux E, Parise B, Teyssier D (2003) The hot core around the Low-mass protostar IRAS 16293-2422: scoundrels rule! Astrophys J Lett 593:L51–L55. doi:10.1086/378038
Cazaux S, Cobut V, Marseille M, Spaans M, Caselli P (2010) Water formation on bare grains: when the chemistry on dust impacts interstellar gas. Astron Astrophys 522:A74. doi:10.1051/0004-6361/201014026
Cazaux S, Caselli P, Spaans M (2011) Interstellar ices as witnesses of star formation: selective deuteration of water and organic molecules unveiled. Astrophys J Lett 741:L34. doi:10.1088/2041-8205/741/2/L34
Ceccarelli C, Hollenbach DJ, Tielens AGGM (1996) Far-Infrared line emission from collapsing protostellar envelopes. Astrophys J 471:400. doi:10.1086/177978
Ceccarelli C, Castets A, Loinard L, Caux E, Tielens AGGM (1998) Detection of doubly deuterated formaldehyde towards the low-luminosity protostar IRAS 16293-2422. Astron Astrophys 338:L43–L46
Ceccarelli C, Castets A, Caux E, Hollenbach D, Loinard L, Molinari S, Tielens AGGM (2000a) The structure of the collapsing envelope around the low-mass protostar IRAS 16293-2422. Astron Astrophys 355:1129–1137
Ceccarelli C, Loinard L, Castets A, Tielens AGGM, Caux E (2000b) The hot core of the solar-type protostar IRAS 16293-2422: H2CO emission. Astron Astrophys 357:L9–L12
Ceccarelli C, Dominik C, Lefloch B, Caselli P, Caux E (2004) Detection of H2D+: measuring the midplane degree of ionization in the disks of DM Tauri and TW Hydrae. Astrophys J Lett 607:L51–L54. doi:10.1086/421461
Ceccarelli C, Dominik C, Caux E, Lefloch B, Caselli P (2005) Discovery of deuterated water in a young protoplanetary disk. Astrophys J Lett 631:L81–L84. doi:10.1086/497028
Ceccarelli C, Caselli P, Herbst E, Tielens AGGM, Caux E (2007) Extreme deuteration and hot corinos: the earliest chemical signatures of Low-Mass star formation. In: Protostars and planets V pp 47–62
Chandler CJ, Koerner DW, Sargent AI, Wood DOS (1995) Dust emission from protostars: the disk and envelope of HH 24 MMS. Astrophys J Lett 449:L139. doi:10.1086/309644
Chapillon E, Dutrey A, Guilloteau S, Piétu V, Wakelam V, Hersant F, Gueth F, Henning T, Launhardt R, Schreyer K, Semenov D (2012a) Chemistry in disks. VII. First detection of HC3N in protoplanetary disks. Astrophys J 756:58. doi:10.1088/0004-637X/756/1/58
Chapillon E, Guilloteau S, Dutrey A, Piétu V, Guélin M (2012b) Chemistry in disks. VI. CN and HCN in protoplanetary disks. Astron Astrophys 537:A60. doi:10.1051/0004-6361/201116762
Charnley SB, Tielens AGGM, Millar TJ (1992) On the molecular complexity of the hot cores in Orion A—grain surface chemistry as ‘The last refuge of the scoundrel’. Astrophys J Lett 399:L71–L74. doi:10.1086/186609
Charnley SB, Tielens AGGM, Rodgers SD (1997) Deuterated methanol in the Orion compact ridge. Astrophys J Lett 482:L203. doi:10.1086/310697
Chaussidon M, Srinivasan G (2012) New constraints on the origin of short-lived 10Be in the early solar system. Meteorit Planet Sci 75:5192
Chiang HF, Looney LW, Tobin JJ (2012) The envelope and embedded disk around the class 0 protostar L1157-mm: dual-wavelength interferometric observations and modeling. Astrophys J 756:168. doi:10.1088/0004-637X/756/2/168
Chiar JE, Pendleton YJ, Allamandola LJ, Boogert ACA, Ennico K, Greene TP, Geballe TR, Keane JV, Lada CJ, Mason RE, Roellig TL, Sandford SA, Tielens AGGM, Werner MW, Whittet DCB, Decin L, Eriksson K (2011) Ices in the quiescent IC 5146 dense cloud. Astrophys J 731:9. doi:10.1088/0004-637X/731/1/9
Choi M, Tatematsu K, Park G, Kang M (2007) Ammonia imaging of the disks in the NGC 1333 IRAS 4A protobinary system. Astrophys J Lett 667:L183–L186. doi:10.1086/522116
Cieza LA, Mathews GS, Williams JP, Ménard FC, Kraus AL, Schreiber MR, Romero GA, Orellana M, Ireland MJ (2012) Submillimeter array observations of the RX J1633.9-2442 transition disk: evidence for multiple planets in the making. Astrophys J 752:75. doi:10.1088/0004-637X/752/1/75
Codella C, Benedettini M, Beltrán MT, Gueth F, Viti S, Bachiller R, Tafalla M, Cabrit S, Fuente A, Lefloch B (2009) Methyl cyanide as tracer of bow shocks in L1157-B1. Astron Astrophys 507:L25–L28. doi:10.1051/0004-6361/200913340
Codella C, Lefloch B, Ceccarelli C, Cernicharo J, Caux E, Lorenzani A, Viti S, Hily-Blant P, Parise B, Maret S, Nisini B, Caselli P, Cabrit S, Pagani L, Benedettini M, Boogert A, Gueth F, Melnick G, Neufeld D, Pacheco S, Salez M, Schuster K, Bacmann A, Baudry A, Bell T, Bergin EA, Blake G, Bottinelli S, Castets A, Comito C, Coutens A, Crimier N, Dominik C, Demyk K, Encrenaz P, Falgarone E, Fuente A, Gerin M, Goldsmith P, Helmich F, Hennebelle P, Henning T, Herbst E, Jacq T, Kahane C, Kama M, Klotz A, Langer W, Lis D, Lord S, Pearson J, Phillips T, Saraceno P, Schilke P, Tielens X, van der Tak F, van der Wiel M, Vastel C, Wakelam V, Walters A, Wyrowski F, Yorke H, Borys C, Delorme Y, Kramer C, Larsson B, Mehdi I, Ossenkopf V, Stutzki J (2010) The CHESS spectral survey of star forming regions: peering into the protostellar shock L1157-B1. I. Shock chemical complexity. Astron Astrophys 518:L112. doi:10.1051/0004-6361/201014582
Codella C, Ceccarelli C, Bottinelli S, Salez M, Viti S, Lefloch B, Cabrit S, Caux E, Faure A, Vasta M, Wiesenfeld L (2012a) First detection of hydrogen chloride toward protostellar shocks. Astrophys J 744:164. doi:10.1088/0004-637X/744/2/164
Codella C, Ceccarelli C, Lefloch B, Fontani F, Busquet G, Caselli P, Kahane C, Lis D, Taquet V, Vasta M, Viti S, Wiesenfeld L (2012b) The Herschel and IRAM CHESS spectral surveys of the protostellar shock L1157-B1: Fossil deuteration. Astrophys J Lett 757:L9. doi:10.1088/2041-8205/757/1/L9
Cordiner MA, Charnley SB, Wirström ES, Smith RG (2012) Organic chemistry of Low-mass Star-forming cores. I. 7 mm spectroscopy of chamaeleon MMS1. Astrophys J 744:131. doi:10.1088/0004-637X/744/2/131
Cortes SR, Meyer MR, Carpenter JM, Pascucci I, Schneider G, Wong T, Hines DC (2009) Grain growth and global structure of the protoplanetary disk associated with the mature classical T Tauri star, PDS 66. Astrophys J 697:1305–1315. doi:10.1088/0004-637X/697/2/1305
Coutens A, Vastel C, Caux E, Ceccarelli C, Bottinelli S, Wiesenfeld L, Faure A, Scribano Y, Kahane C (2012) A study of deuterated water in the low-mass protostar IRAS 16293-2422. Astron Astrophys 539:A132. doi:10.1051/0004-6361/201117627
Crapsi A, Caselli P, Walmsley CM, Myers PC, Tafalla M, Lee CW, Bourke TL (2005) Probing the evolutionary status of starless cores through N2H+ and N2D+ observations. Astrophys J 619:379–406. doi:10.1086/426472
Crapsi A, Caselli P, Walmsley MC, Tafalla M (2007) Observing the gas temperature drop in the high-density nucleus of L 1544. Astron Astrophys 470:221–230. doi:10.1051/0004-6361:20077613
Crimier N, Ceccarelli C, Lefloch B, Faure A (2009) Physical structure and water line spectrum predictions of the intermediate mass protostar OMC2-FIR4. Astron Astrophys 506:1229–1241. doi:10.1051/0004-6361/200911651
Crimier N, Ceccarelli C, Maret S, Bottinelli S, Caux E, Kahane C, Lis DC, Olofsson J (2010) The solar type protostar IRAS16293-2422: new constraints on the physical structure. Astron Astrophys 519:A65. doi:10.1051/0004-6361/200913112
Crovisier J, Biver N, Bockelée-Morvan D, Boissier J, Colom P, Lis DC (2009) The chemical diversity of comets: synergies between space exploration and ground-based radio observations. Earth Moon Planets 105:267–272. doi:10.1007/s11038-009-9293-z
Cuppen HM, Herbst E (2005) Monte Carlo simulations of H2 formation on grains of varying surface roughness. Mon Not R Astron Soc 361:565–576. doi:10.1111/j.1365-2966.2005.09189.x
Cuppen HM, van Dishoeck EF, Herbst E, Tielens AGGM (2009) Microscopic simulation of methanol and formaldehyde ice formation in cold dense cores. Astron Astrophys 508:275–287. doi:10.1051/0004-6361/200913119
Czaja AD (2010) Early earth: microbes and the rise of oxygen. Nat Geosci 3:522–523. doi:10.1038/ngeo929
D’Alessio P, Calvet N, Hartmann L, Lizano S, Cantó J (1999) Accretion disks around young objects. II. Tests of well-mixed models with ISM dust. Astrophys J 527:893–909. doi:10.1086/308103
D’Alessio P, Calvet N, Hartmann L (2001) Accretion disks around young objects. III. Grain growth. Astrophys J 553:321–334. doi:10.1086/320655
D’Alessio P, Calvet N, Hartmann L, Franco-Hernández R, Servín H (2006) Effects of dust growth and settling in T Tauri disks. Astrophys J 638:314–335. doi:10.1086/498861
Dalgarno A, Lepp S (1984) Deuterium fractionation mechanisms in interstellar clouds. Astrophys J Lett 287:L47–L50. doi:10.1086/184395
Dauphas N (2003) The dual origin of the terrestrial atmosphere. Icarus 165:326–339. doi:10.1016/S0019-1035(03)00198-2
Dauphas N, Chaussidon M (2011) A perspective from extinct radionuclides on a young stellar object: the sun and its accretion disk. Annu Rev Earth Planet Sci 39:351–386. doi:10.1146/annurev-earth-040610-133428
Dauphas N, Robert F, Marty B (2000) The late asteroidal and cometary bombardment of earth as recorded in water deuterium to Protium ratio. Icarus 148:508–512. doi:10.1006/icar.2000.6489
Delsemme AH (1992) Cometary origin of carbon and water on the terrestrial planets. Adv Space Res 12:5–12. doi:10.1016/0273-1177(92)90147-P
D’Hendecourt LB, Allamandola LJ, Baas F, Greenberg JM (1982) Interstellar grain explosions—molecule cycling between gas and dust. Astron Astrophys 109:L12–L14
Dominik C, Blum J, Cuzzi JN, Wurm G (2007) Growth of dust as the initial step toward planet formation. In: Protostars and planets V, pp 783–800
Dones L, Weissman PR, Levison HF, Duncan MJ (2004) Oort cloud formation and dynamics, pp 153–174
Doty SD, Neufeld DA (1997) Models for dense molecular cloud cores. Astrophys J 489:122. doi:10.1086/304764
Dubernet ML, Cernicharo J, Daniel F, Debray B, Faure A, Feautrier N, Flower D, Grosjean A, Roueff E, Spielfiedel A, Stoecklin T, Valiron P (2004) Ro-vibrational collisional excitation database: BASECOL. In: Combes F, Barret D, Contini T, Meynadier F, Pagani L (eds) SF2A-2004: Semaine de l’astrophysique Francaise, p 525. http://www.obspm.fr/basecol
Duchêne G, Ménard F, Stapelfeldt K, Duvert G (2003) A layered edge-on circumstellar disk around HK Tau B. Astron Astrophys 400:559–565. doi:10.1051/0004-6361:20021906
Dulieu F, Amiaud L, Congiu E, Fillion JH, Matar E, Momeni A, Pirronello V, Lemaire JL (2010) Experimental evidence for water formation on interstellar dust grains by hydrogen and oxygen atoms. Astron Astrophys 512:A30. doi:10.1051/0004-6361/200912079
Dullemond CP, Monnier JD (2010) The inner regions of protoplanetary disks. Annu Rev Astron Astrophys 48:205–239. doi:10.1146/annurev-astro-081309-130932
Dullemond CP, Henning T, Visser R, Geers VC, van Dishoeck EF, Pontoppidan KM (2007a) Dust sedimentation in protoplanetary disks with polycyclic aromatic hydrocarbons. Astron Astrophys 473:457–466. doi:10.1051/0004-6361:20077581
Dullemond CP, Hollenbach D, Kamp I, D’Alessio P (2007b) Models of the structure and evolution of protoplanetary disks. In: Protostars and planets V, pp 555–572
