Abstract
Purpose
To estimate the incidence density, point prevalence and outcome of severe sepsis and septic shock in German intensive care units (ICUs).
Methods
In a prospective, multicentre, longitudinal observational study, all patients already on the ICU at 0:00 on 4 November 2013 and all patients admitted to a participating ICU between 0:00 on 4 November 2013 and 2359 hours on 1 December 2013 were included. The patients were followed up for the occurrence of severe sepsis or septic shock (SEPSIS-1 definitions) during their ICU stay.
Results
A total of 11,883 patients from 133 ICUs at 95 German hospitals were included in the study, of whom 1503 (12.6 %) were diagnosed with severe sepsis or septic shock. In 860 cases (57.2 %) the infections were of nosocomial origin. The point prevalence was 17.9 % (95 % CI 16.3–19.7).The calculated incidence rate of severe sepsis or septic shock was 11.64 (95 % CI 10.51–12.86) per 1000 ICU days. ICU mortality in patients with severe sepsis/septic shock was 34.3 %, compared with 6 % in those without sepsis. Total hospital mortality of patients with severe sepsis or septic shock was 40.4 %. Classification of the septic shock patients using the new SEPSIS-3 definitions showed higher ICU and hospital mortality (44.3 and 50.9 %).
Conclusions
Severe sepsis and septic shock continue to be a frequent syndrome associated with high hospital mortality. Nosocomial infections play a major role in the development of sepsis. This study presents a pragmatic, affordable and feasible method for the surveillance of sepsis epidemiology. Implementation of the new SEPSIS-3 definitions may have a major effect on future epidemiological data.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Sepsis is a frequent syndrome associated with high morbidity, mortality [1] and long-term disability [2]. Valid epidemiological data on sepsis are important to monitor the burden for the healthcare system and to allocate finances to patient care and research. A systematic review of studies addressing sepsis epidemiology worldwide revealed a highly variable incidence of 13–300 per 100,000 inhabitants per year for severe sepsis and 11 per 100,000 inhabitants per year for septic shock [3]. These data are in line with several European studies reporting an incidence of 66–114 per 100,000 inhabitants per year [4–6].
It was reported that the incidence of sepsis has increased during recent decades because of factors such as advancing age, immunosuppression and multidrug-resistant infections [7, 8]. The change over time in sepsis epidemiology has mainly been studied in the USA and Australia/New Zealand but not in European countries [1, 7–9]. The sequential assessment of sepsis epidemiology is important because it was shown that the burden for rehabilitation facilities has increased in the USA [8], whereas patients in Australia/New Zealand were more often discharged to home [9]. Studies addressing the evolution of sepsis epidemiology over years are usually of a retrospective nature and use surveying patient records [1] or healthcare databases [7–9]. This approach carries the risk of variations in sepsis awareness and coding over time [10, 11]. In addition, the new definition of sepsis by the SEPSIS-3 consensus conference may have major impact on epidemiology of sepsis and septic shock [12].
In the German prevalence study undertaken a decade ago, the calculated incidence of severe sepsis was 76 per 100,000 inhabitants per year [7]. This sepsis incidence was only roughly estimated from prevalence data. Therefore, this study was undertaken, first, to assess the 1-day point prevalence in different ICUs 10 years after the first German prevalence study, and secondly, to estimate the incidence density of severe sepsis and septic shock in German ICUs by prospective data acquisition of a large sample of critically ill patients in a pragmatic and repeatable way. An additional goal was to compare retrospectively clinical data of patients with septic shock according to SEPSIS-1 and SEPSIS-3 definitions.
Materials and methods
This study was approved by all local ethics committees of the participating hospitals. The need for informed consent was waived because of the purely observational nature of the study.
Study design and conduction
This prospective, multicentre, epidemiological and longitudinal observational study was carried out by SepNet Critical Care Trials Group (SepNet) in 2013, a decade after the same group studied the prevalence of severe sepsis and septic shock in German ICUs [13]. Data in this study were collected prospectively. All SepNet members were asked to participate in the INSEP study. Voluntary SepNet members served as regional monitors taking care for additional ICUs not affiliated to SepNet. The regional monitors coached their local centres and met with their staff for screening and documentation training. A participating centre was registered as one organizational unit. This unit could be a distinct ICU out of several ICUs within a large-sized hospital or the general ICU of a hospital. Facilities limited to intermediate care, rehabilitation and paediatric ICUs were excluded. Details of their structural data were recorded.
