Abstract
Purpose
Adjunctive immunoadjuvant therapies are now proposed in the treatment of septic patients that develop immune dysfunctions. However, a prerequisite is to identify patients at high risk of death that would benefit from such therapy. Knowing that rhIL-7 is a putative candidate for septic shock treatment, we evaluated the association between increased plasmatic level of soluble CD127 (sCD127, IL-7 receptor alpha chain) and mortality after septic shock.
Methods
sCD127 plasmatic level was measured in 70 septic shock patients sampled at day 1–2 (D1) and day 3–4 (D3) after the onset of shock and 41 healthy volunteers.
Results
Compared with survivors, non-survivors presented with significantly higher sCD127 concentrations at D1 and D3 (p < 0.001 and p = 0.002). At D1, the area under the receiver operating characteristic curve for sCD127 level association with mortality was 0.846 (p < 0.0001). Kaplan–Meier survival curves illustrated that mortality was significantly different after stratification based on D1 sCD127 level (log rank test, hazard ratio 9.10, p < 0.0001). This association was preserved in multivariate logistic regression analysis including clinical confounders (age, SAPS II and SOFA scores, odds ratio 12.71, p = 0.003). Importantly, patient stratification on both D1 sCD127 value and SAPS II score improved this predictive capacity (log rank test, p = 0.0001).
Conclusions
Increased sCD127 plasmatic level enables the identification of a group of septic shock patients at high risk of death. After confirmation in a larger cohort, this biomarker may be of interest for patient stratification in future clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite improvement in patient care and the implementation of Surviving Sepsis Campaign guidelines, septic syndromes are still associated with a high risk of death in intensive care units (ICU) [1]. Indeed, a recent epidemiological study including more than 25,000 patients in the USA and in Europe showed that overall hospital mortality from severe sepsis or septic shock is still over 30 % [2]. In line with this, one in 1,200 Americans will die of severe sepsis this year and this is despite numerous clinical trials testing various adjuvant therapies and including thousands of patients [3].
One proposed explanation for the failure of these clinical trials was the absence of patient selection before the initiation of such adjunctive therapies. Indeed septic syndromes represent a group of heterogeneous diseases (from chronic pulmonary infection to purpura fulminans or peritonitis) with various germs (Gram-positive or Gram-negative bacteria or even fungi) occurring in patients with various clinical history (age, comorbidities like type I diabetes or hypertension). Moreover, host immune response to septic shock is heterogeneous as well: from massive cytokine release and cytokine storm to immune suppression [3–7]. Therefore, biomarker-based patient stratification is now strongly recommended as a prerequisite to drug administration in the design of future clinical trials testing adjunctive therapies in septic shock [8]. Such biomarkers of host response to infection should help the clinicians to (1) identify the most severe patients (at high risk of death or secondary nosocomial infection), (2) stratify patients before the initiation of targeted therapy, and (3) follow response to treatment.
Among adjunctive treatments now proposed in the therapeutic arsenal of sepsis are immunoadjuvant therapies [4, 8, 9]. Indeed, it is now well accepted that septic patients develop immune alterations that affect both innate and adaptive immune responses and whose intensity and duration are linked to increased risk of death and ICU-acquired infections [10]. In particular, one such therapy is recombinant human IL-7 (rhIL-7) as four studies in mice recently described its beneficial effects on mortality and sepsis-induced immune dysfunctions [11–14]. rhIL-7 is currently undergoing clinical trials notably to treat patients with cancer or chronic viral infections such as HIV or HBV infections. So far, this immunostimulant treatment has been well tolerated [15]. Moreover, we recently showed that ex vivo rhIL-7 treatment of septic patients’ cells significantly restored sepsis-induced lymphocyte functions to a normal response [16].
The biological activities of IL-7 are mediated by its binding to a specific cell surface receptor partly consisting of the IL-7Rα chain (CD127). Soluble forms of IL-7Rα (sCD127) have been reported and recent studies associated their concentration with the pathogenesis of autoimmune diseases [17–19]. As IL-7 is to be tested in septic patients, we investigated whether its soluble receptor (i.e., sCD127) may be associated with clinical deleterious outcomes, and thus could constitute a potential biomarker for patient stratification. In the current study, we assessed the potential value of sCD127 as a predictor of mortality or nosocomial infection.