Durisen RH, Boss AP, Mayer L, Nelson AF, Quinn T, Rice WKM (2007) Gravitational instabilities in gaseous protoplanetary disks and implications for giant planet formation. In: Protostars and planets V, pp 607–622
Dutrey A, Guilloteau S, Duvert G, Prato L, Simon M, Schuster K, Menard F (1996) Dust and gas distribution around T Tauri stars in Taurus-Auriga. I. Interferometric 2.7 mm continuum and 13CO J = 1-0 observations. Astron Astrophys 309:493–504
Dutrey A, Guilloteau S, Guelin M (1997) Chemistry of protosolar-like nebulae: the molecular content of the DM Tau and GG Tau disks. Astron Astrophys 317:L55–L58
Dutrey A, Guilloteau S, Ho P (2007a) Interferometric spectroimaging of molecular gas in protoplanetary disks. In: Protostars and planets V, pp 495–506
Dutrey A, Henning T, Guilloteau S, Semenov D, Piétu V, Schreyer K, Bacmann A, Launhardt R, Pety J, Gueth F (2007b) Chemistry in disks. I. Deep search for N2H+ in the protoplanetary disks around LkCa 15, MWC 480, and DM Tauri. Astron Astrophys 464:615–623. doi:10.1051/0004-6361:20065385
Dutrey A, Wakelam V, Boehler Y, Guilloteau S, Hersant F, Semenov D, Chapillon E, Henning T, Piétu V, Launhardt R, Gueth F, Schreyer K (2011) Chemistry in disks. V. Sulfur-bearing molecules in the protoplanetary disks surrounding LkCa15, MWC480, DM Tauri, and GO Tauri. Astron Astrophys 535:A104. doi:10.1051/0004-6361/201116931
Elsila JE, Glavin DP, Dworkin JP (2009) Cometary glycine detected in samples returned by stardust. Meteorit Planet Sci 44:1323–1330. doi:10.1111/j.1945-5100.2009.tb01224.x
Enoch ML, Corder S, Duchêne G, Bock DC, Bolatto AD, Culverhouse TL, Kwon W, Lamb JW, Leitch EM, Marrone DP, Muchovej SJ, Pérez LM, Scott SL, Teuben PJ, Wright MCH, Zauderer BA (2011) Disk and envelope structure in class 0 protostars. II. High-resolution millimeter mapping of the Serpens sample. Astrophys J Suppl Ser 195:21. doi:10.1088/0067-0049/195/2/21
Evans NJ II, Rawlings JMC, Shirley YL, Mundy LG (2001) Tracing the mass during Low-Mass star formation. II. Modeling the submillimeter emission from preprotostellar cores. Astrophys J 557:193–208. doi:10.1086/321639
Fedele D, van den Ancker ME, Acke B, van der Plas G, van Boekel R, Wittkowski M, Henning T, Bouwman J, Meeus G, Rafanelli P (2008) The structure of the protoplanetary disk surrounding three young intermediate mass stars. II. Spatially resolved dust and gas distribution. Astron Astrophys 491:809–820. doi:10.1051/0004-6361:200810126
Fedele D, Bruderer S, van Dishoeck EF, Herczeg GJ, Evans NJ, Bouwman J, Henning T, Green J (2012) Warm H2O and OH in the disk around the Herbig star HD 163296. Astron Astrophys 544:L9. doi:10.1051/0004-6361/201219615
Feigelson ED, Montmerle T (1999) High-Energy processes in young stellar objects. Annu Rev Astron Astrophys 37:363–408. doi:10.1146/annurev.astro.37.1.363
Fisher DE (1982) Implications of terrestrial Ar-40/Ar-36 for atmospheric and mantle evolutionary models. Phys Earth Planet Inter 29:242–251. doi:10.1016/0031-9201(82)90015-2
Flower DR, Pineau Des Forêts G, Walmsley CM (2006) The importance of the ortho: para H2 ratio for the deuteration of molecules during pre-protostellar collapse. Astron Astrophys 449:621–629. doi:10.1051/0004-6361:20054246
Fogel JKJ, Bethell TJ, Bergin EA, Calvet N, Semenov D (2011) Chemistry of a protoplanetary disk with grain settling and Lyα radiation. Astrophys J 726:29. doi:10.1088/0004-637X/726/1/29
Fouchet T, Irwin PGJ, Parrish P, Calcutt SB, Taylor FW, Nixon CA, Owen T (2004) Search for spatial variation in the Jovian 15N/14N ratio from Cassini/CIRS observations. Icarus 172:50–58. doi:10.1016/j.icarus.2003.11.011
France K, Schindhelm E, Herczeg GJ, Brown A, Abgrall H, Alexander RD, Bergin EA, Brown JM, Linsky JL, Roueff E, Yang H (2012) A Hubble space telescope survey of H2 emission in the circumstellar environments of young stars. Astrophys J 756:171. doi:10.1088/0004-637X/756/2/171
Franklin J, Snell RL, Kaufman MJ, Melnick GJ, Neufeld DA, Hollenbach DJ, Bergin EA (2008) SWAS observations of water in molecular outflows. Astrophys J 674:1015–1031. doi:10.1086/524924
Frau P, Galli D, Girart JM (2011) Comparing star formation models with interferometric observations of the protostar NGC 1333 IRAS 4A. I. Magnetohydrodynamic collapse models. Astron Astrophys 535:A44. doi:10.1051/0004-6361/201117813
Friberg P, Hjalmarson A, Madden SC, Irvine WM (1988) Methanol in dark clouds. Astron Astrophys 195:281–289
Friesen RK, Di Francesco J, Shimajiri Y, Takakuwa S (2010) The initial conditions of clustered star formation. II. N2H+ observations of the Ophiuchus B core. Astrophys J 708:1002–1024. doi:10.1088/0004-637X/708/2/1002
Fuchs GW, Cuppen HM, Ioppolo S, Romanzin C, Bisschop SE, Andersson S, van Dishoeck EF, Linnartz H (2009) Hydrogenation reactions in interstellar CO ice analogues. A combined experimental/theoretical approach. Astron Astrophys 505:629–639. doi:10.1051/0004-6361/200810784
Fuente A, Cernicharo J, Agúndez M, Berné O, Goicoechea JR, Alonso-Albi T, Marcelino N (2010) Molecular content of the circumstellar disk in AB Aurigae. First detection of SO in a circumstellar disk. Astron Astrophys 524:A19. doi:10.1051/0004-6361/201014905
Furlan E, Hartmann L, Calvet N, D’Alessio P, Franco-Hernández R, Forrest WJ, Watson DM, Uchida KI, Sargent B, Green JD, Keller LD, Herter TL (2006) A survey and analysis of Spitzer infrared spectrograph spectra of T Tauri stars in Taurus. Astrophys J Suppl Ser 165:568–605. doi:10.1086/505468
Furuya K, Aikawa Y, Tomida K, Matsumoto T, Saigo K, Tomisaka K, Hersant F, Wakelam V (2012) Chemistry in the first hydrostatic core stage adopting three-dimensional radiation hydrodynamic simulations. ArXiv e-prints
Galli D, Shu FH (1993a) Collapse of magnetized molecular cloud cores. I. Semianalytical solution. Astrophys J 417:220. doi:10.1086/173305
Galli D, Shu FH (1993b) Collapse of magnetized molecular cloud cores. II. Numerical results. Astrophys J 417:243. doi:10.1086/173306
García RJM, Carnerup A, Christy AG, Welham NJ, Hyde ST (2002) Morphology: an ambiguous indicator of biogenicity. Astrobiology 2:353–369. doi:10.1089/153110702762027925
Garrod RT, Herbst E (2006) Formation of methyl formate and other organic species in the warm-up phase of hot molecular cores. Astron Astrophys 457:927–936. doi:10.1051/0004-6361:20065560
Garrod RT, Pauly T (2011) On the formation of CO2 and other interstellar ices. Astrophys J 735:15. doi:10.1088/0004-637X/735/1/15
Garrod RT, Wakelam V, Herbst E (2007) Non-thermal desorption from interstellar dust grains via exothermic surface reactions. Astron Astrophys 467:1103–1115. doi:10.1051/0004-6361:20066704
Garrod RT, Weaver SLW, Herbst E (2008) Complex chemistry in star-forming regions: an expanded gas-grain warm-up chemical model. Astrophys J 682:283–302. doi:10.1086/588035
Garrod RT, Vasyunin AI, Semenov DA, Wiebe DS, Henning T (2009) A new modified-rate approach for Gas-Grain chemistry: comparison with a unified large-scale Monte Carlo simulation. Astrophys J Lett 700:L43–L46. doi:10.1088/0004-637X/700/1/L43
Geers VC, Augereau JC, Pontoppidan KM, Dullemond CP, Visser R, Kessler-Silacci JE, Evans NJ II, van Dishoeck EF, Blake GA, Boogert ACA, Brown JM, Lahuis F, Merín B (2006) C2D Spitzer-IRS spectra of disks around T Tauri stars. II. PAH emission features. Astron Astrophys 459:545–556. doi:10.1051/0004-6361:20064830
Geiss J, Gloeckler G (1998) Abundances of deuterium and Helium-3 in the protosolar cloud. Space Sci Rev 84:239–250
Gerakines PA, Schutte WA, Ehrenfreund P (1996) Ultraviolet processing of interstellar ice analogs. I. Pure ices. Astron Astrophys 312:289–305
Gerin M, Marcelino N, Biver N, Roueff E, Coudert LH, Elkeurti M, Lis DC, Bockelée-Morvan D (2009) Detection of 15NH2D in dense cores: a new tool for measuring the 14N/15N ratio in the cold ISM. Astron Astrophys 498:L9–L12. doi:10.1051/0004-6361/200911759
Girart JM, Rao R, Marrone DP (2006) Magnetic fields in the formation of sun-like stars. Science 313:812–814. doi:10.1126/science.1129093
Glassgold AE, Najita J, Igea J (1997) X-Ray ionization of protoplanetary disks. Astrophys J 480:344. doi:10.1086/303952
Goldsmith PF (2001) Molecular depletion and thermal balance in dark cloud cores. Astrophys J 557:736–746. doi:10.1086/322255
Gomes R, Levison HF, Tsiganis K, Morbidelli A (2005) Origin of the cataclysmic late heavy bombardment period of the terrestrial planets. Nature 435:466–469. doi:10.1038/nature03676
Goodman AA, Benson PJ, Fuller GA, Myers PC (1993) Dense cores in dark clouds. VIII. Velocity gradients. Astrophys J 406:528–547. doi:10.1086/172465
Gorti U, Hollenbach D (2004) Models of chemistry, thermal balance, and infrared spectra from intermediate-aged disks around G and K stars. Astrophys J 613:424–447. doi:10.1086/422406
Gorti U, Hollenbach D (2008) Line emission from gas in optically thick dust disks around young stars. Astrophys J 683:287–303. doi:10.1086/589616
Goto M, Carmona A, Linz H, Stecklum B, Henning T, Meeus G, Usuda T (2012) Kinematics of ionized gas at 0.01 AU of TW Hya. Astrophys J 748:6. doi:10.1088/0004-637X/748/1/6
Gounelle M, Meibom A (2008) The origin of Short-lived radionuclides and the astrophysical environment of solar system formation. Astrophys J 680:781–792. doi:10.1086/587613
Gratton RG, Carretta E, Bragaglia A (2012) Multiple populations in globular clusters. Lessons learned from the Milky Way globular clusters. Astron Astrophys Rev 20:50. doi:10.1007/s00159-012-0050-3
Gredel R, Lepp S, Dalgarno A, Herbst E (1989) Cosmic-ray-induced photodissociation and photoionization rates of interstellar molecules. Astrophys J 347:289–293. doi:10.1086/168117
Guelin M, Langer WD, Snell RL, Wootten HA (1977) Observations of DCO/plus/—the electron abundance in dark clouds. Astrophys J Lett 217:L165–L168. doi:10.1086/182562
Guhathakurta P, Draine BT (1989) Temperature fluctuations in interstellar grains. I. Computational method and sublimation of small grains. Astrophys J 345:230–244. doi:10.1086/167899
Guilloteau S, Piétu V, Dutrey A, Guélin M (2006) Deuterated molecules in DM Tauri: DCO+, but no HDO. Astron Astrophys 448:L5–L8. doi:10.1051/0004-6361:200600005
Güttler C, Blum J, Zsom A, Ormel CW, Dullemond CP (2010) The outcome of protoplanetary dust growth: pebbles, boulders, or planetesimals? I. Mapping the zoo of laboratory collision experiments. Astron Astrophys 513:A56. doi:10.1051/0004-6361/200912852
Habart E, Boulanger F, Verstraete L, Walmsley CM, Pineau des Forêts G (2004a) Some empirical estimates of the H2 formation rate in photon-dominated regions. Astron Astrophys 414:531–544. doi:10.1051/0004-6361:20031659
Habart E, Natta A, Krügel E (2004b) PAHs in circumstellar disks around Herbig Ae/Be stars. Astron Astrophys 427:179–192. doi:10.1051/0004-6361:20035916
Habart E, Natta A, Testi L, Carbillet M (2006) Spatially resolved PAH emission in the inner disks of Herbig Ae/Be stars. Astron Astrophys 449:1067–1075. doi:10.1051/0004-6361:20052994