Patients
All patients already in the participating ICU on 4 November 2013 at 0:00 and all patients admitted in the following 4 weeks starting from 4 November 2013 at 0:00 to 1 December 2013 at 23:59 were included into the study. Outcome data were collected until 2 February 2014. Included patients were screened daily for symptoms of severe sepsis or septic shock during their entire stay in the ICU. According to the SEPSIS-1 definitions of the ACCP/SCCM Consensus Conference Committee [14] severe sepsis was defined as sepsis in combination with at least one organ dysfunction related to infection defined as follows: (a) acute encephalopathy (reduced vigilance, restlessness, disorientation, and delirium without influence of psychotropic drugs), (b) thrombocytopenia (platelets at most 100,000/µl or a drop in platelets greater than 30 % within 24 h not due to blood loss), (c) arterial hypoxemia (PaO2 less than 10 kPa (75 mmHg) under room air, PaO2/FiO2 at most 33 kPa (250 mmHg) not resulting from pulmonary or cardiac disease), (d) renal dysfunction (urinary output at most 0.5 ml/kg/h for at least 1 h despite adequate volume resuscitation and/or increased serum creatinine at least two times the reference level of the respective laboratory) and (e) metabolic acidosis (base deficit at least 5.0 mEq/l or plasma lactate levels at least 1.5 times the reference level of the respective laboratory). Septic shock was defined as severe sepsis combined with arterial hypotension, despite adequate volume resuscitation for at least 2 h, a systolic blood pressure at most 90 mmHg or a mean arterial pressure at most 70 mmHg, or the need for vasopressors at any dose to keep systolic blood pressure greater than or equal to 90 mmHg or mean arterial pressure at least 70 mmHg. Occurrence of septic shock was assessed at or 24 h after onset of sepsis. In addition, clinical data were also used to define septic shock according to SEPSIS-3 definitions, where septic shock was defined as the need of vasopressor therapy to maintain mean arterial pressure of 65 mmHg or greater and having serum lactate levels greater than 2 mmol/l persisting after fluid resuscitation [12].
In all patients under study demographic data including ICU and hospital length of stay were collected. Additional data, such as SIRS criteria, serum lactate concentration, underlying infections and organ dysfunctions during the first 24 h were recorded only if severe sepsis or septic shock occurred during the period of observation. Severity of organ dysfunctions was assessed by SOFA score [15] at day 1 and day 7 after onset of severe sepsis and at discharge from the ICU. It was determined whether infections were community-acquired or nosocomial. Nosocomial infection was defined as an infection diagnosed 48 h or later after hospital admission, 3 days after discharge or in a healthcare facility. Data were collected via Internet-based case report forms (OpenClinica®, LLC, Waltham, MA, USA).
Statistical analysis
The 1-day point prevalence was assessed on 4 November 2013 and was calculated by dividing the number of patients who had severe sepsis or septic shock on that day by the number of all patients in the ICUs. The 95 % confidence interval for the prevalence was calculated using the Wilson score method. Prevalence between different strata was compared by Chi square test.
The incidence density of severe sepsis or septic shock was assessed within a 4-week period from 4 November until 1 December 2013. The individual observation time started at ICU admission, but not earlier than 4 November 2013 (start of the study). The individual observation time ended at the time of ICU discharge, death, second episode of sepsis or at 1 December 2013 (end of the study). The incidence density was calculated by dividing the number of incident sepsis cases by the cumulative sum of the individual observation days. A case was defined as incident if severe sepsis or septic shock was newly diagnosed during the individual observation time in the ICU. We analysed cases of ICU admission as every patient was considered to be at risk for developing severe sepsis or septic shock following admission to the ICU. A 95 % confidence interval for the incidence rate was constructed assuming a Poisson distribution.
Patients admitted with pre-existing severe sepsis or septic shock were defined as prevalent on ICU admission. Similar to our previous study [13], hospitals were categorised into five strata as follows: strata 1–4 comprised ICUs in all non-university hospitals with at most 200, 201–400, 401–600 and more than 600 beds, respectively, and stratum 5 comprised all ICUs in university hospitals. The incidence ratios of different strata were compared by pairwise rate ratio test (R 3.1.2).
