Abstract
Purpose
Oliguria is a common symptom in critically ill patients and puts patients in a high risk category for further worsening renal function (WRF). We performed this study to explore the predictive value of biomarkers to predict WRF in oliguric intensive care unit (ICU) patients.
Patients and methods
Single-center prospective observational study. ICU patients were included when they presented a first episode of oliguria. Plasma and urine biomarkers were measured: plasma and urine neutrophil gelatinase-associated lipocalin (pNGAL and uNGAL), urine α1-microglobulin, urine γ-glutamyl transferase, urine indices of tubular function, cystatin C, C terminal fragment of pro-arginine vasopressin (CT-ProAVP), and proadrenomedullin (MR-ProADM).
Results
One hundred eleven patients formed the cohort, of whom 43 had worsening renal function. Simplified Acute Physiology Score (SAPS) II was 41 (31–51). WRF was associated with increased mortality (hazard ratio 8.65 [95 % confidence interval (CI) 3.0–24.9], p = 0.0002). pNGAL, MR-ProADM, and cystatin C had the best odds ratio and area under the receiver-operating characteristic curve (AUC-ROC: 0.83 [0.75–0.9], 0.82 [0.71–0.91], and 0.83 [0.74–0.90]), but not different from serum creatinine (Screat, 0.80 [0.70–0.88]). A clinical model that included age, sepsis, SAPS II, and Screat had AUC-ROC of 0.79 [0.69–0.87]; inclusion of pNGAL increased the AUC-ROC to 0.86 (p = 0.03). The category-free net reclassification index improved with pNGAL (total net reclassification index for events to higher risk 61 % and nonevents to lower 82 %).
Conclusions
All episodes of oliguria do not carry the same risk. No biomarker further improved prediction of WRF compared with Screat in this selected cohort of patients at increased risk defined by oliguria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urine output is considered as a key marker of kidney function as well as a therapeutic goal and a trigger for therapeutic intervention in critically ill patients [1]. Oliguria appears to be an early marker of acute kidney injury (AKI), anticipating the onset of elevation of Screat, which requires a longer time. While oliguria can be observed within a few hours, the rise in creatinine requires a longer time. Current definition of AKI includes both a “creatinine” and “urine output” criterion [2]. On the one hand, many oliguric patients do not develop AKI based on elevation of Screat [3] and there is accumulating evidence that oliguria and rise in serum creatinine (Screat) do not carry the same risk of death in ICU. Transient episode of oliguria appears to be associated with low risk of dying in ICU [4, 5]. These episodes of oliguria could therefore represent a response to systemic stress in critical illness but not always reflect a decrease of glomerular filtration rate [6]. Low urine output is therefore both a biomarker of severity in ICU patients and a trigger for therapeutic interventions in critically ill patients to prevent occurrence or progression of AKI. Because of this, identification of oliguric patients at risk of further renal function deterioration and poor outcome is of tremendous importance [7]. Early detection of patients at risk may help to decide for adapted therapy to improve their prognosis and adapt ICU resources, especially renal replacement therapy [8–10]. On the other hand, biomarkers of renal injury have failed to accurately identify those at risk of AKI in several ICU populations. This may be due to the low pre-test probability of AKI in patients included. The aim of this study is to assess the diagnostic performance of a set of plasmatic and urinary biomarkers in ICU oliguric patients with high pre-test probability of worsening renal function.
Patients and methods
Patient selection
This study was approved by Institutional Review Board of Paris North Hospitals, Paris 7 University, deciding to waive written informed consent (N 12,000). Data from patients admitted in a mixed ICU of a university hospital, with oliguria were collected. According to the kidney disease: improving global outcomes (KDIGO) definition, oliguria was defined by diuresis less than 0.5 ml/h/kg for more than six consecutive hours. Patients were then screened for oliguria by 2-h blocks. Blood and urine samples were collected at time of oliguria diagnosis together with demographic data, Simplified Acute Physiology Score (SAPS) II, clinical characteristics at ICU admission, and vital parameters as routine laboratory measurements at inclusion. The study period covered the time between the first sample and ICU discharge. After inclusion, the therapeutic decision when oliguria was present (crystalloid or colloid administration, diuretic, vasopressor infusion, transfusion) was at the discretion of the physician in charge.