Hartogh P, Lis DC, Bockelée-Morvan D, de Val-Borro M, Biver N, Küppers M, Emprechtinger M, Bergin EA, Crovisier J, Rengel M, Moreno R, Szutowicz S, Blake GA (2011) Ocean-like water in the Jupiter-family comet 103P/Hartley 2. Nature 478:218–220. doi:10.1038/nature10519
Hasegawa TI, Herbst E, Leung CM (1992) Models of gas-grain chemistry in dense interstellar clouds with complex organic molecules. Astrophys J Suppl Ser 82:167–195. doi:10.1086/191713
Hassel GE, Herbst E, Garrod RT (2008) Modeling the lukewarm corino phase: is L1527 unique? Astrophys J 681:1385–1395. doi:10.1086/588185
Hassel GE, Harada N, Herbst E (2011) Carbon-chain species in warm-up models. Astrophys J 743:182. doi:10.1088/0004-637X/743/2/182
Heinzeller D, Nomura H, Walsh C, Millar TJ (2011) Chemical evolution of protoplanetary disks—the effects of viscous accretion, turbulent mixing, and disk winds. Astrophys J 731:115. doi:10.1088/0004-637X/731/2/115
Hennebelle P, Fromang S (2008) Magnetic processes in a collapsing dense core. I. Accretion and ejection. Astron Astrophys 477:9–24. doi:10.1051/0004-6361:20078309
Henning T, Semenov D, Guilloteau S, Dutrey A, Hersant F, Wakelam V, Chapillon E, Launhardt R, Piétu V, Schreyer K (2010) Chemistry in disks. III. Photochemistry and X-ray driven chemistry probed by the ethynyl radical (CCH) in DM Tau, LkCa 15, and MWC 480. Astrophys J 714:1511–1520. doi:10.1088/0004-637X/714/2/1511
Herbst E, Klemperer W (1973) The formation and depletion of molecules in dense interstellar clouds. Astrophys J 185:505–534. doi:10.1086/152436
Herbst E, van Dishoeck EF (2009) Complex organic interstellar molecules. Annu Rev Astron Astrophys 47:427–480. doi:10.1146/annurev-astro-082708-101654
Hersant F, Wakelam V, Dutrey A, Guilloteau S, Herbst E (2009) Cold CO in circumstellar disks. On the effects of photodesorption and vertical mixing. Astron Astrophys 493:L49–L52. doi:10.1051/0004-6361:200811082
Hily-Blant P, Walmsley M, Pineau Des Forêts G, Flower D (2010) Nitrogen chemistry and depletion in starless cores. Astron Astrophys 513:A41. doi:10.1051/0004-6361/200913200
Hirahara Y, Suzuki H, Yamamoto S, Kawaguchi K, Kaifu N, Ohishi M, Takano S, Ishikawa SI, Masuda A (1992) Mapping observations of sulfur-containing carbon-chain molecules in Taurus molecular cloud 1 (TMC-1). Astrophys J 394:539–551. doi:10.1086/171605
Hiraoka K, Miyagoshi T, Takayama T, Yamamoto K, Kihara Y (1998) Gas-grain processes for the formation of CH4 and H2O: reactions of H atoms with C, O, and CO in the solid phase at 12 K. Astrophys J 498:710. doi:10.1086/305572
Hiraoka K, Sato T, Sato S, Sogoshi N, Yokoyama T, Takashima H, Kitagawa S (2002) Formation of formaldehyde by the tunneling reaction of H with solid CO at 10 K revisited. Astrophys J 577:265–270. doi:10.1086/342132
Hogerheijde MR, Bergin EA, Brinch C, Cleeves LI, Fogel JKJ, Blake GA, Dominik C, Lis DC, Melnick G, Neufeld D, Panić O, Pearson JC, Kristensen L, Yıldız UA, van Dishoeck EF (2011) Detection of the water reservoir in a forming planetary system. Science 334:338. doi:10.1126/science.1208931
Hollenbach D, McKee CF (1989) Molecule formation and infrared emission in fast interstellar shocks. III. Results for J shocks in molecular clouds. Astrophys J 342:306–336. doi:10.1086/167595
Hollenbach D, Salpeter EE (1971) Surface recombination of hydrogen molecules. Astrophys J 163:155. doi:10.1086/150754
Hollenbach D, Kaufman MJ, Bergin EA, Melnick GJ (2009) Water, O2, and ice in molecular clouds. Astrophys J 690:1497–1521. doi:10.1088/0004-637X/690/2/1497
Honda M, Inoue AK, Fukagawa M, Oka A, Nakamoto T, Ishii M, Terada H, Takato N, Kawakita H, Okamoto YK, Shibai H, Tamura M, Kudo T, Itoh Y (2009) Detection of water ice grains on the surface of the circumstellar disk around HD 142527. Astrophys J Lett 690:L110–L113. doi:10.1088/0004-637X/690/2/L110
Horner J, Mousis O, Hersant F (2007) Constraints on the formation regions of comets from their D:H ratios. Earth Moon Planets 100:43–56. doi:10.1007/s11038-006-9096-4
Hughes AM, Andrews SM, Espaillat C, Wilner DJ, Calvet N, D’Alessio P, Qi C, Williams JP, Hogerheijde MR (2009) A spatially resolved inner hole in the disk around GM Aurigae. Astrophys J 698:131–142. doi:10.1088/0004-637X/698/1/131
Hughes AM, Wilner DJ, Andrews SM, Williams JP, Su KYL, Murray-Clay RA, Qi C (2011) Resolved submillimeter observations of the HR 8799 and HD 107146 debris disks. Astrophys J 740:38. doi:10.1088/0004-637X/740/1/38
Ilee JD, Boley AC, Caselli P, Durisen RH, Hartquist TW, Rawlings JMC (2011) Chemistry in a gravitationally unstable protoplanetary disc. Mon Not R Astron Soc 417:2950–2961. doi:10.1111/j.1365-2966.2011.19455.x
Ilgner M, Henning T, Markwick AJ, Millar TJ (2004) Transport processes and chemical evolution in steady accretion disk flows. Astron Astrophys 415:643–659. doi:10.1051/0004-6361:20034061
Ioppolo S, Cuppen HM, Romanzin C, van Dishoeck EF, Linnartz H (2008) Laboratory evidence for efficient water formation in interstellar ices. Astrophys J 686:1474–1479. doi:10.1086/591506
Ioppolo S, Palumbo ME, Baratta GA, Mennella V (2009) Formation of interstellar solid CO2 after energetic processing of icy grain mantles. Astron Astrophys 493:1017–1028. doi:10.1051/0004-6361:200809769
Ioppolo S, van Boheemen Y, Cuppen HM, van Dishoeck EF, Linnartz H (2011) Surface formation of CO2 ice at low temperatures. Mon Not R Astron Soc 413:2281–2287. doi:10.1111/j.1365-2966.2011.18306.x
Irvine WM, Friberg P, Kaifu N, Kawaguchi K, Kitamura Y, Matthews HE, Minh Y, Saito S, Ukita N, Yamamoto S (1989) Observations of some oxygen-containing and sulfur-containing organic molecules in cold dark clouds. Astrophys J 342:871–875. doi:10.1086/167643
Isella A, Testi L, Natta A (2006) Large dust grains in the inner region of circumstellar disks. Astron Astrophys 451:951–959. doi:10.1051/0004-6361:20054647
Jehin E, Manfroid J, Hutsemékers D, Arpigny C, Zucconi JM (2009) Isotopic ratios in comets: status and perspectives. Earth Moon Planets 105:167–180. doi:10.1007/s11038-009-9322-y
Jing D, He J, Brucato J, De Sio A, Tozzetti L, Vidali G (2011) On water formation in the interstellar medium: laboratory study of the O+D reaction on surfaces. Astrophys J Lett 741:L9. doi:10.1088/2041-8205/741/1/L9
Jones AP, Williams DA (1985) Time-dependent sticking coefficients and mantle growth on interstellar grains. Mon Not R Astron Soc 217:413–421
Joos M, Hennebelle P, Ciardi A (2012) Protostellar disk formation and transport of angular momentum during magnetized core collapse. Astron Astrophys 543:A128. doi:10.1051/0004-6361/201118730
Jørgensen JK, van Dishoeck EF (2010a) The HDO/H2O ratio in gas in the inner regions of a low-mass protostar. Astrophys J Lett 725:L172–L175. doi:10.1088/2041-8205/725/2/L172
Jørgensen JK, van Dishoeck EF (2010b) Water vapor in the inner 25 AU of a young disk around a Low-Mass protostar. Astrophys J Lett 710:L72–L76. doi:10.1088/2041-8205/710/1/L72
Jørgensen JK, Schöier FL, van Dishoeck EF (2002) Physical structure and CO abundance of low-mass protostellar envelopes. Astron Astrophys 389:908–930. doi:10.1051/0004-6361:20020681
Jørgensen JK, Schöier FL, van Dishoeck EF (2005) Molecular freeze-out as a tracer of the thermal and dynamical evolution of pre- and protostellar cores. Astron Astrophys 435:177–182. doi:10.1051/0004-6361:20042092
Jørgensen JK, Bourke TL, Myers PC, Di Francesco J, van Dishoeck EF, Lee CF, Ohashi N, Schöier FL, Takakuwa S, Wilner DJ, Zhang Q (2007) PROSAC: a submillimeter array survey of low-mass protostars. I. Overview of program: envelopes, disks, outflows, and hot cores. Astrophys J 659:479–498. doi:10.1086/512230
Jørgensen JK, van Dishoeck EF, Visser R, Bourke TL, Wilner DJ, Lommen D, Hogerheijde MR, Myers PC (2009) PROSAC: a submillimeter array survey of low-mass protostars. II. The mass evolution of envelopes, disks, and stars from the class 0 through I stages. Astron Astrophys 507:861–879. doi:10.1051/0004-6361/200912325
Jørgensen JK, Bourke TL, Nguyen Luong Q, Takakuwa S (2011) Arcsecond resolution images of the chemical structure of the low-mass protostar IRAS 16293-2422. An overview of a large molecular line survey from the submillimeter array. Astron Astrophys 534:A100. doi:10.1051/0004-6361/201117139
Jørgensen JK, Favre C, Bisschop SE, Bourke TL, van Dishoeck EF, Schmalzl M (2012) Detection of the simplest sugar, glycolaldehyde, in a solar-type protostar with ALMA. Astrophys J Lett 757:L4. doi:10.1088/2041-8205/757/1/L4
Kaifu N, Ohishi M, Kawaguchi K, Saito S, Yamamoto S, Miyaji T, Miyazawa K, Ishikawa SI, Noumaru C, Harasawa S, Okuda M, Suzuki H (2004) A 8.8–50GHz complete spectral line survey toward TMC-1 I. Survey data. Publ Astron Soc Jpn 56:69–173
Kamp I (2011) Evolution of PAHs in protoplanetary disks. In: Joblin C, Tielens AGGM (eds) EAS publications series. EAS publications series, vol 46, pp 271–283. doi:10.1051/eas/1146029
Kamp I, Woitke P, Pinte C, Tilling I, Thi WF, Menard F, Duchene G, Augereau JC (2011) Continuum and line modelling of discs around young stars. II. Line diagnostics for GASPS from the DENT grid. Astron Astrophys 532:A85. doi:10.1051/0004-6361/201016399
Kaufman MJ, Neufeld DA (1996) Far-Infrared water emission from magnetohydrodynamic shock waves. Astrophys J 456:611. doi:10.1086/176683
Kavelaars JJ, Mousis O, Petit JM, Weaver HA (2011) On the formation location of Uranus and Neptune as constrained by dynamical and chemical models of comets. Astrophys J Lett 734:L30. doi:10.1088/2041-8205/734/2/L30
Kawamoto T (1996) Experimental constraints on differentiation and H2O abundance of calc-alkaline magmas. Earth Planet Sci Lett 144:577–589. doi:10.1016/S0012-821X(96)00182-3
Keller LP, Messenger S, Flynn GJ, Clemett S, Wirick S, Jacobsen C (2004) The nature of molecular cloud material in interplanetary dust. Geochim Cosmochim Acta 68:2577–2589. doi:10.1016/j.gca.2003.10.044
Keller LD, Sloan GC, Forrest WJ, Ayala S, D’Alessio P, Shah S, Calvet N, Najita J, Li A, Hartmann L, Sargent B, Watson DM, Chen CH (2008) PAH emission from Herbig Ae/Be stars. Astrophys J 684:411–429. doi:10.1086/589818
Kessler-Silacci JE, Hillenbrand LA, Blake GA, Meyer MR (2005) 8–13 μm spectroscopy of young stellar objects: evolution of the silicate feature. Astrophys J 622:404–429. doi:10.1086/427793
Keto E, Caselli P (2008) The different structures of the two classes of starless cores. Astrophys J 683:238–247. doi:10.1086/589147
Keto E, Caselli P (2010) Dynamics and depletion in thermally supercritical starless cores. Mon Not R Astron Soc 402:1625–1634. doi:10.1111/j.1365-2966.2009.16033.x
Kita NT, Togashi S, Morishita Y, Terashima S, Yurimoto H (1998) Search for 60Ni excesses in MET-78008 ureilite: an ion microprobe study. Antarct Meteor Res 11:103
Kita NT, Nagahara H, Togashi S, Morishita Y (2000) A short duration of chondrule formation in the solar nebula: evidence from 26Al in Semarkona ferromagnesian chondrules. Geochim Cosmochim Acta 64:3913–3922. doi:10.1016/S0016-7037(00)00488-9