Methods
Study population
Seventy consecutive septic shock patients, according to the diagnostic criteria of the American College of Chest Physician/Society of Critical Care Medicine [20], who were alive 3 days after the onset of shock were included in this study (Table 1). This inclusion criterion was chosen because IL-7 (or any other potential immunoadjuvant therapy) is thought to be used in a delayed step of the disease (i.e., after day 3) [4]. Additional information is available in the electronic supplementary material (ESM).
Biological analyses were performed on residual blood after completing routine follow-up performed in the ICU. EDTA-anticoagulated tubes were collected at two time points after septic shock onset: day 1–2 (D1) and day 3–4 (D3). Reference values and control samples were obtained from a cohort of 41 healthy volunteers after informed consent was given.
Results
Seventy consecutive septic shock patients were included in this study. Fifty-six (80 %) were still alive after 28-day follow-up (10 in-ICU and 4 in-hospital deaths). No statistical difference was observed regarding gender, number of comorbidities, McCabe score, ventilation, use of vasopressors, or renal replacement therapy between survivors and non-survivors (Table 1). However, a significant difference was observed between these patients when considering age (p = 0.041), initial SAPS II (p = 0.001) and SOFA scores (p = 0.026). Reference values and control samples were obtained from a cohort of 41 healthy volunteers (median age 42 years, 23 women, 18 men).
As a first step, we measured plasmatic IL-7 concentration to assess whether it can predict, by itself, mortality or nosocomial infection in septic shock patients. Although very close to the detection limit of our technique, plasmatic IL-7 concentrations were significantly increased in septic shock patients versus healthy volunteers at D1 and D3 (p < 0.001, Fig. 1a in ESM). However, no evolution of IL-7 concentrations over time could be detected between D1 and D3 in patients and no difference was observed between survivors and non-survivors, or between patients developing or not developing secondary ICU-acquired infection (data not shown).
Despite the absence of difference at D1 (p = 0.99), we observed a small decrease of plasmatic sCD127 levels between healthy volunteers and septic shock patients at D3 (p = 0.03). Interestingly, a significant decrease in sCD127 concentrations was noted between D1 and D3 after septic shock (p < 0.001, Fig. 1a).
Plasmatic concentration of soluble IL-7R α-chain (sCD127) in septic shock patients. Plasmatic sCD127 concentrations were measured in septic shock patients (n = 70) at day 1–2 (D1–2, red circles) and day 3–4 (D3–4, red squares) after the onset of shock in comparison with healthy volunteers (HV, black triangles, n = 41) (a), and in survivors (S, blue, n = 56) versus non-survivors (NS, green, n = 14) (b). Non-parametric Mann–Whitney U test was used to compare results between groups. Non-parametric Wilcoxon paired test was used to evaluate overtime evolution within a group
No correlation between plasmatic sCD127 and IL-7 concentrations was found at day D1 or D3 (respective Spearman’s rank correlation coefficients, r = −0.148 and r = −0.066). Similarly, no significant correlation between sCD127 levels and mHLA-DR, SOFA or SAPS II scores was observed at D1 or D3 (data not shown). Moreover, no statistical difference was observed for sCD127 measurements when comparing patients with or without comorbidities and with nosocomial or community-acquired septic shock at both time points.
When comparing sCD127 concentrations between patients developing or not developing secondary ICU-acquired infections, no statistical difference was observed at D1 (p = 0.60) or D3 (p = 0.28, Fig. 1b in ESM). However, non-survivors presented with significantly higher sCD127 concentrations at D1 (p < 0.001) and at D3 (p = 0.002) compared with survivors (Fig. 1b).
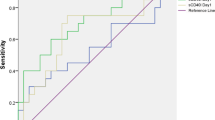
The areas under the receiver operating characteristic curves (AUC) for prediction of 28-day mortality were calculated for sCD127, SAPS II, and SOFA scores. The best AUC was observed for sCD127 concentration at D1 (0.846, [95 % CI] 0.741–0.951, p < 0.001, Fig. 2). At D3, the AUC for sCD127 was 0.774 ([95 % CI] 0.618–0.930, p = 0.002). The AUC for SOFA and SAPS II scores were, respectively 0.692 ([95 % CI] 0.531–0.853, p = 0.027) and 0.770 ([95 % CI] 0.644–0.897, p = 0.002).