Definitions
Baseline Screat level was determined from blood samples before hospital admission when available or on admission when estimated glomerular filtration rate was ≥90 ml/min. In cases where baseline creatinine level or glomerular filtration rate (GFR) was not available, the baseline creatinine level was estimated by using the Modification of Diet in Renal Disease equation with a normal GFR value of 75 ml/min/1.73 m2 [n = 11 (10 %)]. The primary endpoint was development of new AKI or persistent AKI during the 7 days following inclusion. New AKI was defined as (1) an increase in Screat level 26.5 mol/l within 48 h, or increase in Screat to 1.5 times baseline ≥26 μmol/l or >50 % compared with baseline value, or (2) need for renal replacement therapy (RRT) in patients who had no AKI upon inclusion. Worsening or persistent AKI was defined as development of AKI or steady or increase in KDIGO classification stage (based on Screat and urine output criteria) between the first 24 h following admission and day 7 in patients with AKI stage ≥1 at time of inclusion in the study (worsening renal function group). Patients with downstaging of AKI between the first 24 h following admission and day 7 [for example, from KDIGO stage 1 to stage 0] and patients without AKI during the study period were classified into the no-worsening renal function group. Patients who died during the first 24 h after admission were excluded.
Urine analysis
Urine samples collected from urinary catheter were stored at −80 °C until performing dosages. The following biomarkers were measured: urine neutrophil gelatinase-associated lipocalin (uNGAL) and urine γ-glutamyl transferase (uGgt). uNGAL samples were measured with a chemiluminescent microparticle immunoassay using an automatic I2000 Architect (Abbott Diagnostics, Rungis, France). Uα1-μg (colorimetry), uGgt (immunoturbidimetry) and urine urea, Na+, K+, and creatinine were measured using an automatic C8000 Architect (Abbott Diagnostics, Rungis, France) from a single urine sample at inclusion. The assays were performed as recommended by the manufacturers. Intra-assay variability was <5 % for each test. Urine urea, Na+, K+, and creatinine were also measured, allowing calculation of excretion fraction of sodium, excretion fraction of urea, urine/plasma creatinine, urine Na/K, and plasma urea/creatinine.
Plasma analysis
Plasma levels of NGAL (pNGAL, Bioporto) and cystatin C were obtained with immunoturbidimetry assays using an automatic C8000 Architect (Abbott Diagnostics, Rungis, France). C terminal fragment of pro-arginine vasopressin (CT-ProAVP) and proadrenomedullin (MR-ProADM) were evaluated by fluorescence polarization immunoassay (FPIA) using an automatic Kryptor compact (Thermo Fisher Scientific, Courtaboeuf, France).
Statistical analysis
Values are expressed as number and percentage or median and first to third quartile. Using worsening renal function (WRF) as the main outcome measure, the cohort size calculation was based on comparison of the area under the ROC curve for pNGAL and the AUC for a clinical model associated with pNGAL to predict WRF. With an expected incidence of WRF of 0.30, 106 subjects were needed to show a difference of AUC at 0.15 with an expected AUC of pNGAL at 0.70. Comparison between groups was performed using χ 2 test or Wilcoxon test as appropriate. Assessment of biomarkers was performed as follows (Fig. S1, supplementary file).
First, the marginal association of each biomarker of interest with WRF was studied by Wilcoxon test. The area under the receiver-operating characteristic curve (AUC-ROC) to predict WRF was determined, and the optimal threshold value was estimated using the “closest top-left” method.
Second, we estimated the association of each biomarker with the outcome with adjustment of previously described predictive factors using logistic regression. We considered the biomarkers either continuously or dichotomized according to the threshold obtained from the ROC curves. The prognostic variables included in the multivariable model were age, creatinine at admission, SAPS II, and presence of sepsis, as described previously [11]. We selected biomarkers remaining significantly associated with WRF after adjustment in both continuous and dichotomized analyses.
