Abstract
Purpose
To summarize evidence on long-term health-related quality-of-life (HRQL) among survivors of acute kidney injury (AKI) in the intensive care unit (ICU).
Methods
We performed a comprehensive search of the literature for studies reporting original data describing HRQL utilizing validated instruments. Search, study selection and data abstraction were performed in duplicate. Study quality was appraised. Due to study heterogeneity, data are primarily summarized qualitatively.
Results
Our search yielded 2193 articles of which 18 were selected for detailed analysis. The quality of these 18 studies was generally good. Numerous HRQL instruments were utilized, and assessment occurred at variable follow-up duration (range 2 months to 14.5 years). HRQL among AKI survivors was reduced when compared to age/sex-matched populations. HRQL among survivors with and without AKI was generally described as similar beyond 6 months. Physical component domains were consistently more impaired than mental component domains. Survivors had considerable limitations in activities of daily living, implying newly acquired disability, with few returning to work. Despite diminished HRQL, patients’ HRQL was generally perceived as satisfactory, and the majority would receive similar treatment again, including renal replacement therapy in the ICU, if necessary.
Conclusions
Among survivors of critical illness complicated by AKI, HRQL was impaired when referenced to population norms, but it was not significantly different from that of survivors without AKI. Physical limitations and disabilities were more commonly exhibited by AKI patients. Importantly, the impaired HRQL was generally perceived as acceptable to patients, most of whom expressed willingness to undergo similar treatment in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute kidney injury (AKI) is a frequent complication of critical illness, with recent large prospective studies reporting incidence rates of between 20 and 60 % [1, 2]. Among those with more severe AKI, approximately one-quarter receive renal replacement therapy (RRT) [1, 2]. Across the spectrum of AKI severity, there is an increased risk of short-term and long-term adverse events [3]. Hospital mortality among critically ill patients with AKI often exceeds 25 % and is higher for those who receive RRT [1, 2]. For survivors of AKI, considerable circumstantial evidence has suggested AKI portends long-term risks, including incident chronic kidney disease (CKD), accelerated progression to end-stage kidney disease (ESKD), major cardiovascular events, sepsis and fracture risk [3–7].
A number of studies have now described the long-term impact of AKI on the health-related quality of life (HRQL) and functional status of survivors of critical illness. However, interpretation of the results of these studies has been challenging due to heterogeneity in study design, tools used to ascertain HRQL and functional status, case-mix and duration of follow-up. This has translated into discordant and conflicting findings and, consequently, clinical uncertainty with respect to long-term HRQL and functional outcomes among survivors of AKI in the ICU.
To address this uncertainty, we performed a systematic review focused on describing the HRQL and functional outcomes among survivors of critical illness complicated by AKI. The aim of this review was to further explore whether a better understanding of long-term HRQL and functional outcomes for critically ill patients with AKI can be used to better inform prognosis and clinical decision-making.
Methods
The methods used for this systematic review are described in detail in a study protocol developed by three of the authors (PMV, EC, SMB) [see Electronic Supplementary Material (ESM) Protocol Document]. This systematic review conforms to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement for reporting [8].
Search strategy
An initial search of MEDLINE and the Cochrane Database of Systematic Reviews was performed to identify and assess any prior systematic reviews on this topic (26 February 2014). PROSPERO (http://www.crd.york.ac.uk/prospero) was also searched for any registered systematic reviews on this topic (30 November 2014). An initial OVID search strategy was used to search the following databases: MEDLINE (1950 to August week 2, 2014) (plus PubMed using “related articles” features), EMBASE (1980 to August 20, 2014) and CENTRAL (Cochrane Central Register of Controlled Trials). Details pertaining to the search strategy are given in ESM Protocol Document. This search was supplemented by a scan of bibliographies of all retrieved studies, a review of the most recent 5 years of selected scientific meetings (American Society of Nephrology, Society of Critical Care Medicine, International Conference on Critical Care Nephrology, International Symposium on Intensive Care and Emergency Medicine, European Society of Intensive Care Medicine), a scan of clinical trial registries for ongoing clinical studies (http://www.controlled-trials.com/mrct/), a scan of the selected Grey Literature according to the Canadian Agency for Drug and Technology in Health Grey Matters document (http://www.cadth.ca/en/resources/grey-matters). Articles that were forwarded by specialists in the field after the search was performed were also included. Only articles published in English were considered eligible for inclusion.