Receiving operating characteristic curves of SAPS II, SOFA, and sCD127 at D1–2 for predicting mortality. Area under receiver operating characteristic curves, 0.846 (p < 0.001) for sCD127 D1, 0.770 (p = 0.002) for SAPS II, and 0.692 (p = 0.027) for SOFA. Regarding sCD127 values, the best threshold calculated on the basis of minimum d (minimum distance between ROC curve and point of sensitivity and specificity = 1) was 48.78 ng/ml at D1. For this cutoff value at day 1–2, positive predictive value was 42 % and negative predictive value was 93 %
Logistic regression analyses were then performed to assess if this marker remained independently associated with mortality after septic shock. When considering independently other usual clinical confounders such as SOFA or SAPS II scores, higher sCD127 concentrations at D1 or D3 remained significantly associated with a higher risk of death after septic shock (Table 2).
Using cutoff values determined on ROC curves based on calculation of the minimum d (sCD127 at D1, 48.78 ng/ml; sCD127 at D3, 41.56 ng/ml; SAPS II, 59), Kaplan–Meier survival curves were established (Fig. 3a, b). The survival rates of septic patients were significantly different when stratified according to sCD127 concentrations at D1 and D3. Patients with lower sCD127 concentration had better survival compared with patients with higher sCD127 concentration (log rank test, at D1, p < 0.001, hazard ratio 9.10, [95 % CI] 2.92–28.35; at D3, p < 0.001, hazard ratio 13.47, [95 % CI] 3.93–46.14). When using cutoff value of 59 for SAPS II, we observed a statistical difference in the survival rates of septic patients (p = 0.0082, log rank test, hazard ratio 4.61, [95 % CI] 1.48–14.31, data not shown).
Survival curves. Kaplan–Meier survival curves were established on the basis of sCD127 cutoff values at day 1–2 (48.78 ng/ml) (a) and day 3–4 (41.56 ng/ml) (b). A significant difference was measured between the two curves (a: p < 0.001, log rank test, hazard ratio 9.10, [95 % CI] 2.92–28.35 at day 1–2. b: p < 0.001, log rank test, hazard ratio 13.47, [95 % CI] 3.93–46.14 at day 3–4)
Besides, using this threshold (sCD127 at D1, 48.78 ng/ml), the sensitivity of sCD127 concentration for differentiating survivors from non-survivors was 79 % and its specificity 73 %. The positive predictive value (PPV) was 42 % and the negative predictive value (NPV) 93 %. In comparison, respective sensitivity and specificity for SAPS II score were 64 and 73 %. PPV and NPV were 38 and 89 %.
Interestingly, at D1, when stratified on the basis of the combination of sCD127 concentration and SAPS II score, the difference between the survival rates of patients was even greater. Indeed, patients with lower sCD127 concentration and lower SAPS II had a significantly better survival than patients with higher sCD127 concentration and higher SAPS II (p < 0.001, log rank test, Fig. 2a in ESM). This was even conserved when sCD127 measurement was delayed until D3 after the onset of shock (p < 0.001, log rank test, Fig. 2b in ESM).
Discussion
The failure of several high-profile clinical trials in sepsis testing various adjuvant therapies represents one of the greatest disappointments of the past 30 years [3]. Among others, one explanation proposed for the failure of these trials is that the heterogeneity of the septic patient population and of their pathophysiologic response was never considered in the design of these clinical studies. Indeed, septic syndrome affects a group of patients with different etiologies (from pneumopathy to peritonitis) caused by different germs (Gram-positive or Gram-negative bacteria or even fungi) and with different medical history (with or without comorbidities). Moreover, it is now clear that the host immune response after septic shock is variable from one patient to another and also evolves over time, being associated with both an overwhelming cytokine release called cytokine storm and the later development of sepsis-induced immune dysfunctions [4, 5]. Therefore, the need for patient stratification before the initiation of any therapy targeting host response is now considered as mandatory in the design of future clinical trials testing such adjunctive therapies in sepsis [8].
To enable such stratification, robust and reliable biomarkers identifying patients at high risk of death or nosocomial infections are mandatory. One example is the decreased expression on circulating monocytes of human leukocyte antigen-DR (mHLA-DR), which has been shown to be associated with increased risk of death or secondary infections in various ICU clinical contexts (septic shock, trauma, burn, pancreatitis, etc.) [8, 21]. Interestingly, the measurement of this marker is now included for patient stratification in the design of clinical trials evaluating immune-stimulating therapies such as GM-CSF [22]. One limitation to the use of this biomarker is that its measurement is performed by flow cytometry which requires strict pre-analytical conditions and is not available 24/7 in most clinical labs.