For those selected biomarkers, we compared AUC-ROC curves versus Screat, considered as the current gold-standard biomarker. Then, the selected biomarkers were added to this clinical model and the change in AUC with and without the biomarker was compared using the DeLong test. Last, a reclassification analysis including both the net reclassification improvement (NRI) and the integrated discrimination index (IDI) was then performed to evaluate the benefit of addition of these biomarkers to the clinical model. A two-sided p value of 0.05 was considered significant. All analyses were performed using R 2.10.1 statistical software (The R Foundation for Statistical Computing, Vienna, Austria). ROC curve analyses were performed using the package “pROC” and reclassification analyses using the “PredictABEL” package.
Results
Patient selection
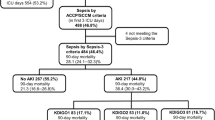
Characteristics of the patients are presented in Table 1; 111 patients were included (63 male, 48 female) with median age of 60 (45–73) years. The main reasons for ICU admission were presence of sepsis (15 %), neurological disorder (34 %), acute respiratory insufficiency (17 %), and hemorrhage/trauma (17 %), with an averaged SAPS II score of 41 (31–51). At inclusion, 34 % of patients received norepinephrine, 3 % epinephrine, and 2 % dobutamine, and 77 % of patients were mechanically ventilated; 43 patients (40 %) had ongoing treatment of infection at inclusion.
Therapeutic interventions
Of patients, 41/111 (37 %) developed WRF. Therapeutic interventions in presence of oliguria were more frequent when WRF occurred (83 versus 47 %, respectively, p = 0.0003). These interventions included fluid administration with crystalloid (63 % in WRF versus 34 % without, p = 0.003) or with colloid (29 % with WRF versus 9 % without, p = 0.004), introduction of vasopressors (15 % with WRF versus 3 % without, p = 0.02) or diuretic administration (22 % with WRF versus 7 % without, p = 0.02).
At day 1, patients with WRF had a more positive fluid balance (1,298.5 [640–2,574.8] ml) than those without WRF (556.5 [46.8–1,230.8] ml, p = 0.0002). The 6-h urine output at inclusion was not significantly different in patients with WRF compared with without WRF (0.3 [0.1–0.3] ml/kg/h versus 0.3 [0.2–0.4] ml/kg/h, respectively, p = 0.05). However, after inclusion, the 6-h urine output was lower in the WRF group versus no-WRF group (0.3 [0.1–0.4] ml/kg/h versus 0.4 [0.3–0.6] ml/kg/h, p = 0.005), a difference which was more pronounced at 24 h (0.3 [0.1–0.5] ml/kg/h versus 0.4 [0.3–0.5] ml/kg/h, p = 0.05).
Outcome
At inclusion, 35/111 patients (32 %) met AKI criteria. Among 41 patients developing WRF, 14/41 had new AKI and 29/41 patients already had AKI at admission. RRT was indicated for 9 (21 %) patients with WRF (Fig. S2, supplementary file). Among patients with WRF, 12 reached KDIGO 1, 7 KDIGO 2, and 22 KDIGO 3. Mortality in ICU was significantly higher in the WRF group compared with the no-WRF group (hazard ratio, HR [95 % CI] at 8.65 [3.0–24.9], p = 0.002; Fig. 1).
Comparison of biomarkers
Biomarker concentrations at inclusion according to the development of WRF or not are detailed in Table 2. The predictive values of these biomarkers are detailed in Table 3. pNGAL and biomarkers of “systemic stress” (CT-ProAVP and MR-ProADM) had the highest odd ratios for WRF. The AUC-ROC values of pNGAL, MR-ProADM, and cystatin C were not statistically different from Screat (0.835 [0.748–0.916], 0.816 [0.711–0.907], and 0.829 [0.740–0.909], respectively, versus 0.80 [0.70–0.88] for Screat).
The c-statistic of the clinical model that included age, creatinine at admission, SAPS II, and presence of sepsis was 0.786 ([0.688–0.872], not significantly different versus Screat; supplementary file).