In addition, statistical analyses for severe sepsis/septic shock described all patients irrespective of whether they were prevalent or incident. Categorical and continuous variables were compared by Chi square or Mann–Whitney U tests, respectively. Categorical data were reported as absolute or relative frequencies, and metric data as median and interquartile ranges. SPSS 22 (IBM Corporation, NY, USA) and R 3.1.2 were used for all data analyses.
Results
Hospitals and ICUs
Twenty-six regional centres and 128 associated local centres initially agreed to participate in this study. One of the regional centres and 20 of the local centres withdrew for personal or capacity reasons, resulting in 133 participating ICUs at 95 hospitals in Germany. The ICUs were located in university hospitals (23.2 %), university-affiliated hospitals (65.3 %) and general hospitals (11.6 %). Less than half of the ICUs were mixed surgical and medical (38.3 %). The ICUs belonged to hospitals of stratum 5 (38.3 %), 18.8 % to stratum 4, 15.8 % to stratum 3, 20.3 % to stratum 2 and 6.8 % to stratum 1.
Patients
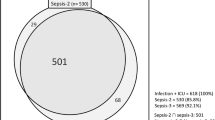
During the 4 weeks of prospective data collection, a total of 11,883 cases admitted to the ICU were included in the study and screened for the occurrence of severe sepsis or septic shock (Fig. 1). Prior to or during the study period, 1503 (12.6 %) cases of severe sepsis or septic shock were diagnosed (Tables 1, 2). Defined by the SEPSIS-1 definitions, severe sepsis occurred in 218 and septic shock in 1285 cases. Defined by the SEPSIS-3 definitions, septic shock was found in 848 cases of those patients with septic shock by SEPSIS-1 definition. The majority of these cases (n = 1403, 93.3 %) developed severe sepsis or septic shock for the first time. Only a minority (6.7 %) suffered from recurrent sepsis episodes. Detailed patient characteristics are given in Table 1. Of patients with severe sepsis or septic shock a greater proportion was male (62.1 %). Out of the total study population, 3924 (33.0 %) patients were admitted for medical reasons and accounted for 49.0 % of the cases of severe sepsis or septic shock. In the course of the same hospital stay, 316 patients were admitted to the ICU twice. Twenty-three patients and three patients were admitted three or four times, respectively.
Point prevalence of severe sepsis or septic shock
On 4 November 2013, 345 out of 1924 patients had severe sepsis or septic shock. The point prevalence was 17.9 % (95 % CI 16.3–19.7) in the entire study population and ranged from 16.0 % (95 % CI 13.9–18.4) to 23.8 % (95 % CI 19.0–29.4) in the different types of hospitals (p = 0.050) (Table 3). A significant difference among strata in the pairwise tests occurred only between strata 3 and 5 with higher prevalence in stratum 3.
Incidence of severe sepsis or septic shock
For calculation of incidence, 388 cases of new severe sepsis or septic shock were prospectively documented in 33,333 patient days. This corresponds to an incidence of 11.64 (95 % CI 10.51–12.86) per 1000 ICU days. Table 4 shows the results for the different types of hospitals. There were no significant differences among the strata in all pairwise rate ratio tests.
Outcome of severe sepsis or septic shock
ICU mortality related to severe sepsis or septic shock was 34.3 %, which was approximately 5.5 times higher than in patients without sepsis (6.0 %). Total hospital mortality of patients with severe sepsis or septic shock was 40.4 % compared with 9.6 % in patients without sepsis. Patients with severe sepsis had ICU and total hospital mortality rates of 16.7 and 23.4 %, respectively. Patients with septic shock had ICU and hospital mortality rates of 37.3 and 43.3 %, respectively. Defined by the new SEPSIS-3 definitions, ICU and hospital mortality of septic shock was 44.3 and 50.9 %, respectively. Kaplan–Meier curves of survival function according to the new sepsis definition is presented in Fig. 2. The median lengths of stay in the ICU and in the hospital were longer in patients with severe sepsis or septic shock (11 and 24 days) than in patients without sepsis (2 and 13 days). The subgroup of patients with septic shock was treated in the ICU for 12 days and in the hospital for 24 days. Details are given in Tables 1 and 2.
Causes of sepsis
Overall, 643 (42.8 %) infections causing sepsis were community-acquired and led to the hospitalization of patients. A total of 860 (57.2 %) infections were of nosocomial origin. Almost half of the nosocomial infections were acquired in the ICU (25.7 % of all infections), followed by infections acquired in the normal ward (21.2 % of all infections). Only a minority of infections were acquired in inpatient care facilities (4.6 %).