Krasnopolsky R, Li ZY, Shang H (2011) Disk formation in magnetized clouds enabled by the hall effect. Astrophys J 733:54. doi:10.1088/0004-637X/733/1/54
Kristensen LE, Visser R, van Dishoeck EF, Yıldı zUA, Doty SD, Herczeg GJ, Liu FC, Parise B, Jørgensen JK, van Kempen TA, Brinch C, Wampfler SF, Bruderer S, Benz AO, Hogerheijde MR, Deul E, Bachiller R, Baudry A, Benedettini M, Bergin EA, Bjerkeli P, Blake GA, Bontemps S, Braine J, Caselli P, Cernicharo J, Codella C, Daniel F, de Graauw T, di Giorgio AM, Dominik C, Encrenaz P, Fich M, Fuente A, Giannini T, Goicoechea JR, Helmich F, Herpin F, Jacq T, Johnstone D, Kaufman MJ, Larsson B, Lis D, Liseau R, Marseille M, McCoey C, Melnick G, Neufeld D, Nisini B, Olberg M, Pearson JC, Plume R, Risacher C, Santiago-García J, Saraceno P, Shipman R, Tafalla M, Tielens AGGM, van der Tak F, Wyrowski F, Beintema D, de Jonge A, Dieleman P, Ossenkopf V, Roelfsema P, Stutzki J (2010) Water in low-mass star-forming regions with Herschel. HIFI spectroscopy of NGC 1333. Astron Astrophys 521:L30. doi:10.1051/0004-6361/201015100
Kristensen LE, van Dishoeck EF, Tafalla M, Bachiller R, Nisini B, Liseau R, Yıldız UA (2011) Water in low-mass star-forming regions with Herschel (WISH-LM). High-velocity H2O bullets in L1448-MM observed with HIFI. Astron Astrophys 531:L1. doi:10.1051/0004-6361/201116975
Kristensen LE, van Dishoeck EF, Bergin EA, Visser R, Yıldız UA, San Jose-Garcia I, Jørgensen JK, Herczeg GJ, Johnstone D, Wampfler SF, Benz AO, Bruderer S, Cabrit S, Caselli P, Doty SD, Harsono D, Herpin F, Hogerheijde MR, Karska A, van Kempen TA, Liseau R, Nisini B, Tafalla M, van der Tak F, Wyrowski F (2012) Water in star-forming regions with Herschel (WISH). II. Evolution of 557 GHz 110-101 emission in low-mass protostars. Astron Astrophys 542:A8. doi:10.1051/0004-6361/201118146
Kwon W, Looney LW, Mundy LG, Chiang HF, Kemball AJ (2009) Grain growth and density distribution of the youngest protostellar systems. Astrophys J 696:841–852. doi:10.1088/0004-637X/696/1/841
Laas JC, Garrod RT, Herbst E, Widicus Weaver SL (2011) Contributions from grain surface and gas phase chemistry to the formation of methyl formate and its structural isomers. Astrophys J 728:71. doi:10.1088/0004-637X/728/1/71
Lada CJ, Bergin EA, Alves JF, Huard TL (2003) The dynamical state of Barnard 68: a thermally supported, pulsating dark cloud. Astrophys J 586:286–295. doi:10.1086/367610
Lahuis F, van Dishoeck EF, Boogert ACA, Pontoppidan KM, Blake GA, Dullemond CP, Evans NJ II, Hogerheijde MR, Jørgensen JK, Kessler-Silacci JE, Knez C (2006) Hot organic molecules toward a young low-mass star: a look at inner disk chemistry. Astrophys J Lett 636:L145–L148. doi:10.1086/500084
Lattelais M, Pauzat F, Ellinger Y, Ceccarelli C (2009) Interstellar complex organic molecules and the minimum energy principle. Astrophys J Lett 696:L133–L136. doi:10.1088/0004-637X/696/2/L133
Laughlin G, Bodenheimer P (1994) Nonaxisymmetric evolution in protostellar disks. Astrophys J 436:335–354. doi:10.1086/174909
Le Guillou C, Rouzaud JN, Bonal L, Quirico E, Derenne S, Remusat L (2012) High resolution TEM of chondritic carbonaceous matter: metamorphic evolution and heterogeneity. Meteorit Planet Sci 47:345–362. doi:10.1111/j.1945-5100.2012.01336.x
Lécuyer C, Simon L, Guy F (2000) Comparison of carbon, nitrogen and water budgets on Venus and the earth. Earth Planet Sci Lett 181:33–40. doi:10.1016/S0012-821X(00)00195-3
Lee T, Shu FH, Shang H, Glassgold AE, Rehm KE (1998) Protostellar cosmic rays and extinct radioactivities in meteorites. Astrophys J 506:898–912. doi:10.1086/306284
Lee JE, Evans NJ II, Bergin EA (2005) Comparisons of an evolutionary chemical model with other models. Astrophys J 631:351–360. doi:10.1086/432531
Lee N, Williams JP, Cieza LA (2011) Protoplanetary disk masses in IC348: a rapid decline in the population of small dust grains after 1 Myr. Astrophys J 736:135. doi:10.1088/0004-637X/736/2/135
Lefloch B, Castets A, Cernicharo J, Langer WD, Zylka R (1998) Cores and cavities in NGC 1333. Astron Astrophys 334:269–279
Lefloch B, Cabrit S, Codella C, Melnick G, Cernicharo J, Caux E, Benedettini M, Boogert A, Caselli P, Ceccarelli C, Gueth F, Hily-Blant P, Lorenzani A, Neufeld D, Nisini B, Pacheco S, Pagani L, Pardo JR, Parise B, Salez M, Schuster K, Viti S, Bacmann A, Baudry A, Bell T, Bergin EA, Blake G, Bottinelli S, Castets A, Comito C, Coutens A, Crimier N, Dominik C, Demyk K, Encrenaz P, Falgarone E, Fuente A, Gerin M, Goldsmith P, Helmich F, Hennebelle P, Henning T, Herbst E, Jacq T, Kahane C, Kama M, Klotz A, Langer W, Lis D, Lord S, Maret S, Pearson J, Phillips T, Saraceno P, Schilke P, Tielens X, van der Tak F, van der Wiel M, Vastel C, Wakelam V, Walters A, Wyrowski F, Yorke H, Bachiller R, Borys C, de Lange G, Delorme Y, Kramer C, Larsson B, Lai R, Maiwald FW, Martin-Pintado J, Mehdi I, Ossenkopf V, Siegel P, Stutzki J (2010) The CHESS spectral survey of star forming regions: peering into the protostellar shock L1157-B1. II. Shock dynamics. Astron Astrophys 518:L113. doi:10.1051/0004-6361/201014630
Lefloch B, Cernicharo J, Pacheco S, Ceccarelli C (2011) Shocked water in the Cepheus E protostellar outflow. Astron Astrophys 527:L3. doi:10.1051/0004-6361/201016247
Leger A, Jura M, Omont A (1985) Desorption from interstellar grains. Astron Astrophys 144:147–160
Levison HF, Duncan MJ, Brasser R, Kaufmann DE (2010) Capture of the sun’s Oort cloud from stars in its birth cluster. Science 329:187. doi:10.1126/science.1187535
Licandro J, Hargrove K, Kelley M, Campins H, Ziffer J, Alí-Lagoa V, Fernández Y, Rivkin A (2012) 5–14 μm Spitzer spectra of Themis family asteroids. Astron Astrophys 537:A73. doi:10.1051/0004-6361/201118142
Lis DC, Wootten A, Gerin M, Roueff E (2010) Nitrogen isotopic fractionation in interstellar ammonia. Astrophys J Lett 710:L49–L52. doi:10.1088/2041-8205/710/1/L49
Liseau R, Ceccarelli C, Larsson B, Nisini B, White GJ, Ade P, Armand C, Burgdorf M, Caux E, Cerulli R, Church S, Clegg PE, Digorgio A, Furniss I, Giannini T, Glencross W, Gry C, King K, Lim T, Lorenzetti D, Molinari S, Naylor D, Orfei R, Saraceno P, Sidher S, Smith H, Spinoglio L, Swinyard B, Texier D, Tommasi E, Trams N, Unger S (1996) Thermal H2O emission from the Herbig–Haro flow HH 54. Astron Astrophys 315:L181–L184
Liu FC, Parise B, Kristensen L, Visser R, van Dishoeck EF, Güsten R (2011) Water deuterium fractionation in the low-mass protostar NGC1333-IRAS2A. Astron Astrophys 527:A19. doi:10.1051/0004-6361/201015519
Loinard L, Castets A, Ceccarelli C, Caux E, Tielens AGGM (2001) Doubly deuterated molecular species in protostellar environments. Astrophys J Lett 552:L163–L166. doi:10.1086/320331
Lommen DJP, van Dishoeck EF, Wright CM, Maddison ST, Min M, Wilner DJ, Salter DM, van Langevelde HJ, Bourke TL, van der Burg RFJ, Blake GA (2010) Grain growth across protoplanetary discs: 10 μm silicate feature versus millimetre slope. Astron Astrophys 515:A77. doi:10.1051/0004-6361/200913150
Looney LW, Mundy LG, Welch WJ (2000) Unveiling the circumstellar envelope and disk: a subarcsecond survey of circumstellar structures. Astrophys J 529:477–498. doi:10.1086/308239
López-Sepulcre A, Kama M, Ceccarelli C, Dominik C, Caux E, Fuente A, Alonso-Albi T (submitted) Astron Astrophys
Machida MN, Inutsuka SI, Matsumoto T (2011) Effect of magnetic braking on circumstellar disk formation in a strongly magnetized cloud. Publ Astron Soc Jpn 63:555
Malbet F, Benisty M, de Wit WJ, Kraus S, Meilland A, Millour F, Tatulli E, Berger JP, Chesneau O, Hofmann KH, Isella A, Natta A, Petrov RG, Preibisch T, Stee P, Testi L, Weigelt G, Antonelli P, Beckmann U, Bresson Y, Chelli A, Dugué M, Duvert G, Gennari S, Glück L, Kern P, Lagarde S, Le Coarer E, Lisi F, Perraut K, Puget P, Rantakyrö F, Robbe-Dubois S, Roussel A, Zins G, Accardo M, Acke B, Agabi K, Altariba E, Arezki B, Aristidi E, Baffa C, Behrend J, Blöcker T, Bonhomme S, Busoni S, Cassaing F, Clausse JM, Colin J, Connot C, Delboulbé A, Domiciano de Souza A, Driebe T, Feautrier P, Ferruzzi D, Forveille T, Fossat E, Foy R, Fraix-Burnet D, Gallardo A, Giani E, Gil C, Glentzlin A, Heiden M, Heininger M, Hernandez Utrera O, Kamm D, Kiekebusch M, Le Contel D, Le Contel JM, Lesourd T, Lopez B, Lopez M, Magnard Y, Marconi A, Mars G, Martinot-Lagarde G, Mathias P, Mège P, Monin JL, Mouillet D, Mourard D, Nussbaum E, Ohnaka K, Pacheco J, Perrier C, Rabbia Y, Rebattu S, Reynaud F, Richichi A, Robini A, Sacchettini M, Schertl D, Schöller M, Solscheid W, Spang A, Stefanini P, Tallon M, Tallon-Bosc I, Tasso D, Vakili F, von der Lühe O, Valtier JC, Vannier M (2007) Disk and wind interaction in the young stellar object MWC 297 spatially resolved with AMBER/VLTI. Astron Astrophys 464:43–53. doi:10.1051/0004-6361:20053924
Mandell AM, Bast J, van Dishoeck EF, Blake GA, Salyk C, Mumma MJ, Villanueva G (2012) First detection of Near-infrared line emission from organics in young circumstellar disks. Astrophys J 747:92. doi:10.1088/0004-637X/747/2/92
Manfroid J, Jehin E, Hutsemékers D, Cochran A, Zucconi JM, Arpigny C, Schulz R, Stüwe JA, Ilyin I (2009) The CN isotopic ratios in comets. Astron Astrophys 503:613–624. doi:10.1051/0004-6361/200911859
Marcelino N, Cernicharo J, Roueff E, Gerin M, Mauersberger R (2005) Deuterated thioformaldehyde in the Barnard 1 cloud. Astrophys J 620:308–320. doi:10.1086/426934
Marcelino N, Cernicharo J, Agúndez M, Roueff E, Gerin M, Martín-Pintado J, Mauersberger R, Thum C (2007) Discovery of interstellar propylene (CH2CHCH3): missing links in interstellar gas-phase chemistry. Astrophys J Lett 665:L127–L130. doi:10.1086/521398
Marcelino N, Brünken S, Cernicharo J, Quan D, Roueff E, Herbst E, Thaddeus P (2010) The puzzling behavior of HNCO isomers in molecular clouds. Astron Astrophys 516:A105. doi:10.1051/0004-6361/200913806
Marhas KK, Goswami JN, Davis AM (2002) Short-Lived nuclides in hibonite grains from Murchison: evidence for solar system evolution. Science 298:2182–2185. doi:10.1126/science.1078322
Marshall CP, Emry JR, Olcott Marshall A (2011) Haematite pseudomicrofossils present in the 3.5-billion-year-old apex chert. Nat Geosci 4:240–243. doi:10.1038/ngeo1084
Marty B (2012) The origins and concentrations of water, carbon, nitrogen and noble gases on earth. Earth Planet Sci Lett 313:56–66. doi:10.1016/j.epsl.2011.10.040
Marty B, Zimmermann L, Burnard PG, Wieler R, Heber VS, Burnett DL, Wiens RC, Bochsler P (2010) Nitrogen isotopes in the recent solar wind from the analysis of genesis targets: evidence for large scale isotope heterogeneity in the early solar system. Geochim Cosmochim Acta 74:340–355