In the current study, knowing that IL-7 is to be assessed in sepsis clinical trials, we tested the hypothesis that plasmatic sCD127 (its soluble receptor) level measurement might represent a robust and easy-to-measure biomarker for identification of patients at high risk of death after septic shock. The main result was to observe the highest sCD127 levels in a group of patients presenting with increased mortality.
CD127 constitutes the alpha chain of the IL-7 receptor, a heterodimer comprised of CD127 and the common cytokine receptor γ-chain (CD132). As with many cytokine receptors, a soluble form of the IL-7Rα chain (sCD127) has been identified [23]. However, the origins (alternative splicing and release of membrane-bound form) and functions of plasmatic sCD127 as well as the mechanisms regulating the level of CD127 expression on the cell surface are not well known. The soluble protein was shown to bind to IL-7 in solution and is known to be involved in several aspects of the immune response including inflammation, cellular proliferation, and apoptosis [24, 25]. In addition, studies have pointed out that sCD127 could be a marker of immunopathogenesis in several diseases such as HIV infection, multiple sclerosis [26], leukemia [26, 27], systemic lupus erythematosus [17], and type 1 diabetes [18].
In the current study, we observed a significant association between increased sCD127 plasmatic level and increased risk of death after septic shock. Moreover, maximal difference in sCD127 concentrations between survivors and non-survivors was observed as early as D1 after the onset of shock. This is interesting because most biomarkers of host immune response usually present with predictive value in regard to deleterious outcome later on after the onset of shock (as is the case for mHLA-DR). Therefore, increased sCD127 could thus prove to be a very early biomarker usable for patient stratification rapidly after septic shock. Moreover, as suggested by experts in the field, as the immune response after sepsis is a rapidly evolving phenomenon, the dynamic measurement of sCD127 over time could be more informative than a single measurement [28]. Although we may hypothesize that sCD127 is a consequence of tremendous lymphocyte activation, the reason for this amplified increase in non-survivors remains to be investigated.
In addition, the association between increased sCD127 and higher risk of death was preserved in multivariate analysis including clinical confounders such as SOFA and SAPS II scores. This suggests that the predictive value of this biomarker is independent of patients’ initial severity and that this marker may be informative by itself. Most interestingly, the combination of sCD127 level and SAPS II improved the predictive value compared with each parameter considered alone. Thus, as proposed in the PIRO (Predisposition, Infection, Response, Organ dysfunction) score, this highlights the interest of combining clinical parameters and biomarkers of host response in a single score to improve patient characterization and thus their stratification [29].
Furthermore, as opposed to other parameters such as mHLA-DR, sCD127 concentration may appear easy to measure in plasma on routine automated tests which are available in emergency laboratories. In fact, ELISA techniques are commonly used for many biological parameters in routine practice and sCD127 measurement may be easily automated.
Thus our results suggest that this parameter may be used for identification of a group of patients with a high risk of death after septic shock. Such stratification strategy is now highly recommended in the design of future clinical trials testing immunoadjuvant therapies in sepsis. Interestingly, one such immunostimulating drug proposed for the treatment of septic shock patients is recombinant human interleukin-7 (rhIL-7) whose receptor partly comprises CD127. Indeed, since T lymphocyte anergy has been shown to be a hallmark of sepsis-induced immune dysfunctions, rhIL-7 has been proposed as a putative therapeutic strategy in sepsis. IL-7 is a pluripotent cytokine and plays a fundamental role in T cell development, peripheral T cell homeostasis, and immune tolerance. Importantly, IL-7 is currently undergoing numerous clinical trials, including in patients with cancer or human immunodeficiency virus 1 (HIV-1), and could represent an innovative therapy in the treatment of sepsis [15]. Indeed, rhIL-7 treatment has been shown to act at multiple levels to improve host immunity during sepsis in different studies in mice such as prevention of T cell apoptosis and improvements in T cell trafficking and functionality after bacterial or fungal sepsis. Beneficial effects on mortality and sepsis-induced immune dysfunctions have been described with rhIL-7 treatment [11–14]. We recently evaluated the interest of ex vivo rhIL-7 administration in septic shock patients. We showed that the IL-7 pathway was not significantly altered and was still functional in septic shock patients. Moreover, in this study, ex vivo rhIL-7 treatment significantly restored sepsis-induced lymphocyte functions to a normal response [16]. Although we did not investigate the correlation between high levels of sCD127 and the response following ex vivo rhIL-7 administration, we believe that this is worth assessing in a further study.