The most frequent sites of infections were the lower respiratory tract (n = 700, 46.6 %), the abdomen including the gastrointestinal tract (n = 431, 28.7 %) and the urogenital tract (n = 190, 12.6 %). Sites of infections are shown in the supplementary material.
Blood cultures were drawn in 82.3 % of all septic patients. Positive blood cultures were found in 449 (29.9 %) cases. In 265 (17.7 %) cases, no blood cultures were taken. Microbiological cultures growing from samples taken at the site of infections were found in 970 (64.6 %) cases. A total of 252 (16.8 %) cases had no microbiological examinations from the suspected site of infection. Blood cultures showed more growth of gram-positive bacteria (55.5 %), whereas cultures of samples from the site of infection tended to show gram-negative bacteria (58.7 %). Yeasts were present in 6.7 % of blood cultures and in 19.3 % of samples from the site of infection. Interestingly, patients with genitourinary infections had a higher rate of positive blood cultures (59.1 %), higher PCT values and lower total hospital mortality (23.0 %) compared with the other groups.
SIRS criteria and biomarkers at the onset of severe sepsis
Tachypnoea (respiratory dysfunction), tachycardia and leucocytosis/leucopenia were the most frequent criteria for SIRS (81.0, 80.9 and 76.7 %), whereas fever or hypothermia was found only in 61.1 % of patients. Biomarkers, such as PCT (procalcitonin), CRP (C-reactive protein) and lactate, were elevated in the majority of patients (Table 2).
Sepsis-induced organ dysfunctions
At onset of sepsis, respiratory dysfunction (66.1 %), septic encephalopathy (43.9 %), oliguria (44.2 %) and metabolic acidosis (43.4 %) had the highest frequencies. After 24 h, the frequency of metabolic acidosis decreased to 28.5 %, whereas the frequencies of respiratory dysfunction and other organ dysfunctions remained unchanged.
Discussion
This large prospective epidemiological study demonstrates that severe sepsis and septic shock continue to be a frequent syndrome with a high mortality rate. For the first time, not only the prevalence but also the incidence density of severe sepsis and septic shock in German ICUs has been assessed prospectively in nearly 12,000 patients.
Although we obtained the incidence density of sepsis in Germany it was not possible to calculate the “population-based incidence” for severe sepsis in Germany in this study because (and in contrast to our earlier German prevalence study [13]), the choice of participating ICUs was not a representative sample of all ICUs in Germany. We would like to emphasize that the proportion of the observed incidence of this study is based on “patient days at risk”, whereas the incidence of our earlier study derived from the observed prevalence in a representative sample leading to a calculated and population-based incidence. Thus, the incidence rates found in the two studies should be compared with caution.
In the German prevalence study, the incidence for severe sepsis in Germany has been estimated at 10.05 per 1000 ICU days (95 % CI 9.45–11.64) regarding underlying prevalence and mean syndrome duration as reported by Freeman [16]. Based on 68,000,000 inhabitants in the total German adult population, the incidence of severe sepsis, including septic shock, has been found to be 76 per 100,000 inhabitants [13]. In similar approaches calculating incidence from prevalence data, an incidence of 51–95 per 100,000 person-years has been reported. A further study revealed 300 per 100,000 suspected cases, but this number was deemed to be overestimated for methodological reasons (as summarized by Engel et al. [13]).
The point prevalence of severe sepsis was higher in this study compared to the German prevalence study [13]. These differing results may be caused, in part, by divergent definitions of organ dysfunctions in the two studies, a problem hampering comparisons of epidemiological studies [17, 18]. In terms of arterial hypoxemia, in the present study more patients were assigned to the severe sepsis group as in the German prevalence study. The opposite holds true for arterial hypotension and metabolic acidosis, where fewer patients were categorized as having severe sepsis in this study compared to the German prevalence study. The fact that point prevalence of severe sepsis in small non-university hospitals (strata 1–3) was two to three times higher in this study may be explained by an increased awareness of sepsis as compared to the awareness a decade ago. The lower prevalence in the ICUs of university hospitals may be because some patients with severe sepsis were treated in intermediate care units not under observation in this study.
In the present study, gram-negative organisms were more commonly isolated than gram-positive organisms from the sites of infections. This is similar to the findings of Vincent et al. who noted the isolation of gram-negative bacteria in 62 % of patients in a study involving 14,000 ICU patients in 75 countries [19]. It is worthwhile to mention that yeasts were found in samples from the site of infection as frequently as in other studies [13, 19].