Mathews GS, Dent WRF, Williams JP, Howard CD, Meeus G, Riaz B, Roberge A, Sandell G, Vandenbussche B, Duchêne G, Kamp I, Ménard F, Montesinos B, Pinte C, Thi WF, Woitke P, Alacid JM, Andrews SM, Ardila DR, Aresu G, Augereau JC, Barrado D, Brittain S, Ciardi DR, Danchi W, Eiroa C, Fedele D, Grady CA, de Gregorio-Monsalvo I, Heras A, Huelamo N, Krivov A, Lebreton J, Liseau R, Martin-Zaidi C, Mendigutía I, Mora A, Morales-Calderon M, Nomura H, Pantin E, Pascucci I, Phillips N, Podio L, Poelman DR, Ramsay S, Rice K, Riviere-Marichalar P, Solano E, Tilling I, Walker H, White GJ, Wright G (2010) GAS in protoplanetary systems (GASPS). I. First results. Astron Astrophys 518:L127. doi:10.1051/0004-6361/201014595
Matrajt G, Messenger S, Brownlee D, Joswiak D (2012) Diverse forms of primordial organic matter identified in interplanetary dust particles. Meteorit Planet Sci 47:525–549. doi:10.1111/j.1945-5100.2011.01310.x
Matthews HE, Sears TJ (1983) The detection of vinyl cyanide in TMC-1. Astrophys J 272:149–153. doi:10.1086/161271
Matthews HE, Friberg P, Irvine WM (1985) The detection of acetaldehyde in cold dust clouds. Astrophys J 290:609–614. doi:10.1086/163018
McCarthy MC, Gottlieb CA, Gupta H, Thaddeus P (2006) Laboratory and astronomical identification of the negative molecular ion C6H−. Astrophys J Lett 652:L141–L144. doi:10.1086/510238
McKeegan KD, Chaussidon M, Robert F (2000) Incorporation of Short-Lived 10Be in a Calcium-Aluminum-Rich inclusion from the Allende meteorite. Science 289:1334–1337. doi:10.1126/science.289.5483.1334
Meibom A, Krot AN, Robert F, Mostefaoui S, Russell SS, Petaev MI, Gounelle M (2007) Nitrogen and carbon isotopic composition of the sun inferred from a high-temperature solar nebular condensate. Astrophys J Lett 656:L33–L36. doi:10.1086/512052
Meier R, Owen TC, Jewitt DC, Matthews HE, Senay M, Biver N, Bockelee-Morvan D, Crovisier J, Gautier D (1998) Deuterium in comet C/1995 O1 (Hale-Bopp): detection of DCN. Science 279:1707. doi:10.1126/science.279.5357.1707
Meijerink R, Glassgold AE, Najita JR (2008) Atomic diagnostics of X-ray-irradiated protoplanetary disks. Astrophys J 676:518–531. doi:10.1086/527411
Mellon RR, Li ZY (2008) Magnetic braking and protostellar disk formation: the ideal MHD limit. Astrophys J 681:1356–1376. doi:10.1086/587542
Merín B, Augereau JC, van Dishoeck EF, Kessler-Silacci J, Dullemond CP, Blake GA, Lahuis F, Brown JM, Geers VC, Pontoppidan KM, Comerón F, Frasca A, Guieu S, Alcalá JM, Boogert ACA, Evans NJ II, D’Alessio P, Mundy LG, Chapman N (2007) Abundant crystalline silicates in the disk of a very low mass star. Astrophys J 661:361–367. doi:10.1086/513092
Messenger S (2000) Identification of molecular-cloud material in interplanetary dust particles. Nature 404:968–971
Milam SN, Charnley SB (2012) Observations of nitrogen fractionation in prestellar cores: nitriles tracing interstellar chemistry. In: Lunar and planetary institute science conference abstracts, Technical Report, vol 43, p 2618, Lunar and Planetary Inst,
Miyauchi N, Hidaka H, Chigai T, Nagaoka A, Watanabe N, Kouchi A (2008) Formation of hydrogen peroxide and water from the reaction of cold hydrogen atoms with solid oxygen at 10 K. Chem Phys Lett 456:27–30. doi:10.1016/j.cplett.2008.02.095
Modica P, Palumbo ME (2010) Formation of methyl formate after cosmic ion irradiation of icy grain mantles. Astron Astrophys 519:A22. doi:10.1051/0004-6361/201014101
Mokrane H, Chaabouni H, Accolla M, Congiu E, Dulieu F, Chehrouri M, Lemaire JL (2009) Experimental evidence for water formation via ozone hydrogenation on dust grains at 10 K. Astrophys J Lett 705:L195–L198. doi:10.1088/0004-637X/705/2/L195
Morbidelli A, Chambers J, Lunine JI, Petit JM, Robert F, Valsecchi GB, Cyr KE (2000) Source regions and time scales for the delivery of water to earth. Meteorit Planet Sci 35:1309–1320. doi:10.1111/j.1945-5100.2000.tb01518.x
Mouschovias TC (1979) Ambipolar diffusion in interstellar clouds—a new solution. Astrophys J 228:475–481. doi:10.1086/156868
Moynier F, Blichert-Toft J, Wang K, Herzog GF, Albarede F (2011) The elusive 60Fe in the solar nebula. Astrophys J 741:71. doi:10.1088/0004-637X/741/2/71
Müller HSP, Schlöder F, Stutzki J, Winnewisser G (2005) The cologne database for molecular spectroscopy, CDMS: a useful tool for astronomers and spectroscopists. J Mol Struct 742:215–227. doi:10.1016/j.molstruc.2005.01.027
Mumma MJ, Charnley SB (2011) The chemical composition of comets: emerging taxonomies and natal heritage. Annu Rev Astron Astrophys 49:471–524. doi:10.1146/annurev-astro-081309-130811
Muñoz Caro GM, Meierhenrich UJ, Schutte WA, Barbier B, Arcones Segovia A, Rosenbauer H, Thiemann WHP, Brack A, Greenberg JM (2002) Amino acids from ultraviolet irradiation of interstellar ice analogues. Nature 416:403–406
Muñoz Caro GM, Meierhenrich U, Schutte WA, Thiemann WHP, Greenberg JM (2004) UV-photoprocessing of interstellar ice analogs: detection of hexamethylenetetramine-based species. Astron Astrophys 413:209–216. doi:10.1051/0004-6361:20031447
Murakawa K, Tamura M, Nagata T (2000) 1–4 micron spectrophotometry of dust in the Taurus dark cloud: water ice distribution in Heiles cloud 2. Astrophys J Suppl Ser 128:603–613. doi:10.1086/313387
Muzerolle J, Calvet N, Hartmann L, D’Alessio P (2003) Unveiling the inner disk structure of T Tauri stars. Astrophys J Lett 597:L149–L152. doi:10.1086/379921
Nakamura T, Noguchi T, Tanaka M, Zolensky ME, Kimura M, Tsuchiyama A, Nakato A, Ogami T, Ishida H, Uesugi M, Yada T, Shirai K, Fujimura A, Okazaki R, Sandford SA, Ishibashi Y, Abe M, Okada T, Ueno M, Mukai T, Yoshikawa M, Kawaguchi J (2011) Itokawa dust particles: a direct link between S-type asteroids and ordinary chondrites. Science 333:1113. doi:10.1126/science.1207758
Natta A, Prusti T, Neri R, Wooden D, Grinin VP, Mannings V (2001) A reconsideration of disk properties in Herbig Ae stars. Astron Astrophys 371:186–197. doi:10.1051/0004-6361:20010334
Natta A, Testi L, Calvet N, Henning T, Waters R, Wilner D (2007) Dust in protoplanetary disks: properties and evolution. In: Protostars and planets V, pp 767–781
Nguyen AN, Stadermann FJ, Zinner E, Stroud RM, Alexander CMO, Nittler LR (2007) Characterization of presolar silicate and oxide grains in primitive carbonaceous chondrites. Astrophys J 656:1223–1240. doi:10.1086/510612
Nisini B, Benedettini M, Giannini T, Codella C, Lorenzetti D, di Giorgio AM, Richer JS (2000) Far infrared mapping of the gas cooling along the L1448 outflow. Astron Astrophys 360:297–310
Noble JA, Dulieu F, Congiu E, Fraser HJ (2011) CO2 formation in quiescent clouds: an experimental study of the CO + OH pathway. Astrophys J 735:121. doi:10.1088/0004-637X/735/2/121
Oba Y, Watanabe N, Kouchi A, Hama T, Pirronello V (2010) Experimental study of CO2 formation by surface reactions of non-energetic OH radicals with CO molecules. Astrophys J Lett 712:L174–L178. doi:10.1088/2041-8205/712/2/L174
Oba Y, Watanabe N, Hama T, Kuwahata K, Hidaka H, Kouchi A (2012) Water formation through a quantum tunneling surface reaction, OH+H2, at 10 K. Astrophys J 749:67. doi:10.1088/0004-637X/749/1/67
Öberg KI, Garrod RT, van Dishoeck EF, Linnartz H (2009a) Formation rates of complex organics in UV irradiated CH3OH-rich ices. I. Experiments. Astron Astrophys 504:891–913. doi:10.1051/0004-6361/200912559
Öberg KI, Linnartz H, Visser R, van Dishoeck EF (2009b) Photodesorption of ices. II. H2O and D2O. Astrophys J 693:1209–1218. doi:10.1088/0004-637X/693/2/1209
Öberg KI, van Dishoeck EF, Linnartz H (2009c) Photodesorption of ices I: CO, N2, and CO2. Astron Astrophys 496:281–293. doi:10.1051/0004-6361/200810207
Öberg KI, Qi C, Fogel JKJ, Bergin EA, Andrews SM, Espaillat C, van Kempen TA, Wilner DJ, Pascucci I (2010a) The disk imaging survey of chemistry with SMA. I. Taurus protoplanetary disk data. Astrophys J 720:480–493. doi:10.1088/0004-637X/720/1/480
Öberg KI, van Dishoeck EF, Linnartz H, Andersson S (2010b) The effect of H2O on ice photochemistry. Astrophys J 718:832–840. doi:10.1088/0004-637X/718/2/832
Öberg KI, Qi C, Fogel JKJ, Bergin EA, Andrews SM, Espaillat C, Wilner DJ, Pascucci I, Kastner JH (2011a) Disk imaging survey of chemistry with SMA. II. Southern sky protoplanetary disk data and full sample statistics. Astrophys J 734:98. doi:10.1088/0004-637X/734/2/98
Öberg KI, Qi C, Wilner DJ, Andrews SM (2011b) The ionization fraction in the DM Tau protoplanetary disk. Astrophys J 743:152. doi:10.1088/0004-637X/743/2/152
Öberg KI, van der Marel N, Kristensen LE, van Dishoeck EF (2011c) Complex molecules toward Low-mass protostars: the Serpens core. Astrophys J 740:14. doi:10.1088/0004-637X/740/1/14
Ohishi M, Kaifu N (1998) Chemical and physical evolution of dark clouds. Molecular spectral line survey toward TMC-1. Faraday Discuss 109:205. doi:10.1039/a801058g
Oliveira CM, Hébrard G, Howk JC, Kruk JW, Chayer P, Moos HW (2003) Interstellar deuterium, nitrogen, and oxygen abundances toward GD 246, WD 2331-475, HZ 21, and Lanning 23: results from the FUSE mission. Astrophys J 587:235–255. doi:10.1086/368019
Olofsson J, Juhász A, Henning T, Mutschke H, Tamanai A, Moór A, Ábrahám P (2012) Transient dust in warm debris disks. Detection of Fe-rich olivine grains. Astron Astrophys 542:A90. doi:10.1051/0004-6361/201118735
Ossenkopf V, Henning T (1994) Dust opacities for protostellar cores. Astron Astrophys 291:943–959
Owen T, Bar-Nun A (1995) Comets, impacts and atmospheres. Icarus 116:215–226. doi:10.1006/icar.1995.1122
Owen T, Mahaffy PR, Niemann HB, Atreya S, Wong M (2001) Protosolar nitrogen. Astrophys J Lett 553:L77–L79. doi:10.1086/320501
Padovani M, Galli D (2011) Effects of magnetic fields on the cosmic-ray ionization of molecular cloud cores. Astron Astrophys 530:A109. doi:10.1051/0004-6361/201116853
Pagani L, Bacmann A, Cabrit S, Vastel C (2007) Depletion and low gas temperature in the L183 (=L134N) prestellar core: the N2H+-N2D+ tool. Astron Astrophys 467:179–186. doi:10.1051/0004-6361:20066670
Pagani L, Vastel C, Hugo E, Kokoouline V, Greene CH, Bacmann A, Bayet E, Ceccarelli C, Peng R, Schlemmer S (2009) Chemical modeling of L183 (L134N): an estimate of the ortho/para H2 ratio. Astron Astrophys 494:623–636. doi:10.1051/0004-6361:200810587
Pagani L, Steinacker J, Bacmann A, Stutz A, Henning T (2010) The ubiquity of Micrometer-Sized dust grains in the dense interstellar medium. Science 329:1622. doi:10.1126/science.1193211
Palumbo ME, Pendleton YJ, Strazzulla G (2000) Hydrogen isotopic substitution studies of the 2165 wavenumber (4.62 micron) “XCN” feature produced by ion bombardment. Astrophys J 542:890–893. doi:10.1086/317061
Parise B, Ceccarelli C, Tielens AGGM, Herbst E, Lefloch B, Caux E, Castets A, Mukhopadhyay I, Pagani L, Loinard L (2002) Detection of doubly-deuterated methanol in the solar-type protostar IRAS 16293-2422. Astron Astrophys 393:L49–L53. doi:10.1051/0004-6361:20021131
Parise B, Castets A, Herbst E, Caux E, Ceccarelli C, Mukhopadhyay I, Tielens AGGM (2004) First detection of triply-deuterated methanol. Astron Astrophys 416:159–163. doi:10.1051/0004-6361:20034490