When pNGAL was added to the clinical model, the AUC-ROC curve slightly increased compared with the clinical model alone (0.86 [0.77–0.93], p = 0.03; Table S3, supplementary file and Fig. 1) but was not different for MR-ProADM and cystatin C (supplementary file). Reclassification with both NRI and IDI resulted in significant reclassification (p < 0.001) of a substantial number of patients with the three selected biomarkers (pNGAL, MR-ProADM, and cystatin C; Table S5). pNGAL had excellent predictive value for RRT (AUC-ROC 0.93 [0.87–0.97], supplementary file; Fig. 2).
Discussion
Key findings
The key findings of our study are that, in a population of oliguric patients with a priori high pre-test probability of worsening renal function, biomarkers of renal injury and systemic stress did not significantly outperform Screat in predicting worsening renal function. We also confirmed that many episodes of oliguria do not translate into sustained decrease in GFR. Although pNGAL may reclassify patients, underlying its potential additional value compared with Screat, these results remain to be confirmed. None of the biomarkers appear accurate enough to predict poor renal outcome in this population.
Relationship with previous literature
In this study, we hypothesized that all episodes of oliguria would not carry the same risk of worsening renal function and poor outcome. In this line, the risk, injury, failure, loss of kidney function, and end-stage kidney disease (RIFLE) definition appropriately identifies patients with transient (short-term) oliguria as the “risk” category of AKI. The study was designed to address the question of identification of high-risk oliguric patients integrating the clinical context and a set of biomarkers.
Low urinary output is often interpreted as an early bedside warning parameter of developing AKI and/or triggering therapeutic interventions (e.g., fluid loading) [12]. However, oliguria may also be secondary to renal function adaptation to frequent situations observed in ICU [8], such as mechanical ventilation with positive pressure breathing [13] or neurohormonal activation in response to systemic stress [14]. As a consequence, a substantial proportion of oliguric patients do not develop AKI based on the Screat criteria [15]. Recently, 80 % of ICU patients meeting the oliguria criteria in a prospective observational cohort study did not increase their Screat level [15]. In another observational study, most episodes of AKI were diagnosed on only urine output criteria [16]. The authors found modest accuracy of oliguria to predict AKI based on the Screat criteria with an AUC of 0.75 (95 % CI 0.64–0.85), and a sensitivity of only 0.21 and a positive predictive value of 0.09 for an episode of oliguria >6 h. Oliguria has also been described as a factor of poor outcome in critically ill patients [3, 5]. The prognostic burden of oliguria varies according to the duration and the association with elevated Screat. Worse outcome with higher mortality rate has been observed mainly with prolonged episodes of oliguria and/or with elevated Screat [5]. In our cohort, WRF after an episode of oliguria was strongly associated with poor outcome.
Biomarkers of renal injury have previously been proposed to identify episodes of prerenal AKI or transient AKI. NGAL, a member of the lipocalin superfamily of proteins, has yielded conflicting results regarding its accuracy in predicting AKI [17, 18]. Nejat et al. [19] found that even patients with transient AKI showed elevated biomarkers of kidney injury, which increased when AKI occurred. Interestingly, in our study, the accuracy of pNGAL was found to be good, but it did not improve the predictive performance of Screat. The predictive performance of pNGAL for RRT appears promising, but should be interpreted very cautiously with respect to the very low incidence of RRT in our cohort [20, 21]. Data regarding reclassification improvement using pNGAL should be regarded as hypothesis-generating in this small cohort.
In this study we also explored other potential biomarkers. Arginine vasopressin (AVP), a stress hormone having arteriolar vasoconstrictive effect and antidiuretic effect, is a potential biomarker for renal dysfunction in critically ill patients [22]. High CT-ProAVP, the C-terminal part of pro-AVP, was shown to be predictive of renal dysfunction after renal transplantation and in patients with autosomal dominant polycystic kidney disease [23–25]. In the present study, CT-ProAVP did not significantly improve the diagnostic accuracy of WRF when compared with Screat but was found to have a good AUC-ROC curve. Proadrenomedullin peptide (PAMP), a surrogate for the ubiquitous angiogenic and vasodilator peptide adrenomedullin, has been proposed as a systemic stress biomarker targeting the kidney [26]. In a recent paper, Wagner et al. [27] observed renal protective effects after blocking adrenomedullin in a septic animal model. MR-ProADM was found to be a good predictor of WRF in our study but did not outperform Screat or pNGAL.