Our study has some limitations. First, these results need to be confirmed in a larger ideally multicentric study including important cohorts of patients. Furthermore, other groups of septic patients should be included, as here we focused on a specific group of patients still alive after day 3. A prerequisite would be the optimization of sCD127 ELISA measurement so as to allow its standardization. In addition, as this study was exploratory and very preliminary, mechanisms responsible for plasmatic sCD127 increase after septic shock (altered clearance in the most severe patients, shedding from activated cells, etc.) as well as the link between plasmatic sCD127 level, lymphopenia, and lymphocyte anergy and/or its restoration by rhIL-7 need to be evaluated in a dedicated study. Understanding how sCD127 measurement could direct rhIL-7 therapy would also deserve investigation in a dedicated study, especially regarding sCD127 measurement and lymphocyte alterations. Finally, healthy volunteers were not age-matched with septic shock patients. Nevertheless, as the main outcome was mortality among septic patients, we hypothesize that this did not interfere with the results.
To conclude, our results strongly support the hypothesis that sCD127 measurement could represent a new attractive biomarker for identification of a group of patients presenting with higher risk of mortality. Upon association studies showing a link between sCD127 measurement and sepsis-induced immune/lymphocyte alterations and/or response to rhIL-7 treatment, we can imagine that in upcoming clinical trials evaluating new immunoadjuvant therapies in sepsis (especially aimed at rejuvenating the adaptive side of immunity), assessment of sCD127 might be of help for patient stratification.
References
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R (2013) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 39:165–228
Levy MM, Artigas A, Phillips GS, Rhodes A, Beale R, Osborn T, Vincent JL, Townsend S, Lemeshow S, Dellinger RP (2012) Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis 12:919–924
Angus DC, van der Poll T (2013) Severe sepsis and septic shock. N Engl J Med 369:840–851
Hotchkiss RS, Monneret G, Payen D (2013) Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis 13:260–268
Hotchkiss RS, Monneret G, Payen D (2013) Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 13:862–874
Guignant C, Venet F, Planel S, Demaret J, Gouel-Cheron A, Nougier C, Friggeri A, Allaouchiche B, Lepape A, Monneret G (2013) Increased MerTK expression in circulating innate immune cells of patients with septic shock. Intensive Care Med 39:1556–1564
Grimaldi D, Louis S, Pene F, Sirgo G, Rousseau C, Claessens YE, Vimeux L, Cariou A, Mira JP, Hosmalin A, Chiche JD (2011) Profound and persistent decrease of circulating dendritic cells is associated with ICU-acquired infection in patients with septic shock. Intensive Care Med 37:1438–1446
Venet F, Lukaszewicz AC, Payen D, Hotchkiss R, Monneret G (2013) Monitoring the immune response in sepsis: a rational approach to administration of immunoadjuvant therapies. Curr Opin Immunol 25:477–483
Hotchkiss RS, Opal S (2010) Immunotherapy for sepsis—a new approach against an ancient foe. N Engl J Med 363:87–89
Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA (2009) The sepsis seesaw: tilting toward immunosuppression. Nat Med 15:496–497
Unsinger J, Burnham CA, McDonough J, Morre M, Prakash PS, Caldwell CC, Dunne WM Jr, Hotchkiss RS (2012) Interleukin-7 ameliorates immune dysfunction and improves survival in a 2-hit model of fungal sepsis. J Infect Dis 206:606–616
Unsinger J, Kazama H, McDonough JS, Griffith TS, Hotchkiss RS, Ferguson TA (2010) Sepsis-induced apoptosis leads to active suppression of delayed-type hypersensitivity by CD8+ regulatory T cells through a TRAIL-dependent mechanism. J Immunol 184:6766–6772
Unsinger J, Kazama H, McDonough JS, Hotchkiss RS, Ferguson TA (2009) Differential lymphopenia-induced homeostatic proliferation for CD4+ and CD8+ T cells following septic injury. J Leukoc Biol 85:382–390
Kasten KR, Prakash PS, Unsinger J, Goetzman HS, England LG, Cave CM, Seitz AP, Mazuski CN, Zhou TT, Morre M, Hotchkiss RS, Hildeman DA, Caldwell CC (2010) Interleukin-7 (IL-7) treatment accelerates neutrophil recruitment through gamma delta T-cell IL-17 production in a murine model of sepsis. Infect Immun 78:4714–4722