Parise B, Caux E, Castets A, Ceccarelli C, Loinard L, Tielens AGGM, Bacmann A, Cazaux S, Comito C, Helmich F, Kahane C, Schilke P, van Dishoeck E, Wakelam V, Walters A (2005) HDO abundance in the envelope of the solar-type protostar IRAS 16293-2422. Astron Astrophys 431:547–554. doi:10.1051/0004-6361:20041899
Parise B, Ceccarelli C, Tielens AGGM, Castets A, Caux E, Lefloch B, Maret S (2006) Testing grain surface chemistry: a survey of deuterated formaldehyde and methanol in low-mass class 0 protostars. Astron Astrophys 453:949–958. doi:10.1051/0004-6361:20054476
Parise B, Belloche A, Du F, Güsten R, Menten KM (2011) Extended emission of D2H+ in a prestellar core. Astron Astrophys 526:A31. doi:10.1051/0004-6361/201015475
Peng R, Yoshida H, Chamberlin RA, Phillips TG, Lis DC, Gerin M (2010) A comprehensive survey of hydrogen chloride in the galaxy. Astrophys J 723:218–228. doi:10.1088/0004-637X/723/1/218
Peng TC, Despois D, Brouillet N, Parise B, Baudry A (2012) Deuterated methanol in orion BN/KL. Astron Astrophys 543:A152. doi:10.1051/0004-6361/201118310
Persson MV, Jørgensen JK, van Dishoeck EF (2012) Subarcsecond resolution observations of warm water toward three deeply embedded low-mass protostars. Astron Astrophys 541:A39. doi:10.1051/0004-6361/201117917
Petit JM, Mousis O, Kavelaars JJ (2012) Formation location of Enceladus and comets from D/H measurements. In: Lunar and planetary institute science conference abstracts, vol 43, p 1937
Pickett HM, Poynter RL, Cohen EA, Delitsky ML, Pearson JC, Müller HSP (1998) Submillimeter, millimeter and microwave spectral line catalog. J Quant Spectrosc Radiat Transf 60:883–890. doi:10.1016/S0022-4073(98)00091-0
Pilling S, Andrade DPP, da Silveira EF, Rothard H, Domaracka A, Boduch P (2012) Formation of unsaturated hydrocarbons in interstellar ice analogues by cosmic rays. Mon Not R Astron Soc 423:2209–2221. doi:10.1111/j.1365-2966.2012.21031.x
Pineda JE, Maury AJ, Fuller GA, Testi L, García-Appadoo D, Peck AB, Villard E, Corder SA, van Kempen TA, Turner JL, Tachihara K, Dent W (2012) The first ALMA view of IRAS 16293-2422. Direct detection of infall onto source B and high-resolution kinematics of source A. Astron Astrophys 544:L7. doi:10.1051/0004-6361/201219589
Pirronello V, Liu C, Roser JE, Vidali G (1999) Measurements of molecular hydrogen formation on carbonaceous grains. Astron Astrophys 344:681–686
Pizzarello S, Holmes W (2009) Nitrogen-containing compounds in two CR2 meteorites: 15N composition, molecular distribution and precursor molecules. Geochim Cosmochim Acta 73:2150–2162
Pizzarello S, Huang Y (2005) The deuterium enrichment of individual amino acids in carbonaceous meteorites: a case for the presolar distribution of biomolecule precursors. Geochim Cosmochim Acta 69:599–605. doi:10.1016/j.gca.2004.07.031
Pizzarello S, Huang Y, Becker L, Poreda RJ, Nieman RA, Cooper G, Williams M (2001) The organic content of the Tagish lake meteorite. Science 293:2236–2239. doi:10.1126/science.1062614
Pizzarello S, Zolensky M, Turk KA (2003) Nonracemic isovaline in the Murchison meteorite: chiral distribution and mineral association. Geochim Cosmochim Acta 67:1589–1595. doi:10.1016/S0016-7037(02)01283-8
Podio L, Kamp I, Flower D, Howard C, Sandell G, Mora A, Aresu G, Brittain S, Dent WRF, Pinte C, White GJ (2012) Herschel/PACS observations of young sources in Taurus: the far-infrared counterpart of optical jets. Astron Astrophys 545:A44. doi:10.1051/0004-6361/201219475
Pollack JB, Hubickyj O, Bodenheimer P, Lissauer JJ, Podolak M, Greenzweig Y (1996) Formation of the giant planets by concurrent accretion of solids and gas. Icarus 124:62–85. doi:10.1006/icar.1996.0190
Pontoppidan KM, Salyk C, Blake GA, Käufl HU (2010a) Spectrally resolved pure rotational lines of water in protoplanetary disks. Astrophys J Lett 722:L173–L177. doi:10.1088/2041-8205/722/2/L173
Pontoppidan KM, Salyk C, Blake GA, Meijerink R, Carr JS, Najita J (2010b) A Spitzer survey of mid-infrared molecular emission from protoplanetary disks. I. Detection rates. Astrophys J 720:887–903. doi:10.1088/0004-637X/720/1/887
Prasad SS, Tarafdar SP (1983) UV radiation field inside dense clouds—its possible existence and chemical implications. Astrophys J 267:603–609. doi:10.1086/160896
Preibisch T, Feigelson ED (2005) The evolution of X-ray emission in young stars. Astrophys J Suppl Ser 160:390–400. doi:10.1086/432094
Qi C, Ho PTP, Wilner DJ, Takakuwa S, Hirano N, Ohashi N, Bourke TL, Zhang Q, Blake GA, Hogerheijde M, Saito M, Choi M, Yang J (2004) Imaging the disk around TW Hydrae with the submillimeter array. Astrophys J Lett 616:L11–L14. doi:10.1086/421063
Qi C, Wilner DJ, Aikawa Y, Blake GA, Hogerheijde MR (2008) Resolving the chemistry in the disk of TW Hydrae. I. Deuterated species. Astrophys J 681:1396–1407. doi:10.1086/588516
Quan D, Herbst E, Osamura Y, Roueff E (2010) Gas-grain modeling of isocyanic acid (HNCO), cyanic acid (HOCN), fulminic acid (HCNO), and isofulminic acid (HONC) in assorted interstellar environments. Astrophys J 725:2101–2109. doi:10.1088/0004-637X/725/2/2101
Rafikov RR (2006) Microwave emission from spinning dust in circumstellar disks. Astrophys J 646:288–296. doi:10.1086/504793
Ratajczak A, Taquet V, Kahane C, Ceccarelli C, Faure A, Quirico E (2011) The puzzling deuteration of methanol in low- to high-mass protostars. Astron Astrophys 528:L13. doi:10.1051/0004-6361/201016402
Raymond SN, O’Brien DP, Morbidelli A, Kaib NA (2009) Building the terrestrial planets: constrained accretion in the inner solar system. Icarus 203:644–662. doi:10.1016/j.icarus.2009.05.016
Redman MP, Rawlings JMC, Nutter DJ, Ward-Thompson D, Williams DA (2002) Molecular gas freeze-out in the pre-stellar core L1689B. Mon Not R Astron Soc 337:L17–L21. doi:10.1046/j.1365-8711.2002.06106.x
Remijan AJ, Hollis JM, Snyder LE, Jewell PR, Lovas FJ (2006) Methyltriacetylene (CH3C6H) toward TMC-1: the largest detected symmetric top. Astrophys J Lett 643:L37–L40. doi:10.1086/504918
Remusat L, Palhol F, Robert F, Derenne S (2005) Hydrogen isotopic composition of aliphatic linkages in carbonaceous chondrites insoluble organic matter. In: Mackwell S, Stansbery E (eds) 36th annual lunar and planetary science conference, lunar and planetary institute science conference abstracts, vol 36, p 1350
Remusat L, Robert F, Meibom A, Mostefaoui S, Delpoux O, Binet L, Gourier D, Derenne S (2009) Proto-planetary disk chemistry recorded by D-rich organic radicals in carbonaceous chondrites. Astrophys J 698:2087–2092. doi:10.1088/0004-637X/698/2/2087
Requena-Torres MA, Marcelino N, Jiménez-Serra I, Martín-Pintado J, Martín S, Mauersberger R (2007) Organic chemistry in the dark clouds L1448 and L183: a unique grain mantle composition. Astrophys J Lett 655:L37–L40. doi:10.1086/511677
Riaz B, Honda M, Campins H, Micela G, Guarcello MG, Gledhill T, Hough J, Martín EL (2012) The radial distribution of dust species in young brown Dwarf discs. Mon Not R Astron Soc 420:2603–2624. doi:10.1111/j.1365-2966.2011.20233.x
Ricci L, Testi L, Maddison ST, Wilner DJ (2012) Fomalhaut debris disk emission at 7 millimeters: constraints on the collisional models of planetesimals. Astron Astrophys 539:L6. doi:10.1051/0004-6361/201118524
Robert F (2003) The D/H ratio in chondrites. Space Sci Rev 106:87–101. doi:10.1023/A:1024629402715
Robert F, Derenne S (2006) The molecular structure and isotopic compositions of the insoluble organic matter in chondrites. Meteorit Planet Sci 41:5259
Roberts H, Herbst E, Millar TJ (2003) Enhanced deuterium fractionation in dense interstellar cores resulting from multiply deuterated \(\mathrm{H}^{+}_{3}\). Astrophys J Lett 591:L41–L44. doi:10.1086/376962
Roberts JF, Rawlings JMC, Viti S, Williams DA (2007) Desorption from interstellar ices. Mon Not R Astron Soc 382:733–742. doi:10.1111/j.1365-2966.2007.12402.x
Robitaille TP, Whitney BA, Indebetouw R, Wood K, Denzmore P (2006) Interpreting spectral energy distributions from young stellar objects. I. A grid of 200,000 YSO model SEDs. Astrophys J Suppl Ser 167:256–285. doi:10.1086/508424
Rodríguez LF, Loinard L, D’Alessio P, Wilner DJ, Ho PTP (2005) IRAS 16293-2422B: a compact, possibly isolated protoplanetary disk in a class 0 object. Astrophys J Lett 621:L133–L136. doi:10.1086/429223
Romanzin C, Ioppolo S, Cuppen HM, van Dishoeck EF, Linnartz H (2011) Water formation by surface O3 hydrogenation. J Chem Phys 134(8):084,504. doi:10.1063/1.3532087
Sakai N, Ikeda M, Morita M, Sakai T, Takano S, Osamura Y, Yamamoto S (2007) Production pathways of CCS and CCCS inferred from their 13C isotopic species. Astrophys J 663:1174–1179. doi:10.1086/518595
Sakai N, Sakai T, Hirota T, Yamamoto S (2008) Abundant Carbon-Chain molecules toward the Low-Mass protostar IRAS 04368+2557 in L1527. Astrophys J 672:371–381. doi:10.1086/523635
Sakai N, Saruwatari O, Sakai T, Takano S, Yamamoto S (2010a) Abundance anomaly of the 13C species of CCH. Astron Astrophys 512:A31. doi:10.1051/0004-6361/200913098
Sakai N, Shiino T, Hirota T, Sakai T, Yamamoto S (2010b) Long Carbon-chain molecules and their anions in the starless core, Lupus-1A. Astrophys J Lett 718:L49–L52. doi:10.1088/2041-8205/718/2/L49
Salyk C, Pontoppidan KM, Blake GA, Lahuis F, van Dishoeck EF, Evans II NJ (2008) H2O and OH gas in the terrestrial planet-forming zones of protoplanetary disks. Astrophys J Lett 676:L49–L52. doi:10.1086/586894
Salyk C, Pontoppidan KM, Blake GA, Najita JR, Carr JS (2011) A Spitzer survey of mid-infrared molecular emission from protoplanetary disks. II. Correlations and local thermal equilibrium models. Astrophys J 731:130. doi:10.1088/0004-637X/731/2/130
Santangelo G, Nisini B, Giannini T, Antoniucci S, Vasta M, Codella C, Lorenzani A, Tafalla M, Liseau R, van Dishoeck EF, Kristensen LE (2012) The Herschel HIFI water line survey in the low-mass proto-stellar outflow L1448. Astron Astrophys 538:A45. doi:10.1051/0004-6361/201118113
Sargent BA, Forrest WJ, Tayrien C, McClure MK, Watson DM, Sloan GC, Li A, Manoj P, Bohac CJ, Furlan E, Kim KH, Green JD (2009) Dust processing and grain growth in protoplanetary disks in the Taurus-Auriga star-forming region. Astrophys J Suppl Ser 182:477–508. doi:10.1088/0067-0049/182/2/477
Schaller EL, Brown ME (2007) Volatile loss and retention on Kuiper belt objects. In: AAS/Division for planetary sciences meeting abstracts. Bulletin of the American astronomical society, vol 39, p 511
Schöier FL, Jørgensen JK, van Dishoeck EF, Blake GA (2002) Does IRAS 16293-2422 have a hot core? Chemical inventory and abundance changes in its protostellar environment. Astron Astrophys 390:1001–1021. doi:10.1051/0004-6361:20020756
Schöier FL, van der Tak FFS, van Dishoeck EF, Black JH (2005) An atomic and molecular database for analysis of submillimetre line observations. Astron Astrophys 432:369–379. doi:10.1051/0004-6361:20041729
Schopf JW, Kudryavtsev AB, Agresti DG, Wdowiak TJ, Czaja AD (2002) Laser-Raman imagery of Earth’s earliest fossils. Nature 416:73–76
Schräpler R, Blum J, Seizinger A, Kley W (2012) The physics of protoplanetesimal dust agglomerates. VII. The low-velocity collision behavior of large dust agglomerates. ArXiv e-prints