Study selection
Two authors (PMV, EC) independently performed an initial screen of all retrieved abstracts. Eligible abstracts were subsequently included for full-text review if they met the following inclusion criteria: (1) study design—observational studies and/or interventional studies, not case reports or review articles; (2) study population—intensive care unit (ICU) survivors aged ≥15 years with a diagnosis of AKI by any validated measure/criteria; (3) outcome—reported HRQL and functional status using a validated instrument. Instruments described included the Short Form-36 Health Survey (SF-36), EuroQol (EQ-5D), Health Utility Index Mark 3 (HUI3), Nottingham Health Profile (NHP), Short Form Health Survey 12 (SF-12), Medical Outcome Study Short Form Health Survey (MOS-SF-20) and Activities of Daily Living (ADL). ESM Table S1 provides a detailed description of these instruments. Disagreements regarding the inclusion of abstracts for full-text review were resolved through discussion; if consensus could not be reached, the decision was adjudicated by a third reviewer (SMB).
Data collection
All data were extracted in duplicate by two authors (EC, PV) on standardized data forms. Any discordance in data was resolved through discussion or resorting to a third data abstractor (SMB). Data forms included details of study design/methodology, measures of study quality, definitions and details of definition of AKI (including whether RRT was used), duration of follow-up and primary and secondary outcomes.
Quality assessment
The methodological quality of the studies was assessed independently by two reviewers (EC, PV) using the Modified Downs and Black checklist [9] (ESM Item S1). We also analyzed the proportion of patients lost to follow-up as a measure of quality (i.e. attrition rate) (ESM Table S3 provides the percentage of patients lost to follow-up in each study).
Outcomes
The primary outcome was HRQL and functional status among ICU survivors of AKI, as measured using any validated instrument. Secondary outcomes were a description of HRQL across various durations of follow-up; HRQL compared to that of the general population; HRQL compared to that of ICU survivors without AKI; physical and mental components scores as assessed by validated instruments; the change in HRQL compared to baseline where reported; self-rated health and perception of care (by any instrument); the impact of dialysis dependence on HRQL.
Statistical analysis
Data analysis was performed using Review Manager, version 5.0 (RevMan; The Nordic Cochrane Centre, The Cochrane Collaboration 2008, Copenhagen, Denmark). Outcomes were summarized according to how they were reported in the included studies, namely, as mean HRQL score with standard deviation or 95 % confidence interval, as median HRQL score with interquartile range (IQR) or descriptively (e.g. the percentage of patients who returned to work following AKI). Due to significant heterogeneity of the reporting tools for HRQL utilized across studies and the variable time-frame for ascertainment, no formal meta-analysis was undertaken.
Results
Study characteristics
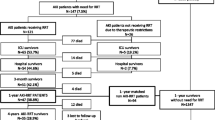
No previous systematic review on this topic was identified during our literature search. The search strategy yielded 2408 citations. Of these, 2193 records remained following removal of duplicate publications. A further 2157 records were found to be ineligible for inclusion in our review (Fig. 1). Two new studies [10, 11] which met the eligibility criteria were published after our initial search and were added, yielding a final total of 18 unique studies after full-text review of the remaining articles.
All of the studies included in our review were published between 1997 and 2015 (Table 1). The SF-36 (n = 8 studies [10, 12–18]) and EQ-5D (n = 5 studies [10, 19–22]) were the most common HRQL instruments used in these studies. The NHP was used in three studies [23–25], and one study each used the HUI3 [26], SF-12 [11] and MOSSF-20 [27]. The timeframe for ascertainment of HRQL after AKI was highly variable (median 10.5 months, IQR 6–33 months, range 2 months to 14.5 years).
