Abstract
Purpose
To assess the feasibility and validity of ultrasonographic measurement of gastric antral cross-sectional area (usCSA) in critically ill patients to predict gastric volume and the use of computed tomography (CT) as a reference to measure gastric volume.
Method
This single-center, prospective, cross-sectional study included 55 critically ill patients who had an abdominal CT scan. usCSA measurements were performed within the hour preceding the CT scan. Gastric volumes were measured on the CT scan using semiautomatic software. The feasibility rate, performing conditions (% “good” and “poor”), internal and external validity of antral usCSA measurements, performed by an ICU physician, were assessed to predict gastric volume.
Results
Antral usCSA measurements were feasible in 95 % of cases and were positively correlated with gastric volume measured by the CT scan when performed in “good” conditions (65 %) (r = 0.43). There was good reproducibility of measurements (intraclass correlation coefficient of 0.97, CI 95 % 0.96–0.99) and there was clinically acceptable agreement between measurements performed by radiologists and intensivists (bias −0.12 cm2). The receiver operating characteristic curve identified a cutoff value of 3.6 cm2 that discriminated an “at-risk stomach” (volume >0.8 mL/kg) at a sensitivity of 76 % and a specificity of 78 %.
Conclusions
Ultrasonographic measurement of antral CSA is feasible and reliable in the majority of critically ill patients. This technique could be useful to manage critically ill patients at risk of aspiration or with enteral feeding.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gastric emptying is commonly disturbed in critically ill patients. Consequently, airway management procedures or inappropriate enteral feeding can cause aspiration, which can lead to devastating complications [1]. Animal experiments have shown that the severity of pulmonary damage is linked to the volume of gastric fluid aspirated [2]. For patients undergoing elective surgery, a 6-h fasting period (2 h for clear fluids) has been recommended to reduce the risk of aspiration during anesthesia [3]. For critically ill patients, gastric emptying is frequently altered and is influenced by many factors, including age, diagnosis at admission [4], nature of illness [5], medications [6, 7], and mechanical ventilation [8]. Thus, a simple, quick, reliable, and noninvasive bedside test to assess gastric volume would be of great interest. Such a test would mean that clinical decisions regarding airway management (intubation, extubation, or orotracheal tube exchange) and enteral feeding could rely on an objective measurement of gastric residual volume.
Studies in healthy volunteers have shown the feasibility of using ultrasound to assess gastric content by measuring the antral cross-sectional area (usCSA) [9, 10]. Perlas et al. [9] reported an almost linear relationship between usCSA and gastric volume in healthy volunteers. Measurements on patients undergoing elective surgery also showed a significant positive relationship between usCSA and the fluid volume aspirated via a nasogastric tube [11]. However, no study has properly evaluated this technique in critically ill patients, whose gastric status is often different from healthy volunteers because of gastrointestinal dysfunction and positive-pressure ventilation [8, 12]. There has been no reliable and easy way to perform a gold standard test to assess gastric volume. Volumetric measurement by tomodensitometry could be an adequate method, as it enables easy access for intensive care patients and gives accurate measurements using a high spatial resolution when the most recent generation of multiple-detector computed tomography (CT) scanners is used [13].
The aim of our study was to assess the feasibility and validity of ultrasound (US) to assess usCSA to predict gastric volume in critically ill patients, and to use CT volumetric measurement as the reference method.
Materials and methods
This prospective observational study was performed in an 18-bed academic intensive care unit (ICU). The protocol was approved by the local institutional review board (Comité d’Evaluation de l’Ethique des projets pour la Recherche Biomédicale, Paris Nord, France N°10-060) and informed consent was obtained from all patients or from their relatives.
Protocol design and data collection
All consecutive patients admitted to the ICU and scheduled to undergo an abdominal contrast-enhanced CT scan were prospectively and consecutively included. Exclusion criteria were being aged less than 18 years, pregnancy, and any medical history of upper gastrointestinal surgery. The whole protocol design is summarized in Fig. 1. The following clinical items were recorded on the day of inclusion: age, gender, body mass index, relevant medical and surgical history, reasons for ICU admission, length of stay in the ICU before inclusion in the study, and the indication for an abdominal CT scan. A simplified acute physiology score [14] (SAPS II) was calculated for all patients on the day of inclusion, and an injury severity score [15] (ISS) was calculated for multiple-trauma patients. The type of ventilation, ongoing medications, and all data regarding feeding were recorded (type of feeding, type of nasogastric tube, fasting time). Patients were considered as fasting if they had a minimum of 6 h fast.