Semenov DA (2011) Chemical evolution of a protoplanetary disk. In: IAU symposium, vol 280, pp 114–126. doi:10.1017/S1743921311024914
Shen CJ, Greenberg JM, Schutte WA, van Dishoeck EF (2004) Cosmic ray induced explosive chemical desorption in dense clouds. Astron Astrophys 415:203–215. doi:10.1051/0004-6361:20031669
Shimajiri Y, Takahashi S, Takakuwa S, Saito M, Kawabe R (2008) Millimeter- and submillimeter-wave observations of the OMC-2/3 region. II. Observational evidence for outflow-triggered star formation in the OMC-2 FIR 3/4 region. Astrophys J 683:255–266. doi:10.1086/588629
Shu FH (1977) Self-similar collapse of isothermal spheres and star formation. Astrophys J 214:488–497. doi:10.1086/155274
Shu FH, Adams FC, Lizano S (1987) Star formation in molecular clouds—observation and theory. Annu Rev Astron Astrophys 25:23–81. doi:10.1146/annurev.aa.25.090187.000323
Sicilia D, Ioppolo S, Vindigni T, Baratta GA, Palumbo ME (2012) Nitrogen oxides and carbon chain oxides formed after ion irradiation of CO:N2 ice mixtures. Astron Astrophys 543:A155. doi:10.1051/0004-6361/201219390
Siebenmorgen R, Heymann F (2012) Polycyclic aromatic hydrocarbons in protoplanetary disks: emission and X-ray destruction. Astron Astrophys 543:A25. doi:10.1051/0004-6361/201219039
Siebenmorgen R, Krügel E (2010) The destruction and survival of polycyclic aromatic hydrocarbons in the disks of T Tauri stars. Astron Astrophys 511:A6. doi:10.1051/0004-6361/200912035
Skrzypczak A, Binet L, Gourier D, Derenne S, Robert F (2003) On the controversial biogenicity of the organic matter in the oldest Archean cherts: can electron paramagnetic resonance provide clues? In: Mackwell S, Stansbery E (eds) Lunar and planetary institute science conference abstracts, lunar and planetary institute science conference abstracts, vol 34, p 1677
Snyder LE, Hollis JM, Jewell PR, Lovas FJ, Remijan A (2006) Confirmation of interstellar methylcyanodiacetylene (CH3C5N). Astrophys J 647:412–417. doi:10.1086/505323
Spitzer L (1978) Physical processes in the interstellar medium
Stäuber P, Doty SD, van Dishoeck EF, Benz AO (2005) X-ray chemistry in the envelopes around young stellar objects. Astron Astrophys 440:949–966. doi:10.1051/0004-6361:20052889
Sturm B, Bouwman J, Henning T, Evans NJ, Acke B, Mulders GD, Waters LBFM, van Dishoeck EF, Meeus G, Green JD, Augereau JC, Olofsson J, Salyk C, Najita J, Herczeg GJ, van Kempen TA, Kristensen LE, Dominik C, Carr JS, Waelkens C, Bergin E, Blake GA, Brown JM, Chen JH, Cieza L, Dunham MM, Glassgold A, Güdel M, Harvey PM, Hogerheijde MR, Jaffe D, Jørgensen JK, Kim HJ, Knez C, Lacy JH, Lee JE, Maret S, Meijerink R, Merín B, Mundy L, Pontoppidan KM, Visser R, Yıldız UA (2010) First results of the Herschel key program “Dust, ice and gas in time” (DIGIT): dust and gas spectroscopy of HD 100546. Astron Astrophys 518:L129. doi:10.1051/0004-6361/201014674
Tafalla M, Myers PC, Caselli P, Walmsley CM (2004) On the internal structure of starless cores. I. Physical conditions and the distribution of CO, CS, N2H+, and NH3 in L1498 and L1517B. Astron Astrophys 416:191–212. doi:10.1051/0004-6361:20031704
Tafalla M, Santiago-García J, Myers PC, Caselli P, Walmsley CM, Crapsi A (2006) On the internal structure of starless cores. II. A molecular survey of L1498 and L1517B. Astron Astrophys 455:577–593. doi:10.1051/0004-6361:20065311
Taquet V, Ceccarelli C, Kahane C (2012a) Formaldehyde and methanol deuteration in protostars: fossils from a past fast high-density pre-collapse phase. Astrophys J Lett 748:L3. doi:10.1088/2041-8205/748/1/L3
Taquet V, Ceccarelli C, Kahane C (2012b) Multilayer modeling of porous grain surface chemistry. I. The GRAINOBLE model. Astron Astrophys 538:A42. doi:10.1051/0004-6361/201117802
Taquet V, Lopez-Sepulcre A, Ceccarelli C, Kahane C (2012c, in print) Arcsecond resolution observations of deuterated water towards low-mass protostar. Astron Astrophys A42. doi:10.1051/0004-6361/201117812
Taquet V, Peters P, Kahane C, Ceccarelli C, Lopez-Sepulcre A, Toubin C, Duflot D, Wiesenfeld L (2012d, in print) Modelling of deuterated water ice formation. Astron Astrophys A42. doi:10.1051/0004-6361/201117802
Tatulli E, Isella A, Natta A, Testi L, Marconi A, Malbet F, Stee P, Petrov RG, Millour F, Chelli A, Duvert G, Antonelli P, Beckmann U, Bresson Y, Dugué M, Gennari S, Glück L, Kern P, Lagarde S, Le Coarer E, Lisi F, Perraut K, Puget P, Rantakyrö F, Robbe-Dubois S, Roussel A, Weigelt G, Zins G, Accardo M, Acke B, Agabi K, Altariba E, Arezki B, Aristidi E, Baffa C, Behrend J, Blöcker T, Bonhomme S, Busoni S, Cassaing F, Clausse JM, Colin J, Connot C, Delboulbé A, Domiciano de Souza A, Driebe T, Feautrier P, Ferruzzi D, Forveille T, Fossat E, Foy R, Fraix-Burnet D, Gallardo A, Giani E, Gil C, Glentzlin A, Heiden M, Heininger M, Hernandez Utrera O, Hofmann KH, Kamm D, Kiekebusch M, Kraus S, Le Contel D, Le Contel JM, Lesourd T, Lopez B, Lopez M, Magnard Y, Mars G, Martinot-Lagarde G, Mathias P, Mège P, Monin JL, Mouillet D, Mourard D, Nussbaum E, Ohnaka K, Pacheco J, Perrier C, Rabbia Y, Rebattu S, Reynaud F, Richichi A, Robini A, Sacchettini M, Schertl D, Schöller M, Solscheid W, Spang A, Stefanini P, Tallon M, Tallon-Bosc I, Tasso D, Vakili F, von der Lühe O, Valtier JC, Vannier M (2007) Constraining the wind launching region in Herbig Ae stars: AMBER/VLTI spectroscopy of HD 104237. Astron Astrophys 464:55–58. doi:10.1051/0004-6361:20065719
Terada H, Tokunaga AT, Kobayashi N, Takato N, Hayano Y, Takami H (2007) Detection of water ice in Edge-on protoplanetary disks: HK Tauri B and HV Tauri C. Astrophys J 667:303–307. doi:10.1086/520951
Testi L, Natta A, Shepherd DS, Wilner DJ (2003) Large grains in the disk of CQ Tau. Astron Astrophys 403:323–328. doi:10.1051/0004-6361:20030362
Thi WF, van Zadelhoff GJ, van Dishoeck EF (2004) Organic molecules in protoplanetary disks around T Tauri and Herbig Ae stars. Astron Astrophys 425:955–972. doi:10.1051/0004-6361:200400026
Thi WF, Mathews G, Ménard F, Woitke P, Meeus G, Riviere-Marichalar P, Pinte C, Howard CD, Roberge A, Sandell G, Pascucci I, Riaz B, Grady CA, Dent WRF, Kamp I, Duchêne G, Augereau JC, Pantin E, Vandenbussche B, Tilling I, Williams JP, Eiroa C, Barrado D, Alacid JM, Andrews S, Ardila DR, Aresu G, Brittain S, Ciardi DR, Danchi W, Fedele D, de Gregorio-Monsalvo I, Heras A, Huelamo N, Krivov A, Lebreton J, Liseau R, Martin-Zaidi C, Mendigutía I, Montesinos B, Mora A, Morales-Calderon M, Nomura H, Phillips N, Podio L, Poelman DR, Ramsay S, Rice K, Solano E, Walker H, White GJ, Wright G (2010a) Herschel-PACS observation of the 10 Myr old T Tauri disk TW Hya. Constraining the disk gas mass. Astron Astrophys 518:L125. doi:10.1051/0004-6361/201014578
Thi WF, Woitke P, Kamp I (2010b) Warm non-equilibrium gas phase chemistry as a possible origin of high HDO/H2O ratios in hot and dense gases: application to inner protoplanetary discs. Mon Not R Astron Soc 407:232–246. doi:10.1111/j.1365-2966.2009.16162.x
Thomas KL, Blanford GE, Keller LP, Klock W, McKay DS (1993) Carbon abundance and silicate mineralogy of anhydrous interplanetary dust particles. Geochim Cosmochim Acta 57:1551–1566. doi:10.1016/0016-7037(93)90012-L
Tielens AGGM (1983) Surface chemistry of deuterated molecules. Astron Astrophys 119:177–184
Tielens AGGM (2005) The physics and chemistry of the interstellar medium
Troland TH, Crutcher RM (2008) Magnetic fields in dark cloud cores: Arecibo OH Zeeman observations. Astrophys J 680:457–465. doi:10.1086/587546
Troscompt N, Faure A, Maret S, Ceccarelli C, Hily-Blant P, Wiesenfeld L (2009) Constraining the ortho-to-para ratio of H2 with anomalous H2CO absorption. Astron Astrophys 506:1243–1247. doi:10.1051/0004-6361/200912770
van der Tak FFS, Schilke P, Müller HSP, Lis DC, Phillips TG, Gerin M, Roueff E (2002) Triply deuterated ammonia in NGC 1333. Astron Astrophys 388:L53–L56. doi:10.1051/0004-6361:20020647
van der Tak FFS, Caselli P, Ceccarelli C (2005) Line profiles of molecular ions toward the pre-stellar core LDN 1544. Astron Astrophys 439:195–203. doi:10.1051/0004-6361:20052792
van Dishoeck EF, Thi WF, van Zadelhoff GJ (2003) Detection of DCO+ in a circumstellar disk. Astron Astrophys 400:L1–L4. doi:10.1051/0004-6361:20030091
van Dishoeck EF, Kristensen LE, Benz AO, Bergin EA, Caselli P, Cernicharo J, Herpin F, Hogerheijde MR, Johnstone D, Liseau R, Nisini B, Shipman R, Tafalla M, van der Tak F, Wyrowski F, Aikawa Y, Bachiller R, Baudry A, Benedettini M, Bjerkeli P, Blake GA, Bontemps S, Braine J, Brinch C, Bruderer S, Chavarría L, Codella C, Daniel F, de Graauw T, Deul E, di Giorgio AM, Dominik C, Doty SD, Dubernet ML, Encrenaz P, Feuchtgruber H, Fich M, Frieswijk W, Fuente A, Giannini T, Goicoechea JR, Helmich FP, Herczeg GJ, Jacq T, Jørgensen JK, Karska A, Kaufman MJ, Keto E, Larsson B, Lefloch B, Lis D, Marseille M, McCoey C, Melnick G, Neufeld D, Olberg M, Pagani L, Panić O, Parise B, Pearson JC, Plume R, Risacher C, Salter D, Santiago-García J, Saraceno P, Stäuber P, van Kempen TA, Visser R, Viti S, Walmsley M, Wampfler SF (2011) Water in Star-forming regions with the Herschel space observatory (WISH). I. Overview of key program and first results. Publ Astron Soc Pac 123:138–170. doi:10.1086/658676
van Kempen TA, van Dishoeck EF, Güsten R, Kristensen LE, Schilke P, Hogerheijde MR, Boland W, Menten KM, Wyrowski F (2009) APEX-CHAMP+ high-J CO observations of low-mass young stellar objects. II. Distribution and origin of warm molecular gas. Astron Astrophys 507:1425–1442. doi:10.1051/0004-6361/200912507
van Zadelhoff GJ, van Dishoeck EF, Thi WF, Blake GA (2001) Submillimeter lines from circumstellar disks around pre-main sequence stars. Astron Astrophys 377:566–580. doi:10.1051/0004-6361:20011137
Vasta M, Codella C, Lorenzani A, Santangelo G, Nisini B, Giannini T, Tafalla M, Liseau R, van Dishoeck EF, Kristensen L (2012) Water emission from the chemically rich outflow L1157. Astron Astrophys 537:A98. doi:10.1051/0004-6361/201118201
Vastel C, Phillips TG, Ceccarelli C, Pearson J (2003) First detection of doubly deuterated hydrogen sulfide. Astrophys J Lett 593:L97–L100. doi:10.1086/378261
Vastel C, Phillips TG, Yoshida H (2004) Detection of D2H+ in the dense interstellar medium. Astrophys J Lett 606:L127–L130. doi:10.1086/421265
Vastel C, Ceccarelli C, Caux E, Coutens A, Cernicharo J, Bottinelli S, Demyk K, Faure A, Wiesenfeld L, Scribano Y, Bacmann A, Hily-Blant P, Maret S, Walters A, Bergin EA, Blake GA, Castets A, Crimier N, Dominik C, Encrenaz P, Gérin M, Hennebelle P, Kahane C, Klotz A, Melnick G, Pagani L, Parise B, Schilke P, Wakelam V, Baudry A, Bell T, Benedettini M, Boogert A, Cabrit S, Caselli P, Codella C, Comito C, Falgarone E, Fuente A, Goldsmith PF, Helmich F, Henning T, Herbst E, Jacq T, Kama M, Langer W, Lefloch B, Lis D, Lord S, Lorenzani A, Neufeld D, Nisini B, Pacheco S, Pearson J, Phillips T, Salez M, Saraceno P, Schuster K, Tielens X, van der Tak F, van der Wiel MHD, Viti S, Wyrowski F, Yorke H, Cais P, Krieg JM, Olberg M, Ravera L (2010) Ortho-to-para ratio of interstellar heavy water. Astron Astrophys 521:L31. doi:10.1051/0004-6361/201015101