Study quality
The median Modified Downs and Black score was 13 (IQR 12–15, range 9–18) [9]. In total, 67 % (n = 12) of included studies were of ‘good’ quality [10–16, 18–22, 26]; the remaining studies were of ‘moderate’ quality (n = 6) [17, 23–25, 27] (ESM Table S2). Across studies, loss to follow-up was significant (range 0–56.3 %), with ≥20 % of patients lost to follow-up for HRQL assessment in 14 of the included studies (78 %) [10, 12, 14, 17–27] (ESM Table S3). There was no significant correlation between time-frame for ascertainment of HRQL and lost to follow-up (correlation coefficient 0.02; p = 0.93).
HRQL according to various instruments
Short Form-36 Health Survey
Eight studies with a total of 536 patients used SF-36 to measure HRQL after AKI [10, 12–18] (Table 2). Population normative data have been standardized to the physical component summary (PCS) and mental component summary (MCS) scores of 50 patients each. SF-36 scores were similar across studies with only a few exceptions. The weighted averages in PCS and MCS were 40.8 (range 32.4–42.1) and 51.2 (range 33.8–53.9), implying that AKI survivors had significantly impaired HRQL, predominantly driven by impairment in the physical domains compared with the mental domains. A comparison of the PCS and MCS consistently showed greater impairment across all studies (except for Abelha et al. [12] where both the PCS and MCS are markedly impaired).
EuroQol
Five studies with a total of 1275 patients used the EQ-5D to measure HRQL after AKI [10, 19–22] (Table 3). Population normative data for the EQ-index and EQ-visual analogue scale (EQ-VAS) are generally 0.80–0.85 and 80–85, respectively. The weighted average EQ-index and EQ-VAS scores of the 1275 patients in these five studies were 0.69 (range 0.46–0.80) and 69.5 (range 65–70), respectively, implying significantly impaired HRQL among AKI survivors. Among studies reporting the EQ-VAS, two studies reported lower scores for patients receiving RRT compared with the scores of those not treated with RRT [10, 19, 21]. Two Finnish studies found that the EQ-VAS scores among AKI survivors were similar to those of the matched general population [10, 19, 21]. EQ-index scores were generally low across all studies (except for the study of Gallagher et al. where the EQ-index was relatively preserved [11, 20, 23–27]).
Other HRQL instruments
Seven studies (n = 1244) used four additional validated instruments to measure HRQL among AKI survivors (NHP, HUI3, MOS-SF-20, SF-12; see ESM Table S4) [11, 20, 23–27]. In general, the majority of studies reported that AKI survivors had impaired HRQL compared to non-AKI survivors or normative data from the general population, with one exception. Landoni et al. (n = 22) found that the HRQL was similar among AKI and non-AKI survivors, with HRQL rated overall as reasonable by survivors [27]. Two studies showed impaired HRQL measured by the SF-12 across both the PCS and MCS domains [11, 20], with one study showing greater reductions in PCS scores among patients requiring maintenance dialysis [11]. The majority of studies reported significant impairment across the physical function domains, including increased physical limitations [24, 25], limited energy [23, 24], diminished mobility [23, 24] or difficulty with ambulation [26], decreased ability to do heavy housework [24] or simply decreased physical fitness [25].
Comparison of HRQL of AKI ICU survivors compared to general population
Of the 18 studies (n = 1624 patients), ten (56 %) compared the HRQL of AKI survivors to normative data from their respective general populations (ESM Table S5). Of these, six used the SF-36 and showed that the HRQL among AKI survivors was consistently lower, in particular for PCS, than that of a matched general population [10, 12–14, 17, 18]. Similarly, the remaining studies using the EQ-5D [10, 19, 21], HUI3 [26] and SF-12 [6] all described significantly impaired HRQL among AKI survivors relative to population normative data.