The nonsuperiority of biomarkers of AKI over Screat calls for several hypothesis. First, the accuracy of biomarkers of AKI was found to be good (i.e., pNGAL) and in accordance with most previously published studies in adult critically ill patients. However, the performance of Screat appeared to be improved in this group of high-risk patients. Altogether, these data reinforce the view that, although tightly associated, renal injury and renal function should be regarded as separate entities. Another hypothesis is that biomarkers were measured too early. However, this appears unlikely, since pNGAL was found high in the group with WRF. Finally, alternative biomarkers of renal injury (i.e., insulin-like growth factor-binding protein 7, metallopeptidase inhibitor 2, liver-type fatty acid-binding protein, etc.) warrant further investigation in this group of patients.
Study implications
The clinical perspective of this study was to identify patients with high risk of poor renal outcome and therefore to better target patients at need for ICU resources such as renal replacement therapy. Accuracy of biomarkers did not outperform Screat. These results underline that not all episodes of oliguria carry the same risk. In this line, elevated Screat with low urine output carries high risk of worsening renal function and in-hospital death. Under the careful application of a protocol for management of oliguric patients [8], outcomes appeared good before rise of Screat occurred.
Strengths and limitations
One of the main contributions of our study is its focus on oliguric patients. Indeed, the rather modest predictive performance of renal injury biomarkers may partially rely on the low pre-test probability in unselected ICU patients. We therefore designed this study to compare the value of biomarkers to identify high-risk oliguric patients for poor renal outcome. This is also the first study exploring systemic stress biomarkers (MR-ProADM and CT-ProAVP) and showing good predictive values. In addition, oliguria persisted despite application of preestablished hemodynamic protocol for optimization. As a consequence, development of WRF was unlikely to be related to hemodynamic alterations, although we acknowledge that not all patients responded favorably to the treatment.
The present study has several limitations. First, the sample size was relatively small, so the observed significant results and reclassification can be seen as preliminary results. Second, the single-center nature of the study may limit its generalization. The relatively small number of events in our population preclude inclusion of too many variables in the model, such as therapeutic decisions. However, the results highlight the complex question of oliguria as a diagnostic biomarker of AKI and the multiple faces of oliguria in critically ill patients. We acknowledge that our results are preliminary and should be validated in a large multicenter study. Also, multiple biomarkers were tested, introducing the risk of type I error. NRI results should be considered with caution and as hypothesis-generating, as very small increases in probability are given equal weighting to very large ones as long as they are in the right direction. The 6 h of oliguria could have been too short to detect patients at highest risk. Indeed, Prowle et al. [16] observed that oliguria persisting longer than 12 h has high predictive value in terms of outcome prediction (AKI defined by increased serum creatinine). However, we chose a 6-h period to detect a potential early therapeutic window for AKI. Finally, we acknowledge the limits of the gold standard we use for renal function (i.e., serum creatinine), which can underestimate decline in GFR. However, it is very unlikely that sustained worsening renal function was missed using the KDIGO definition because of the 7 days of follow-up [28]. This point is further underlined by the worse outcome we observed in patients with WRF.
Conclusions
The present study including critically ill patients with new-onset oliguria strongly suggests that not all episodes of oliguria carry the same risk. Oliguria with elevated Screat carries high risk of poor outcome. pNGAL, MR-ProADM, and cystatin C had good performance but did not significantly outperform Screat to identify oliguric patients with poor renal outcome. The search for new biomarkers should continue.