We found a high rate of nosocomial infections (57.2 %) causing sepsis. Almost half of these infections were acquired during the ICU stay. These numbers are consistent with our earlier findings of the German prevalence study [13]. In a Canadian study, a close association between mortality, location and time of acquisition of sepsis was described. The nosocomial infection rate was as high as 67 %. Compared with community-acquired infection, nosocomial infections show a odds ratio of death of 1.69 [1]. Kollef and coworkers demonstrated that 63.7 % of positive blood cultures were healthcare associated. The risk of death in these patients was threefold higher than in patients with community-acquired blood stream infections [20]. In an analysis of the German Hospital Infection Surveillance System (KISS), Geffers and Gastmeier calculated the impressive number of 57,900 nosocomial infections per year for approximately 7,000,000 patient days in German ICUs [21].
The observed total hospital mortality of severe sepsis or septic shock (40.4 %) is comparable to other findings in European ICUs reporting mortality rates between 36.3 and 42.8 % [22–24]. Control groups in recent European sepsis trials also showed mortality rates of severe sepsis approximating 43 % [25, 26]. The reasons for this high mortality in European ICUs compared with US or Australian ICUs are still a matter of debate. They may be related to differences in the number of ICU beds available per inhabitant, to the admission criteria and to the degree of care in critical illness [23]. Other reasons may be discrepancies in definitions and methods of database abstraction [27].
An interesting finding in this study was the decrease in ICU and hospital mortality of sepsis (34.3 and 40.4 %, respectively) compared to the German prevalence study [13]. A decline of sepsis mortality within the last decade has also been found in other studies [5, 9]. This may be related to increased awareness of sepsis resulting in increased numbers of less severely ill cases, which was recently discussed as the “Will Rogers phenomenon” [2]. Therefore, it is of upmost importance to discuss mortality rates in relation to the true incidence of a disease. We found an almost similar incidence of severe sepsis compared with the calculated incidence 10 years ago in the German sepsis prevalence study [13]. This suggests that mortality rates of severe sepsis or septic shock have decreased in Germany within the last decade but remain as high as 23.4 % for severe sepsis and 43.3 % for septic shock.
In this prospective study, sepsis was defined according to the SEPSIS-1 definitions (infection plus at least two SIRS criteria, severe sepsis as sepsis plus organ dysfunction). In the new SEPSIS-3 definition, sepsis is defined as “life-threatening organ dysfunction due to a dysregulated host response to infection” which is associated with an in-hospital mortality greater than 10 % [12]. Organ dysfunction is assessed by the SOFA score of 2 points or greater [12]. Thus, “severe sepsis patients” according to SEPSIS-1 approximately meet the new SEPSIS-3 criteria of “sepsis”. In SEPSIS-3, the SIRS criteria and the term “severe sepsis” have been eliminated because one in eight patients admitted to critical care units with infection and new organ failure did not meet the condition of at least two SIRS criteria. Therefore, we may have missed about 12.5 % of patients with infections with less than two SIRS criteria. As a result of our prospectively generated and for SEPSIS-1 optimized database, we are not able to report on sepsis incidence as defined by the new SEPSIS-3 definitions retrospectively.
Applying the new SEPSIS-3 definition of septic shock to data from this study, only 848 patients of the 1285 septic shock patients according the SEPSIS-1 definition were found to be in septic shock. Again, we may have missed patients on vasopressor therapy and less than two SIRS criteria. With this shortcoming in mind, this demonstrates that the profound change from SIRS criteria to organ dysfunction in the new SEPSIS-3 definition of sepsis or septic shock may have a major impact on frequency and mortality rates [17, 18].
The present study shows that collection of data on a voluntary basis is a pragmatic, affordable and feasible method in sepsis epidemiology. It enables one to measure effects of quality improvement programmes in the future. This method, however, cannot provide an exact “population-based incidence”.