Comparison of HRQL among survivors with and without AKI
Six studies (n = 813) compared the HRQL among survivors with and without AKI [10, 12, 14, 21, 22, 27] (Table 4). In a large Finnish study, Vaara et al. compared the HRQL of 313 critically ill patients with AKI receiving RRT with that of 5415 survivors without AKI [22]. At 6 months, there was no clinically important difference in the HRQL, as measured by the EQ-5D, between the two groups. In a subsequent Finnish study, Nisula et al. also compared the HRQL measured by the EQ-5D among 327 AKI survivors (85 of whom received RRT) with that among non-AKI survivors [21]. Again, no clinically important difference in HRQL at 6 months was found (EQ-5D score: 0.68 for AKI group, 0.68 for RRT group, 0.69 for non-AKI group). Landoni et al. compared the HRQL among survivors receiving or not receiving RRT after cardiac surgery using the MOS-SF-20 [27]. These authors found no difference in HRQL between the two groups at 3.5 years. Oeyen et al., using the SF-36 and EQ-5D, also found no difference in HRQL at 4 years when comparing survivors who were matched to whether they received and did not receive RRT [by age, sex, admission diagnosis, APACHE II (Acute Physiology and Chronic Health Evaluation II) score] [10]. Only two of the studies included in this review found differences in HRQL among critically ill survivors who did and did not have AKI [12, 14]. Abelha et al. compared 50 post-operative patients with AKI to 737 patients who did not have AKI and found that those with AKI had worse SF-36 scores at 6 months across the physical function, role physical, general health and role emotional domains [12]. Hofhuis et al. similarly found that AKI survivors had lower scores for the vitality and general health domains of the SF-36 at 6 months than those without AKI, although aggregate PCS and MCS were not significantly different between the two groups [14].
HRQL associated with non-recovery and dialysis dependence
Only two studies evaluated the impact of non-recovery and dialysis dependence after AKI on HRQL [11, 26]. Wang et al., using the SF-12 at 3.5 years, found that the PCS scores were lower in AKI survivors receiving maintenance dialysis than in those who became dialysis independent (34.3 vs. 40.3, respectively; P = 0.04); however, MCS scores were similar (51.6 vs. 49.7; P = 0.5) [11]. In contrast, Johansen et al., using the HUI3 to assess patients at 60 days, found no significant difference in scores between those who were dialysis dependent and those who had recovered [26]. While scores in general were significantly impaired, there was no significant difference between these patients and critically ill survivors without AKI, with the exception of worse cognition.
Impact of AKI on ADL and return to work
Activities of daily living
Five studies (n = 184) specifically evaluated ADL among survivors of AKI [12, 13, 15, 23, 24] (Table 5). Across studies, new disability in at least one ADL occurred in 20–42 % of AKI survivors at 6 months.
Return to work
Only two studies (n = 191) assessed the proportion of AKI survivors able to return work [16, 25]. Morgera et al. reported that 69 % of patients who were employed prior to critical illness were able to return to work [25]. In a more recent study, Morsch et al. found only 13 of 46 (28 %) of AKI survivors had returned to work by 9 months [16].
Comparison of pre- and post-ICU HRQL and disability
Six studies (n = 939 patients) compared HRQL and disability among AKI survivors relative to baseline status [10, 14–16, 21, 22] (Table 6). Two Finnish studies which used the EQ-5D for baseline and 6-month assessment of HRQL found no significant or clinically important changes over time [21, 22]. Notably, in both of these studies, baseline EQ-5D scores were significantly impaired HRQL relative to population normative data. In two studies using the SF-36, AKI survivors showed a significant decline in PCS scores but the MCS scores were unchanged [10, 14]. Oeyen et al. reported that relative to baseline, AKI survivors had a greater incident disability associated to mobilization, ability to perform usual activities and anxiety/depression [10]. Similarly, Maynard et al. found the incidence of new disability in ADL was 42 % at 6 months [15].