Standardized performance of ultrasonography
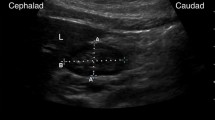
US measurements of usCSA were performed by two experienced intensivists (PG) and (SH), who had undergone 4 h of practical training (12 cases), and an abdominal radiologist (MR). Measurements were standardized and performed within 60 min before undergoing an abdominal CT scan. Patients were placed in a semi-upright position with the head of the bed raised to 30°, or in a 30° inclined straight supine position for patients with a spinal or pelvic fracture. The antrum position was scanned with a curvilinear probe (2–5 Hz, Acuson, Siemens, Malvern, PA, USA) in a parasagittal plane in the epigastric area. The left lobe of the liver and the abdominal aorta were used as internal and posterior landmarks. Once these landmarks were identified, the probe was rotated to obtain the smallest cross-sectional view of the antrum (Fig. 2) [9]. Anteroposterior (Dap) and craniocaudal (Dcc) diameters were then measured three times to estimate usCSA using the following formula [9]:
\( {\text{usCSA}}=({\text{mean\,Dap}} \times {\text{mean\,Dcc}}\times\pi)/4.\)
While performing the US examination, operators were asked to note the conditions in which US was performed as “good”, “poor”, or “impossible”. The operator also noted if the nasogastric tube was seen. Immediately afterwards, a second set of measurements was performed by an abdominal radiologist unaware of the intensivists’ US measurements to determine external validity. If the radiologist was unable to attend, the CT scan was performed without the second set of measurements to limit the delay between the US and CT measurements.
Methods used for CT scan measurements
CT analysis was performed using a 64-row detector CT (GE Healthcare, Waukesha, WI, USA). Data were analyzed on a dedicated workstation (Advantage Windows, VolumeShare 5, GE Healthcare, Waukesha, WI, USA), and volume measurements were performed by a senior radiologist (MR) using semiautomatic dedicated software. The radiologist was blinded to the clinical data and US measurements (Volume Viewer, GE Healthcare, Waukesha, WI, USA). Each slice of the stomach acquired in the portal phase was traced with a cursor, and the computer then calculated the corresponding volume. The following volumes were measured using a three-dimensional technique: antral cross-sectional area (ctCSA), total gastric volume (air and fluid: GV total), and fluid volume alone (GV fluid). All measurements were performed in triplicate and final volumes were calculated as the mean of these three measurements. Scanographic measurements were considered as the reference for stomach content volume. In our study patients with an “at-risk stomach” were defined as those with a total gastric volume exceeding 0.8 mL/kg [2].
Endpoints
The primary endpoint of this study was to assess the validity of the ultrasound technique, used by ICU physicians, to measure gastric usCSA to predict gastric volume measured on the CT scan in critically ill patients.
The secondary endpoints were estimation of the feasibility of ultrasonography at the patient’s bedside (as % “good” or “poor” conditions), evaluation of external validity by comparing measurements taken by ICU physicians and radiologists, and determination of a cutoff value for usCSA that could discriminate an “at-risk stomach” from an “empty stomach” using a threshold gastric volume of greater than 0.8 mL/kg of liquid.
Statistical analyses
Data are presented as frequencies and percentages for categorical variables, and as medians (25–75th percentile) for quantitative variables. The Kolmogorov–Smirnov test was used to assess whether continuous data were normally distributed. Interdependence of US and CT measurements was evaluated using the nonparametric Spearman’s correlation coefficient due to the skewed distribution of the variables. To identify associations between the patients’ characteristics and gastric volume, a logistic regression model was used. Factors selected for the multivariable regression model had p values less than 0.05 in the univariate analyses.
Categorical variables were compared using Fisher’s exact test and continuous variables were analyzed using Wilcoxon’s rank-sum test. The intraclass correlation coefficient was used to measure intraobserver variability of the intensivists to measure usCSA.
The performance of usCSA to discriminate an “at-risk stomach” (volume >0.8 mL/kg) was evaluated from receiver operating characteristic (ROC) curves and calculation of the areas under the curves (ROCAUC). Sensitivity, specificity, positive predictive value, and negative predictive value were calculated at the cutoff value of discrimination for usCSA (Youden index maximization). All tests were two-tailed and statistical significance was set at the p < 0.05 level. All analyses were performed using R software version 3.02 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 55 patients were included in the study. Clinical and demographic characteristics are shown in Table 1. An abdominal CT was performed in all patients within 44 min following completion of the US measurements (31 min [23–44]). US performance conditions were reported as “impossible” in three patients (6 %) because ileus and gas screen impeded penetration of the US. Of the remaining 52 patients (94 %), 36 series of measurements were reported as “good” conditions (65 %) and 16 as “poor” conditions (29 %).
Correlations between usCSA and gastric volume measured by a CT scan in different conditions are presented in Fig. 3 (and Electronic supplementary material 1). The logistic regression model did not show any significant association between gastric volume and age, gender, body mass index, mechanical ventilation, or vasopressor infusion.
Intraobserver reproducibility of ICU physicians, measured using the intraclass correlation coefficient to assess usCSA measurements, was 0.97 (95 % CI 0.96–0.99). Concerning external validity, agreement between intensivists and radiologists was analyzed in 11 patients (20 %) (9 were “good” and 2 were “poor” conditions), leading to 30 pairs of measurements. The Bland–Altman diagram estimated the systematic bias between usCSA measurements made by intensivists and radiologist at –0.12 cm2 with limits of agreement of [–2.21; 1.96] (Electronic supplementary material 2). All measurements outside the limits of agreement were obtained from measurements performed in “poor” conditions.