Our study has some limitations. First, differences in organ dysfunctions compared with preceding studies may hamper the comparability between the German prevalence study and this study. Second, as a result of the voluntary design of the study, there was no sample size calculation and we were not able to provide a representative random sample of German hospitals. The distribution of ICUs in our study does not exactly mirror the distribution in Germany. A total of 133 ICUs in 95 hospitals participated in our study representing 8 % of all German hospitals and 11 % of all German ICUs. In Germany, 1198 hospitals have one or several ICUs with 33 % of all ICUs located in hospitals with at least 800 beds, 21 % in hospitals with 500–799 beds, 32 % in hospitals with 200–499 beds and the remaining 14 % in hospitals with less than 200 beds. In the present study, hospitals were categorised into five strata as follows: strata 1–4 comprised ICUs in all non-university hospitals with at most 200, 201–400, 401–600 and more than 600 beds, respectively, and stratum 5 comprised all ICUs in university hospitals. In the present study, 38.3 % of all ICUs located in university hospitals, 18.8 % in hospitals with more than 600 beds, 15.8 % hospitals with 401–600 beds, 20.3 % in hospitals with 200–400 beds and the remaining 6.8 % in hospitals with less than 200 beds. Third, as a result of study structure and overlap of confidence intervals, we cannot exclude centre effects. Fourth, different policies of admission to the ICUs or differences in end of life care may impact study results. Fifth, differences in implementation of sepsis bundles may influence the physician’s awareness of sepsis and thus impact study results. All these factors may have contributed to the observed differences in the strata of this study and the German prevalence study.
Conclusion
This study showed that sepsis remains a frequent and life-threatening syndrome in German ICUs associated with a total hospital mortality of 40 %. Nosocomial infections caused 57 % of all sepsis cases, almost half of them acquired in the ICU. The new definition of septic shock identifies more severely ill patients with a higher mortality rate. The presented study design may serve as a feasible, pragmatic and affordable method to survey epidemiology of sepsis over distinct time periods. There is a need to analyse changes of epidemiological data due to assessment using SEPSIS-1 and -3 definitions in future prospective studies.
Change history
01 December 2017
The members of the SepNet Critical Care Trials Group were provided in such a way that they could not be indexed as collaborators on PubMed. The publisher apologizes for this error and is pleased to list the members of the group here:
References
Martin GS, Mannino DM, Eaton S, Moss M (2003) The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348:1546–1554
Iwashyna TJEE, Smith DM, Langa KM (2010) Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 304:1787–1794
Jawad I, Luksic I, Rafnsson SB (2012) Assessing available information on the burden of sepsis: global estimates of incidence, prevalence and mortality. J Glob Health 2:010404
Andreu Ballester JC, Ballester F, Gonzalez Sanchez A, Almela Quilis A, Colomer Rubio E, Penarroja Otero C (2008) Epidemiology of sepsis in the Valencian Community (Spain), 1995–2004. Infect Control Hosp Epidemiol 29:630–634
Harrison DA, Welch CA, Eddleston JM (2006) The epidemiology of severe sepsis in England, Wales and Northern Ireland, 1996 to 2004: secondary analysis of a high quality clinical database, the ICNARC Case Mix Programme Database. Crit Care 10:R42
van Gestel A, Bakker J, Veraart CP, van Hout BA (2004) Prevalence and incidence of severe sepsis in Dutch intensive care units. Crit Care 8:R153–R162
Dombrovskiy VY, Martin AA, Sunderram J, Paz HL (2007) Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med 35:1244–1250
Kumar G, Kumar N, Taneja A, Kaleekal T, Tarima S, McGinley E, Jimenez E, Mohan A, Khan RA, Whittle J, Jacobs E, Nanchal R, Milwaukee Initiative in Critical Care Outcomes Research Group of Investigators (2011) Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest 140:1223–1231
Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R (2014) Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA 311:1308–1316
Rothberg MB, Pekow PS, Priya A, Lindenauer PK (2014) Variation in diagnostic coding of patients with pneumonia and its association with hospital risk-standardized mortality rates: a cross-sectional analysis. Ann Intern Med 160:380–388
Lindenauer PK, Lagu T, Shieh MS, Pekow PS, Rothberg MB (2012) Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003–2009. JAMA 307:1405–1413