Vasyunin AI, Semenov D, Henning T, Wakelam V, Herbst E, Sobolev AM (2008) Chemistry in protoplanetary disks: a sensitivity analysis. Astrophys J 672:629–641. doi:10.1086/523887
Vasyunin AI, Wiebe DS, Birnstiel T, Zhukovska S, Henning T, Dullemond CP (2011) Impact of grain evolution on the chemical structure of protoplanetary disks. Astrophys J 727:76. doi:10.1088/0004-637X/727/2/76
Villeneuve J, Chaussidon M, Libourel G (2009) Homogeneous distribution of 26Al in the solar system from the Mg isotopic composition of chondrules. Science 325:985. doi:10.1126/science.1173907
Visser R, Geers VC, Dullemond CP, Augereau JC, Pontoppidan KM, van Dishoeck EF (2007) PAH chemistry and IR emission from circumstellar disks. Astron Astrophys 466:229–241. doi:10.1051/0004-6361:20066829
Visser R, van Dishoeck EF, Doty SD, Dullemond CP (2009) The chemical history of molecules in circumstellar disks. I. Ices. Astron Astrophys 495:881–897. doi:10.1051/0004-6361/200810846
Visser R, Doty SD, van Dishoeck EF (2011) The chemical history of molecules in circumstellar disks. II. Gas-phase species. Astron Astrophys 534:A132. doi:10.1051/0004-6361/201117249
Visser R, Kristensen LE, Bruderer S, van Dishoeck EF, Herczeg GJ, Brinch C, Doty SD, Harsono D, Wolfire MG (2012) Modelling Herschel observations of hot molecular gas emission from embedded low-mass protostars. Astron Astrophys 537:A55. doi:10.1051/0004-6361/201117109
Viti S, Collings MP, Dever JW, McCoustra MRS, Williams DA (2004) Evaporation of ices near massive stars: models based on laboratory temperature programmed desorption data. Mon Not R Astron Soc 354:1141–1145. doi:10.1111/j.1365-2966.2004.08273.x
Vorobyov EI (2011) Embedded protostellar disks around (sub-)solar stars. II. Disk masses, sizes, densities, temperatures, and the planet formation perspective. Astrophys J 729:146. doi:10.1088/0004-637X/729/2/146
Wakelam V, Herbst E (2008) Polycyclic aromatic hydrocarbons in dense cloud chemistry. Astrophys J 680:371–383. doi:10.1086/587734
Wakelam V, Herbst E, Selsis F (2006) The effect of uncertainties on chemical models of dark clouds. Astron Astrophys 451:551–562. doi:10.1051/0004-6361:20054682
Wakelam V, Herbst E, Loison JC, Smith IWM, Chandrasekaran V, Pavone B, Adams NG, Bacchus-Montabonel MC, Bergeat A, Béroff K, Bierbaum VM, Chabot M, Dalgarno A, van Dishoeck EF, Faure A, Geppert WD, Gerlich D, Galli D, Hébrard E, Hersant F, Hickson KM, Honvault P, Klippenstein SJ, Le Picard S, Nyman G, Pernot P, Schlemmer S, Selsis F, Sims IR, Talbi D, Tennyson J, Troe J, Wester R, Wiesenfeld L (2012) A KInetic database for astrochemistry (KIDA). Astrophys J Suppl Ser 199:21. doi:10.1088/0067-0049/199/1/21
Walmsley CM, Jewell PR, Snyder LE, Winnewisser G (1984) Detection of interstellar methyldiacetylene (CH3C4H) in the dark dust cloud TMC 1. Astron Astrophys 134:L11–L14
Walsh C, Nomura H, Millar TJ, Aikawa Y (2012) Chemical processes in protoplanetary disks. II. On the importance of photochemistry and X-ray ionization. Astrophys J 747:114. doi:10.1088/0004-637X/747/2/114
Ward-Thompson D, Scott PF, Hills RE, Andre P (1994) A submillimetre continuum survey of pre protostellar cores. Mon Not R Astron Soc 268:276
Ward-Thompson D, Motte F, Andre P (1999) The initial conditions of isolated star formation. III. Millimetre continuum mapping of pre-stellar cores. Mon Not R Astron Soc 305:143–150. doi:10.1046/j.1365-8711.1999.02412.x
Wardle M (2004) Star formation and the hall effect. Astrophys Space Sci 292:317–323. doi:10.1023/B:ASTR.0000045033.80068.1f
Watanabe N, Kouchi A (2002) Efficient formation of formaldehyde and methanol by the addition of hydrogen atoms to CO in H2O-CO ice at 10 K. Astrophys J Lett 571:L173–L176. doi:10.1086/341412
Watson WD (1974) Ion-molecule reactions, molecule formation, and hydrogen-isotope exchange in dense interstellar clouds. Astrophys J 188:35–42. doi:10.1086/152681
Weidenschilling SJ (1977) Aerodynamics of solid bodies in the solar nebula. Mon Not R Astron Soc 180:57–70
Whittet DCB (2010) Oxygen depletion in the interstellar medium: implications for grain models and the distribution of elemental oxygen. Astrophys J 710:1009–1016. doi:10.1088/0004-637X/710/2/1009
Whittet DCB, Duley WW (1991) Carbon monoxide frosts in the interstellar medium. Astron Astrophys Rev 2:167–189. doi:10.1007/BF00872766
Whittet DCB, Cook AM, Herbst E, Chiar JE, Shenoy SS (2011) Observational constraints on methanol production in interstellar and preplanetary ices. Astrophys J 742:28. doi:10.1088/0004-637X/742/1/28
Wienen M, Wyrowski F, Schuller F, Menten KM, Walmsley CM, Bronfman L, Motte F (2012) Ammonia from cold high-mass clumps discovered in the inner galactic disk by the ATLASGAL survey. Astron Astrophys 544:A146. doi:10.1051/0004-6361/201118107
Willacy K, Langer WD (2000) The importance of photoprocessing in protoplanetary disks. Astrophys J 544:903–920. doi:10.1086/317236
Willacy K, Millar TJ (1998) Desorption processes and the deuterium fractionation in molecular clouds. Mon Not R Astron Soc 298:562–568. doi:10.1046/j.1365-8711.1998.01648.x
Willacy K, Langer WD, Velusamy T (1998) Dust emission and molecular depletion in L1498. Astrophys J Lett 507:L171–L175. doi:10.1086/311695
Willacy K, Langer W, Allen M, Bryden G (2006) Turbulence-driven diffusion in protoplanetary disks: chemical effects in the outer regions. Astrophys J 644:1202–1213. doi:10.1086/503702
Williams JP, Cieza LA (2011) Protoplanetary disks and their evolution. Annu Rev Astron Astrophys 49:67–117. doi:10.1146/annurev-astro-081710-102548
Wilner DJ, D’Alessio P, Calvet N, Claussen MJ, Hartmann L (2005) Toward planetesimals in the disk around TW Hydrae: 3.5 centimeter dust emission. Astrophys J Lett 626:L109–L112. doi:10.1086/431757
Windmark F, Birnstiel T, Ormel CW, Dullemond CP (2012) Breaking through: the effects of a velocity distribution on barriers to dust growth. Astron Astrophys 544:L16. doi:10.1051/0004-6361/201220004
Wirström ES, Charnley SB, Cordiner MA, Milam SN (2012) Isotopic anomalies in primitive solar system matter: spin-state-dependent fractionation of nitrogen and deuterium in interstellar clouds. Astrophys J Lett 757:L11. doi:10.1088/2041-8205/757/1/L11
Woitke P, Pinte C, Tilling I, Ménard F, Kamp I, Thi WF, Duchêne G, Augereau JC (2010) Continuum and line modelling of discs around young stars. I. 300000 disc models for HERSCHEL/GASPS. Mon Not R Astron Soc 405:L26–L30. doi:10.1111/j.1745-3933.2010.00852.x
Wood K, Lada CJ, Bjorkman JE, Kenyon SJ, Whitney B, Wolff MJ (2002) Infrared signatures of protoplanetary disk evolution. Astrophys J 567:1183–1191. doi:10.1086/338662
Woodall J, Agúndez M, Markwick-Kemper AJ, Millar TJ (2007) The UMIST database for astrochemistry 2006. Astron Astrophys 466:1197–1204. doi:10.1051/0004-6361:20064981
Wooden DH, Harker DE, Woodward CE, Butner HM, Koike C, Witteborn FC, McMurtry CW (1999) Silicate mineralogy of the dust in the inner coma of comet C/1995 01 (Hale-Bopp) pre- and postperihelion. Astrophys J 517:1034–1058. doi:10.1086/307206
Wooden DH, Woodward CE, Harker DE (2004) Discovery of crystalline silicates in comet C/2001 Q4 (NEAT). Astrophys J Lett 612:L77–L80. doi:10.1086/424593
Wootten A (1989) The duplicity of IRAS 16293-2422: a protobinary star? Astrophys J 337:858. doi:10.1086/167156
Wootten A, Snell R, Glassgold AE (1979) The determination of electron abundances in interstellar clouds. Astrophys J 234:876–880. doi:10.1086/157569
Wouterloot JGA, Henkel C, Brand J, Davis GR (2008) Galactic interstellar 18O/17O ratios—a radial gradient? Astron Astrophys 487:237–246. doi:10.1051/0004-6361:20078156
Wyatt MC (2008) Evolution of debris disks. Annu Rev Astron Astrophys 46:339–383. doi:10.1146/annurev.astro.45.051806.110525
Xie T, Allen M, Langer WD (1995) Turbulent diffusion and its effects on the chemistry of molecular clouds. Astrophys J 440:674. doi:10.1086/175305
Yamaguchi T, Takano S, Sakai N, Sakai T, Sheng-Yuan TL, Su YN, Hirano N, Takakuwa S, Aikawa Y, Nomura H, Yamamoto S (2011) Detection of phosphorus nitride in the lynds 1157 B1 shocked region. Publ Astron Soc Jpn 63:L37–L41
Yang L, Ciesla FJ (2012) The effects of disk building on the distributions of refractory materials in the solar nebula. Meteorit Planet Sci 47:99–119. doi:10.1111/j.1945-5100.2011.01315.x
Yıldız UA, Kristensen LE, van Dishoeck EF, Belloche A, van Kempen TA, Hogerheijde MR, Güsten R, van der Marel N (2012) APEX-CHAMP+ high-J CO observations of low-mass young stellar objects. III. NGC 1333 IRAS 4A/4B envelope, outflow, and ultraviolet heating. Astron Astrophys 542:A86. doi:10.1051/0004-6361/201118368
Young ED, Gounelle M, Smith RL, Morris MR, Pontoppidan KM (2011) Astronomical oxygen isotopic evidence for supernova enrichment of the solar system birth environment by propagating star formation. Astrophys J 729:43. doi:10.1088/0004-637X/729/1/43
Zolensky M, Nakamura-Messenger K, Rietmeijer F, Leroux H, Mikouchi T, Ohsumi K, Simon S, Grossman L, Stephan T, Weisberg M, Velbel M, Zega T, Stroud R, Tomeoka K, Ohnishi I, Tomioka N, Nakamura T, Matrajt G, Joswiak D, Brownlee D, Langenhorst F, Krot A, Kearsley A, Ishii H, Graham G, Dai ZR, Chi M, Bradley J, Hagiya K, Gounelle M, Bridges J (2008) Comparing wild 2 particles to chondrites and IDPs. Meteorit Planet Sci 43:261–272. doi:10.1111/j.1945-5100.2008.tb00621.x
Zsom A, Ormel CW, Dullemond CP, Henning T (2011) The outcome of protoplanetary dust growth: pebbles, boulders, or planetesimals? III. Sedimentation driven coagulation inside the snowline. Astron Astrophys 534:A73. doi:10.1051/0004-6361/201116515
Acknowledgements
We wish to thank our many colleagues for enlightening discussions over the years: L. Bizzocchi, A. Boley, S. Bottinelli, E. Caux, S. Cazaux, C. Codella, A. Crapsi, L. Dore, T. Douglas, E. Herbst, A. Faure, F. Fontani, J. Henshaw, P. Hily-Blant, J. Ilee, I. Jimenez-Serra, T. Hartquist, C. Kahane, E. Keto, B. Lelfoch, P. Myers, J. Pineda, M. Spaans, M. Tafalla, J. Tan, V. Taquet, C. Vastel, M. Walmsley and L. Wiesenfeld. In addition, C. Ceccarelli acknowledges the stimulating discussions with M. Chaussidon, B. Marty and F. Robert on the meteorites. P. Caselli acknowledges the financial support of successive rolling grants awarded by the UK Science and Technology Funding Council. C. Ceccarelli acknowledges the financial support from the Agence Nationale pour la Recherche (ANR), France (project FORCOMS, contracts ANR-08-BLAN-022, and the CNES (Centre National d’Etudes Spatiales).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caselli, P., Ceccarelli, C. Our astrochemical heritage. Astron Astrophys Rev 20, 56 (2012). https://doi.org/10.1007/s00159-012-0056-x
Received:
Published:
DOI: https://doi.org/10.1007/s00159-012-0056-x