References
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent J-L, Moreno R, Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup (2013) Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 39:165–228. doi:10.1007/s00134-012-2769-8
Hoste EAJ, De Corte W (2013) Implementing the kidney disease: improving global outcomes/acute kidney injury guidelines in ICU patients. Curr Opin Crit Care 19:544–553. doi:10.1097/MCC.0000000000000039
Macedo E, Malhotra R, Bouchard J, Wynn SK, Mehta RL (2011) Oliguria is an early predictor of higher mortality in critically ill patients. Kidney Int 80:760–767. doi:10.1038/ki.2011.150
Payen D, Legrand M (2011) Can we identify prerenal physiology and does it matter? Contrib Nephrol 174:22–32. doi:10.1159/000329230
Mandelbaum T, Lee J, Scott DJ, Mark RG, Malhotra A, Howell MD, Talmor D (2013) Empirical relationships among oliguria, creatinine, mortality, and renal replacement therapy in the critically ill. Intensive Care Med 39:414–419. doi:10.1007/s00134-012-2767-x
Cruz DN, Bagshaw SM, Ronco C, Ricci Z (2010) Acute kidney injury: classification and staging. Contrib Nephrol 164:24–32. doi:10.1159/000313717
Basu RK, Zappitelli M, Brunner L, Wang Y, Wong HR, Chawla LS, Wheeler DS, Goldstein SL (2014) Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int 85:659–667. doi:10.1038/ki.2013.349
Legrand M, Payen D (2013) Case scenario: hemodynamic management of post-operative acute kidney injury. Anesthesiology 118(6):1446–1454. doi:10.1097/ALN.0b013e3182923e8a
Legrand M, Januzzi JL Jr, Mebazaa A (2013) Critical research on biomarkers: what’s new? Intensive Care Med 39:1824–1828. doi:10.1007/s00134-013-3008-7
Schneider AG, Bellomo R (2013) Acute kidney injury: new studies. Intensive Care Med 39:569–571. doi:10.1007/s00134-013-2860-9
Parr SK, Clark AJ, Bian A, Shintani AK, Wickersham NE, Ware LB, Ikizler TA, Siew ED (2014) Urinary L-FABP predicts poor outcomes in critically ill patients with early acute kidney injury. Kidney Int. doi:10.1038/ki.2014.301
Legrand M, Payen D (2011) Understanding urine output in critically ill patients. Ann Intensive Care 1:13. doi:10.1186/2110-5820-1-13
Farge D, De la Coussaye JE, Beloucif S, Fratacci MD, Payen DM (1995) Interactions between hemodynamic and hormonal modifications during PEEP-induced antidiuresis and antinatriuresis. Chest 107:1095–1100
Matot I, Paskaleva R, Eid L, Cohen K, Khalaileh A, Elazary R, Keidar A (2012) Effect of the volume of fluids administered on intraoperative oliguria in laparoscopic bariatric surgery: a randomized controlled trial. Arch Surg 147:228–234. doi:10.1001/archsurg.2011.308
Macedo E, Malhotra R, Claure-Del Granado R, Fedullo P, Mehta RL (2011) Defining urine output criterion for acute kidney injury in critically ill patients. Nephrol Dial Transplant 26:509–515. doi:10.1093/ndt/gfq332
Prowle JR, Liu Y-L, Licari E, Bagshaw SM, Egi M, Haase M, Haase-Fielitz A, Kellum JA, Cruz D, Ronco C, Tsutsui K, Uchino S, Bellomo R (2011) Oliguria as predictive biomarker of acute kidney injury in critically ill patients. Crit Care 15:R172. doi:10.1186/cc10318
Glassford NJ, Schneider AG, Xu S, Eastwood GM, Young H, Peck L, Venge P, Bellomo R (2013) The nature and discriminatory value of urinary neutrophil gelatinase-associated lipocalin in critically ill patients at risk of acute kidney injury. Intensive Care Med 39:1714–1724. doi:10.1007/s00134-013-3040-7
Valette X, Savary B, Nowoczyn M, Daubin C, Pottier V, Terzi N, Seguin A, Fradin S, Charbonneau P, Hanouz JL, du Cheyron D (2013) Accuracy of plasma neutrophil gelatinase-associated lipocalin in the early diagnosis of contrast-induced acute kidney injury in critical illness. Intensive Care Med 39:857–865. doi:10.1007/s00134-013-2826-y
Nejat M, Pickering JW, Devarajan P, Bonventre JV, Edelstein CL, Walker RJ, Endre ZH (2012) Some biomarkers of acute kidney injury are increased in pre-renal acute injury. Kidney Int 81:1254–1262. doi:10.1038/ki.2012.23