HRQL for assessing patient satisfaction using non-standardized HRQL instruments
Four studies (n = 192) used non-validated or simplified questionnaires to directly ask AKI survivors to rate their HRQL [15, 23, 25, 27] (ESM Table S6). Across these studies, 69–100 % of AKI survivors rated their current HRQL and overall health status as satisfactory. Four studies (n = 160) also asked AKI survivors whether they were satisfied with the care they received and whether they would undergo the same treatment again, specifically RRT, if they suffered a subsequent episode of critical illness [10, 15, 23, 25] (ESM Table S7). The vast majority of AKI survivors (71.4–98.5 %) indicated that they rated their treatment as worthwhile and that they would receive similar care again (including RRT) if deemed medically necessary to survive. Notably, in the study by Oeyen et al., when asked at 1 year after ICU, 81.8 % of RRT treated survivors would accept readmission; however, this declined to 71.4 % at 4 years [10].
Discussion
Summary of findings
Our systematic review included 18 unique studies of moderate to good quality evaluating the HRQL and functional status among survivors of AKI. To summarize:
-
First, we found that HRQL was markedly impaired among survivors of AKI, in particular in the context of critical illness, in 17 of the 18 studies included in this review. This finding was consistent across studies using a number of validated tools to capture HRQL and at variable durations of follow-up.
-
Second, we found HRQL among AKI survivors was universally impaired when compared with general population norms; however, the magnitude of impairment was comparable to that of ICU survivors who did not have AKI or receive RRT. Paradoxically, despite impaired HRQL, the overwhelming majority of AKI survivors were satisfied with their care and would be willing to again undergo similar treatment in the ICU again if necessary.
-
Third, we found impaired HRQL was predominantly driven by impairment in physical domains rather than mental domains across studies and across HRQL instruments. AKI survivors described greater occurrence of limitations in physical function, mobility and ambulation when compared to psycho-social domains [10, 12–18, 23–25].
-
Fourth, we found AKI survivors commonly had newly documented disabilities and dependency for ADL, with few of these returning to baseline function; however, this was assessed in few studies. Moreover, limited data showed very few AKI survivors were able to return to work [16, 25]. Morgera et al. [25] reported that 69 % of survivors who were previously employed returned to work when assessed at approximately 2.5 years, while Morsch et al. [16] reported that only 28 % of survivors returned to work at 9 months after hospital discharge. Whether this wide disparity is related in part to differences in socio-demographic factors (i.e. Germany vs. Brazil), case-mix, baseline illness severity (APACHE II score: 21 in the study of Morgera et al. [25] vs. 25 in the study of Morsch et al. [16]) or timing of ascertainment remains uncertain and warrants further evaluation. Prior data among survivors of critical illness found only 55 % returned to work; however, among those who did, HRQL was significantly higher at 1 year compared to those not returning to work [28]. However, few studies have focused on describing modifiable factors that predict return to work, the extent to which survivors are capable of re-engaging in their usual activities prior to critical illness and the relative timing of their return to work.
-
Finally, we found that HRQL among AKI survivors, similar to that among non-AKI survivors, was often significantly impaired at baseline. This may, in part, explain the finding that in studies which evaluated baseline and follow-up HRQL among AKI survivors, any clinically important difference, if present at all, was marginal. However, an important limitation of these studies is that baseline HRQL was often determined by proxy or by patient recall, which may itself be an important source of bias [29].
HRQL after AKI relative to other conditions
Our evidence synthesis would suggest that critically ill survivors with AKI have HRQL comparable to that of ICU survivors in general or to those with other ICU syndromes, such as sepsis; however, it is more impaired compared to the HRQL of ICU survivors of acute respiratory distress syndrome (ESM Table S8). The critical challenge in comparing and interpreting these data is in estimating the attributable impairment in HRQL related to a specific syndrome experienced in the ICU, such as AKI rather than sepsis, which commonly co-exist, and in establishing how this may be causally related. Alternatively, it may be more relevant to characterize the longer term HRQL and functional status among AKI survivors who remain dialysis dependent or rapidly progress to ESKD, as ESKD has been associated with impaired HRQL and health utility [30, 31]. Similarly, our data imply that AKI survivors often describe a HRQL comparable to that of many patients with chronic illness, such as heart failure.