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC (2016) The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315:801–810
Engel C, Brunkhorst F, Bone H-G, Brunkhorst R, Gerlach H, Grond S, Gruendling M, Huhle G, Jaschinski U, John S, Mayer K, Oppert M, Olthoff D, Quintel M, Ragaller M, Rossaint R, Stuber F, Weiler N, Welte T, Bogatsch H et al (2007) Epidemiology of sepsis in Germany: results from a national prospective multicenter study. Intensive Care Med 33:606–618
Committee ASCC (1992) Definition for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 20:864–874
Vincent J-L, Moreno R, Takala J, Willatts S, Mendonca AD, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
Freeman J, Hutchison GB (1980) Prevalence, incidence and duration. Am J Epidemiol 112:707–723
Weiss M, Huber-Lang M, Taenzer M, Kron M, Hay B, Nass M, Huber M, Schneider M (2010) How many variables need to be fulfilled when defining sepsis due to the 2003 SCCM/ESICM/ACCP/ATS/SIS definitions in critically ill surgical patients: a retrospective observational study. BMC Anesthesiol 10:22
Weiss M, Huber-Lang M, Taenzer M, Traeger K, Altherr J, Kron M, Hay B, Schneider M (2009) Different patient case mix by applying the 2003 SCCM/ESICM/ACCP/ATS/SIS sepsis definitions instead of the 1992 ACCP/SCCM sepsis definitions in surgical patients: a retrospective observational study. BMC Med Inform Decis Mak 9:25
Vincent J, Rello J, Marshall J et al (2009) International study of the prevalence and outcomes of infection in intensive care units. JAMA 302:2323–2329
Kollef MH, Zilberberg MD, Shorr AF, Vo L, Schein J, Micek ST, Kim M (2011) Epidemiology, microbiology and outcomes of healthcare-associated and community-acquired bacteremia: a multicenter cohort study. J Infect 62:130–135
Geffers C, Gastmeier P (2011) Nosocomial infections and multidrugresistant organisms in Germany epidemiological data from KISS (The Hospital Infection Surveillance System). Dtsch Arztebl Int 108:87–93
Heublein SHM, Hagel S, Hutagalung R, Brunkhorst FM (2013) Epidemiology of sepsis in German hospitals derived from administrative databases. Infection 41:41
Levy MM, Artigas A, Phillips GS, Rhodes A, Beale R, Osborn T, Vincent JL, Townsend S, Lemeshow S, Dellinger RP (2012) Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis 12:919–924
Beale RRK, Brunkhorst FM, Dobb G, Levy M, Martin G et al (2009) Promoting Global Research Excellence in Severe Sepsis (PROGRESS): lessons from an international sepsis registry. Infection 37:222–232
Asfar P, Meziani F, Hamel JF, Grelon F, Megarbane B, Anguel N, Mira JP, Dequin PF, Gergaud S, Weiss N, Legay F, Le Tulzo Y, Conrad M, Robert R, Gonzalez F, Guitton C, Tamion F, Tonnelier JM, Guezennec P, Van Der Linden T, Vieillard-Baron A, Mariotte E, Pradel G, Lesieur O, Ricard JD, Herve F, du Cheyron D, Guerin C, Mercat A, Teboul JL, Radermacher P, Investigators S (2014) High versus low blood-pressure target in patients with septic shock. N Engl J Med 370:1583–1593
Caironi P, Tognoni G, Masson S, Fumagalli R, Pesenti A, Romero M, Fanizza C, Caspani L, Faenza S, Grasselli G, Iapichino G, Antonelli M, Parrini V, Fiore G, Latini R, Gattinoni L (2014) Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med 370:1412–1421
Gaieski DF, Edwards JM, Kallan MJ, Carr BG (2013) Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 41:1167–1174
Acknowledgements
Writing Committee: Gernot Marx, Melanie Schäfer, Josef Briegel, Tobias Schuerholz, Stefan Schröder, Friedhelm Bach, Ulrich Jaschinski, Manfred Weiß, Stefan Kluge, Holger Bogatsch, Frank Bloos, Norbert Weiler, and Michael Oppert.
Study design and protocol: Gernot Marx, Frank Bloos, Josef Briegel, Herwig Gerlach, Matthias Gründling, Axel Nierhaus, Michael Oppert, Konrad Reinhart, Rolf Rossaint, Michael Quintel, Norbert Weiler, Manfred Weiß, and Christoph Engel.
Statistical Analysis: Holger Bogatsch performed the full statistical analysis.
Database: Matthias Loebe.
Author information
Authors and Affiliations
Consortia
Additional information
A full list of SepNet Critical Care Trials Group (SepNet) authors and investigators is presented in the supplementary material (ESM2).
A correction to this article is available online at https://doi.org/10.1007/s00134-017-4980-0.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
SepNet Critical Care Trials Group. Incidence of severe sepsis and septic shock in German intensive care units: the prospective, multicentre INSEP study. Intensive Care Med 42, 1980–1989 (2016). https://doi.org/10.1007/s00134-016-4504-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-016-4504-3