Cruz DN, de Cal M, Garzotto F, Perazella MA, Lentini P, Corradi V, Piccinni P, Ronco C (2010) Plasma neutrophil gelatinase-associated lipocalin is an early biomarker for acute kidney injury in an adult ICU population. Intensive Care Med 36:444–451. doi:10.1007/s00134-009-1711-1
Pipili C, Ioannidou S, Tripodaki ES, Parisi M, Douka E, Vasileiadis I, Joannidis M, Nanas S (2014) Prediction of the renal replacement therapy requirement in mechanically ventilated critically ill patients by combining biomarkers for glomerular filtration and tubular damage. J Crit Care 29:692.e7–692.e13. doi:.1016/j.jcrc.2014.02.011
Albert M, Losser M-R, Hayon D, Faivre V, Payen D (2004) Systemic and renal macro- and microcirculatory responses to arginine vasopressin in endotoxic rabbits. Crit Care Med 32:1891–1898
Boertien WE, Meijer E, Zittema D, van Dijk MA, Rabelink TJ, Breuning MH, Struck J, Bakker SJL, Peters DJM, de Jong PE, Gansevoort RT (2012) Copeptin, a surrogate marker for vasopressin, is associated with kidney function decline in subjects with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 27:4131–4137. doi:10.1093/ndt/gfs070
Meijer E, Bakker SJL, de Jong PE, Homan van der Heide JJ, van Son WJ, Struck J, Lems SPM, Gansevoort RT (2009) Copeptin, a surrogate marker of vasopressin, is associated with accelerated renal function decline in renal transplant recipients. Transplantation 88:561–567. doi:10.1097/TP.0b013e3181b11ae4
Morgenthaler NG (2010) Copeptin: a biomarker of cardiovascular and renal function. Congest Heart Fail 16:S37–S44. doi:10.1111/j.1751-7133.2010.00177.x
Nishikimi T (2007) Adrenomedullin in the kidney-renal physiological and pathophysiological roles. Curr Med Chem 14:1689–1699
Wagner K, Wachter U, Vogt JA, Scheuerle A, McCook O, Weber S, Gröger M, Stahl B, Georgieff M, Möller P, Bergmann A, Hein F, Calzia E, Radermacher P, Wagner F (2013) Adrenomedullin binding improves catecholamine responsiveness and kidney function in resuscitated murine septic shock. Intensive Care Med Exp 1:2
Pickering JW, Ralib AM, Endre ZH (2013) Combining creatinine and volume kinetics identifies missed cases of acute kidney injury following cardiac arrest. Crit Care 17:R7. doi:10.1186/cc1193
Acknowledgments
We acknowledge funding from institutional grants from Université Paris VII and from the Ministère de la Recherche plan quadriennal EA 3509. Assays for Ct-ProAVP and MR-ProADM were provided by Thermo Fisher.
Author contributions
All co-authors contributed to the manuscript and approved the submission.
Prior publication or overlapping content
Neither this manuscript nor any significant part of it is under consideration for publication elsewhere or published or available elsewhere in a manner that could be construed as a prior or duplicate publication of the same or substantially overlapping content.
Conflicts of interest
Matthieu Legrand received lectures fees from Alere. All other authors have no conflict of interest related to this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Take-home message: Although oliguria is used to detect acute kidney injury, only a small proportion of oliguric patients subsequently show a sustained decrease of glomerular filtration rate. In this study, biomarkers of renal function injury and systemic stress could substantially improve our ability to detect oliguric patients at risk of poor renal outcome when compared with clinical presentation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Legrand, M., Jacquemod, A., Gayat, E. et al. Failure of renal biomarkers to predict worsening renal function in high-risk patients presenting with oliguria. Intensive Care Med 41, 68–76 (2015). https://doi.org/10.1007/s00134-014-3566-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-014-3566-3