Limitations/strengths
While we believe our review synthesizes a wide array of knowledge on the HRQL among ICU survivors whose course was complicated by AKI, there are notable limitations that warrant consideration. Wide variability in study design, case-mix and tools to capture HRQL, variable duration of follow-up and significant patient attrition due to high mortality rates present challenges for drawing clear inferences. As such, it was generally not feasible to perform pooled analysis of aggregate data across studies. Moreover, poor HRQL, along with new and/or severe disability, may have been disproportionately experienced by those with early death after hospitalization prior to any opportunity to measure HRQL. This along with patients with more severely impaired HRQL suffering greater likelihood of attrition across studies may have introduced bias and/or contributed to the perception of a more favorable HRQL among survivors in whom HRQL was measured [32]. Similarly, there was considerable heterogeneity across studies in the “control” populations used to compare HRQL, such a non-AKI patients, non-RRT-treated patients and population normative data, with very few performing robust methodology to match cohorts. This also represents a source of bias, prohibits detailed pooled analysis and presents challenges for making clear inferences. Despite these limitations, we believe our evidence synthesis is strengthened by our rigorous methodology, including literature search, screening for eligibility and systematic evaluation of the study quality.
Implications for policy/future research priorities
We believe our review provides a strong anchor for further evaluation of HRQL among survivors of critical illness and ICU admission, specifically those with AKI. Ideally, we believe future studies should use widely available, non-proprietary and standardized HRQL instruments (i.e. EuroQol) with the aim to assess pre-AKI baseline data and evaluate HRQL at relatively fixed durations of follow-up (i.e. 90 days to correspond to transition to CKD, and between 6 and 12 months based on the observation of relatively minimal incremental gain between these assessments) to better enable synthesis and comparisons across populations. Numerous studies have evaluated HRQL in patients relatively early following hospital discharge (<90 days), when the attributable impairment in HRQL related to complications of AKI (i.e. ESKD) may not be discernable (if any) from the residual impairment due to recovery from critical illness in general. In addition, relatively few studies have evaluated the impact of non-recovery of kidney function on longer term HRQL, specifically across the subgroups that were dialysis dependent, who developed new CKD or who later developed accelerated ESKD. Similarly, further studies should aim to integrate the modifying impact of complications occurring among AKI survivors that may be temporally related to AKI or non-recovery of function, such as major cardiovascular events or sepsis.
At the present time, clinicians and policy-makers should consider that patients with AKI in the studies included in our review generally reported being satisfied with their ICU care and that the majority were generally willing to undergo aggressive care in the ICU again, including RRT. This would appear to be particularly relevant in the context that survivors of AKI have HRQL that is comparable to that of survivors without AKI. These findings should be used to help inform prognosis, survival expectations and clinical decision-making for patients, families and clinicians when confronted with critical illness complicated by AKI.
Conclusions
Health-related quality of life among critically ill survivors who developed AKI was generally impaired at baseline and was markedly lower than the general population; however, it was not significantly more impaired than that of critically ill survivors without AKI. Survivors’ impaired HRQL was predominantly characterized by physical limitations, new disabilities and functional limitations, while mental domains appeared to be largely preserved.