Mackall CL, Fry TJ, Gress RE (2011) Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol 11:330–342
Venet F, Foray AP, Villars-Mechin A, Malcus C, Poitevin-Later F, Lepape A, Monneret G (2012) IL-7 restores lymphocyte functions in septic patients. J Immunol 189:5073–5081
Badot V, Luijten RK, van Roon JA, Depresseux G, Aydin S, Van den Eynde BJ, Houssiau FA, Lauwerys BR (2013) Serum soluble interleukin 7 receptor is strongly associated with lupus nephritis in patients with systemic lupus erythematosus. Ann Rheum Dis 72:453–456
Monti P, Brigatti C, Krasmann M, Ziegler AG, Bonifacio E (2013) Concentration and activity of the soluble form of the Interleukin-7 receptor alpha in type 1 diabetes identifies an interplay between hyperglycemia and immune function. Diabetes 62:2500–2508
Lundstrom W, Highfill S, Walsh ST, Beq S, Morse E, Kockum I, Alfredsson L, Olsson T, Hillert J, Mackall CL (2013) Soluble IL7Ralpha potentiates IL-7 bioactivity and promotes autoimmunity. Proc Natl Acad Sci USA 110:E1761–E1770
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101:1644–1655
Monneret G, Venet F, Pachot A, Lepape A (2008) Monitoring immune dysfunctions in the septic patient: a new skin for the old ceremony. Mol Med 14:64–78
Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, Weber-Carstens S, Hasper D, Keh D, Zuckermann H, Reinke P, Volk HD (2009) Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med 180:640–648
Pleiman CM, Gimpel SD, Park LS, Harada H, Taniguchi T, Ziegler SF (1991) Organization of the murine and human interleukin-7 receptor genes: two mRNAs generated by differential splicing and presence of a type I-interferon-inducible promoter. Mol Cell Biol 11:3052–3059
Crawley AM, Angel JB (2012) The influence of HIV on CD127 expression and its potential implications for IL-7 therapy. Semin Immunol 24:231–240
Crawley AM, Faucher S, Angel JB (2010) Soluble IL-7R alpha (sCD127) inhibits IL-7 activity and is increased in HIV infection. J Immunol 184:4679–4687
McKay FC, Swain LI, Schibeci SD, Rubio JP, Kilpatrick TJ, Heard RN, Stewart GJ, Booth DR (2008) Haplotypes of the interleukin 7 receptor alpha gene are correlated with altered expression in whole blood cells in multiple sclerosis. Genes Immun 9:1–6
Korte A, Kochling J, Badiali L, Eckert C, Andreae J, Geilen W, Kebelmann-Betzing C, Taube T, Wu S, Henze G, Seeger K (2000) Expression analysis and characterization of alternatively spliced transcripts of human IL-7Ralpha chain encoding two truncated receptor proteins in relapsed childhood all. Cytokine 12:1597–1608
Gouel-Cheron A, Allaouchiche B, Guignant C, Davin F, Floccard B, Monneret G (2012) Early interleukin-6 and slope of monocyte human leukocyte antigen-DR: a powerful association to predict the development of sepsis after major trauma. PLoS One 7:e33095
Rubulotta F, Marshall JC, Ramsay G, Nelson D, Levy M, Williams M (2009) Predisposition, insult/infection, response, and organ dysfunction: a new model for staging severe sepsis. Crit Care Med 37:1329–1335
Acknowledgments
The authors would like to thank Anne Portier and Caroline Guignant from the Immunology Laboratory of Hôpital E. Herriot—Lyon for their help in performing pre-analytical handling of samples; Nathalie Panel and Marion Provent (Clinical Research Center, Lyon-Sud) for their work on patient inclusion and clinical data acquisition. This work was supported by funds from the Hospices Civils de Lyon to AL, GM, and FV.
Author information
Authors and Affiliations
Corresponding author
Additional information
Take-home message: Increased plasmatic level of soluble IL-7 receptor (CD127) is associated with elevated mortality in septic shock patients. This may be of interest for patient stratification in future clinical trials testing immunoadjuvant therapies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Demaret, J., Villars-Méchin, A., Lepape, A. et al. Elevated plasmatic level of soluble IL-7 receptor is associated with increased mortality in septic shock patients. Intensive Care Med 40, 1089–1096 (2014). https://doi.org/10.1007/s00134-014-3346-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-014-3346-0