References
Bouchard J, Acharya A, Cerda J, Maccariello ER, Madarasu RC, Tolwani AJ, Liang X, Fu P, Liu ZH, Mehta RL (2015) A prospective international multicenter study of AKI in the intensive care unit. Clin J Am Soc Nephrol 10:1324–1331
Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honore PM, Joannes-Boyau O, Joannidis M, Korhonen AM, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, Kellum JA (2015) Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 41:1411–1423
Chawla LS, Eggers PW, Star RA, Kimmel PL (2014) Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 371:58–66
Gammelager H, Christiansen CF, Johansen MB, Tonnesen E, Jespersen B, Sorensen HT (2014) Three-year risk of cardiovascular disease among intensive care patients with acute kidney injury: a population-based cohort study. Crit Care 18:492
Lai TS, Wang CY, Pan SC, Huang TM, Lin MC, Lai CF, Wu CH, Wu VC, Chien KL, National Taiwan University Hospital Study Group on Acute Renal Failure (2013) Risk of developing severe sepsis after acute kidney injury: a population-based cohort study. Crit Care 17:R231
Wang WJ, Chao CT, Huang YC, Wang CY, Chang CH, Huang TM, Lai CF, Huang HY, Shiao CC, Chu TS, Chen YM, Wu VC, Ko WJ, Wu KD, National Taiwan University Study Group on Acute Renal F (2014) The impact of acute kidney injury with temporary dialysis on the risk of fracture. J Bone Miner Res 29:676–684
Wu VC, Wu PC, Wu CH, Huang TM, Chang CH, Tsai PR, Ko WJ, Chen L, Wang CY, Chu TS, Wu KD, National Taiwan University Study Group on Acute Renal Failure G (2014) The impact of acute kidney injury on the long-term risk of stroke. J Am Heart Assoc 3:e000933
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700
Downs SH, Black N (1998) The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 52:377–384
Oeyen S, De Corte W, Benoit D, Annemans L, Dhondt A, Vanholder R, Decruyenaere J, Hoste E (2015) Long-term quality of life in critically ill patients with acute kidney injury treated with renal replacement therapy: a matched cohort study. Crit Care 19:289
Wang AY, Bellomo R, Cass A, Finfer S, Gattas D, Myburgh J, Chadban S, Hirakawa Y, Ninomiya T, Li Q, Lo S, Barzi F, Sukkar L, Jardine M, Gallagher MP, Investigators P-RS, the ACTG (2015) Health-related quality of life in survivors of acute kidney injury: the prolonged outcomes study of the randomized evaluation of normal versus augmented level replacement therapy study outcomes. Nephrology 20:492–498
Abelha FJ, Botelho M, Fernandes V, Barros H (2009) Outcome and quality of life of patients with acute kidney injury after major surgery. Nefrologia 29:404–414
Delannoy B, Floccard B, Thiolliere F, Kaaki M, Badet M, Rosselli S, Ber CE, Saez A, Flandreau G, Guerin C (2009) Six-month outcome in acute kidney injury requiring renal replacement therapy in the ICU: a multicentre prospective study. Intensive Care Med 35:1907–1915
Hofhuis JG, van Stel HF, Schrijvers AJ, Rommes JH, Spronk PE (2013) The effect of acute kidney injury on long-term health-related quality of life: a prospective follow-up study. Crit Care 17:R17
Maynard SE, Whittle J, Chelluri L, Arnold R (2003) Quality of life and dialysis decisions in critically ill patients with acute renal failure. Intensive Care Med 29:1589–1593
Morsch C, Thome FS, Balbinotto A, Guimaraes JF, Barros EG (2011) Health-related quality of life and dialysis dependence in critically ill patient survivors of acute kidney injury. Ren Fail 33:949–956
Noble JS, Simpson K, Allison ME (2006) Long-term quality of life and hospital mortality in patients treated with intermittent or continuous hemodialysis for acute renal and respiratory failure. Ren Fail 28:323–330
Van Berendoncks AM, Elseviers MM, Lins RL, Group SS (2010) Outcome of acute kidney injury with different treatment options: long-term follow-up. Clin J Am Soc Nephrol 5:1755–1762
Ahlstrom A, Tallgren M, Peltonen S, Rasanen P, Pettila V (2005) Survival and quality of life of patients requiring acute renal replacement therapy. Intensive Care Med 31:1222–1228
Gallagher M, Cass A, Bellomo R, Finfer S, Gattas D, Lee J, Lo S, McGuinness S, Myburgh J, Parke R, Rajbhandari D, Investigators P-RS, the ACTG (2014) Long-term survival and dialysis dependency following acute kidney injury in intensive care: extended follow-up of a randomized controlled trial. PLoS Med 11:e1001601
Nisula S, Vaara ST, Kaukonen KM, Reinikainen M, Koivisto SP, Inkinen O, Poukkanen M, Tiainen P, Pettila V, Korhonen AM, Group F-QS (2013) Six-month survival and quality of life of intensive care patients with acute kidney injury. Crit Care 17:R250
Vaara ST, Pettila V, Reinikainen M, Kaukonen KM, Finnish Intensive Care C (2012) Population-based incidence, mortality and quality of life in critically ill patients treated with renal replacement therapy: a nationwide retrospective cohort study in Finnish intensive care units. Crit Care 16:R13
Gopal I, Bhonagiri S, Ronco C, Bellomo R (1997) Out of hospital outcome and quality of life in survivors of combined acute multiple organ and renal failure treated with continuous venovenous hemofiltration/hemodiafiltration. Intensive Care Med 23:766–772
Korkeila M, Ruokonen E, Takala J (2000) Costs of care, long-term prognosis and quality of life in patients requiring renal replacement therapy during intensive care. Intensive Care Med 26:1824–1831
Morgera S, Kraft AK, Siebert G, Luft FC, Neumayer HH (2002) Long-term outcomes in acute renal failure patients treated with continuous renal replacement therapies. Am J Kidney Dis 40:275–279
Johansen KL, Smith MW, Unruh ML, Siroka AM, O’Connor TZ, Palevsky PM, Network VNARFT (2010) Predictors of health utility among 60-day survivors of acute kidney injury in the Veterans affairs/national institutes of health acute renal failure trial network study. Clin J Am Soc Nephrol 5:1366–1372
Landoni G, Zangrillo A, Franco A, Aletti G, Roberti A, Calabro MG, Slaviero G, Bignami E, Marino G (2006) Long-term outcome of patients who require renal replacement therapy after cardiac surgery. Eur J Anaesthesiol 23:17–22
Myhren H, Ekeberg O, Stokland O (2010) Health-related quality of life and return to work after critical illness in general intensive care unit patients: a 1-year follow-up study. Crit Care Med 38:1554–1561
Dinglas VD, Gifford JM, Husain N, Colantuoni E, Needham DM (2013) Quality of life before intensive care using EQ-5D: patient versus proxy responses. Crit Care Med 41:9–14
Evans RW, Manninen DL, Garrison LP Jr, Hart LG, Blagg CR, Gutman RA, Hull AR, Lowrie EG (1985) The quality of life of patients with end-stage renal disease. N Engl J Med 312:553–559
Weisbord SD, Carmody SS, Bruns FJ, Rotondi AJ, Cohen LM, Zeidel ML, Arnold RM (2003) Symptom burden, quality of life, advance care planning and the potential value of palliative care in severely ill haemodialysis patients. Nephrol Dial Transplant 18:1345–1352
Sacket DL, Richardson WS, Rosenberg W (1997) Evidence-based medicine: how to practice and teach EBM. Churchill Livingstone, New York
Acknowledgments
S.M. Bagshaw is supported by a Canada Research Chair in Critical Care Nephrology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
S.M. Bagshaw has consulted for and received speaking fees and unrestricted grants from Baxter. The other authors declare that they have no conflicts of interest.
Additional information
Take-home message: Health-related quality-of-life among survivors of acute kidney injury in the intensive care unit is lower than population norms but not significantly different from that of critically ill survivors without acute kidney injury.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Villeneuve, PM., Clark, E.G., Sikora, L. et al. Health-related quality-of-life among survivors of acute kidney injury in the intensive care unit: a systematic review. Intensive Care Med 42, 137–146 (2016). https://doi.org/10.1007/s00134-015-4151-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-015-4151-0