The ultrasonographic diagnosis of an “at-risk stomach”, defined as a gastric volume exceeding 0.8 mL/kg, showed an area under the ROC curve of 0.799 (Electronic supplementary material 3). For measurements of usCSA obtained in “good” conditions, a cutoff value of 3.6 cm2 was defined, with a sensitivity of 76 % and a specificity of 78 % (Table 2).
Thirty-five nasogastric tubes out of 38 (92 %) were seen during the ultrasound examinations, of which 23 (60 %) were performed in “good” and 15 (40 %) in “poor” conditions.
Discussion
The main original finding of this study is that usCSA measurements are feasible in the majority of critically ill patients; they are positively correlated with gastric volume in all conditions, and are more strongly correlated in good conditions. Our results suggest that adequate measurements can be obtained in 65 % of cases (“good” conditions). These measurements allowed good discrimination between an “at-risk” stomach, defined as a gastric volume exceeding 0.8 mL/kg, and an “empty stomach” using a cutoff value for usCSA of 3.6 cm2. Although the correlation between ultrasound and CT was not particularly strong, the interest of a dynamic bedside test would rather be to answer pragmatic questions such as how is enteral nutrition tolerated, does the patient need propulsant medication, or should a gastric tube be placed prior to intubation?
Internal repeatability was good and external validity showed a clinically acceptable bias. Moreover, the five pairs of measurements with the highest negative differences made by the intensivists and the radiologist were obtained in “poor” conditions. If one only considers measurements realized in “good” conditions as reliable, this observation allowed us to conclude that external validity was excellent (Spearman’s ratio 0.94 and bias –0.08). In our study, the antral cross-sectional area could not be accurately measured in one case out of three. For conditions labeled as “poor”, there were no actual technical impossibilities, but the low sharpness of the imaged structures did not permit reliable measurements. This failure rate is comparable to that described in pregnant women (40 %) [16], but is higher than in healthy volunteers or in patients in a preoperative setting (0–2.5 %) [10, 11].
The use of US to assess gastric contents by measuring antral cross-sectional area has already been studied in healthy volunteers. In a preoperative setting it showed a higher rate of feasibility (98.5–100 %) than in our critically ill patients. To our knowledge, Koenig et al. [17] have published the only study designed to qualitatively assess the gastric contents of patients that require urgent endotracheal intubation. They demonstrated that a rapid (<2 min) left upper-quadrant US examination could distinguish patients with a full stomach, and whose gastric aspiration through a nasogastric tube then showed a mean volume of 553 ± 290 mL. Nevertheless no systematic assessment of gastric volume was conducted on other patients [17]. Perlas et al. [9] constructed a mathematical model to predict gastric volume as a function of usCSA, height, and age of healthy volunteers. However, this formula was not applicable for our cohort of critically ill patients because there was no association between gastric volume and the patient’s characteristics (age, gender, body mass index, mechanical ventilation, or ongoing medications). As a consequence, this mathematical model led to erratic results in our patients.
To date, there is no gold standard to evaluate gastric volume. The techniques that have been proposed, such as scintigraphy, gastric impedance [18], or paracetamol absorption [19], are not accurate or are invasive and difficult to perform in critically ill patients. The use of gastric aspiration has also been proposed to assess gastric volume [11, 17]. Koenig et al. [17] simply aspirated through a nasogastric tube and then controlled, with a second US, how much gastric content had been removed. Bouvet et al. [11] provided further details about their protocol: they aspirated gently for at least 15 min while moving the tube backwards and forwards, massaging the patient’s epigastrium and placing the patients in supine, supine with head up, Trendelenburg, and supine with right and left lateral tilt positions.
In the absence of a universally accepted gold standard, the “reference” technique we chose in our study to accurately assess gastric volume was a multiple-detector CT scan. Experimental imaging strategies have assessed gastric volume in rats using a CT scan and ingested contrast agents [20]. The technique had been validated after comparison with classical postmortem methods (stomach phenol red content and stomach wet weight) [21]. Three-dimensional multi-slice CT has also been described to assess gastric pouch volume in patients after bariatric surgery. However, the technique described for this population is invasive as it combines oral administration of an ionic contrast agent and intravenous administration of butylscopolamine [22]. We chose a 64-row detector contrast-enhanced CT scan and three-dimensional semiautomatic volumetric analyses as the reference for our patients, as it allowed accurate measurements at the millimeter scale [13, 23, 24]. No oral contrast agent was used to avoid adding any invasive procedure to our protocol.
Our study had several limitations. Firstly, it is a descriptive study that allowed us to assess the feasibility and validity of usCSA measurements to evaluate gastric contents in ICU patients, in a cohort of limited size. The gender disproportion could be explained by the fact that almost half the patients of the cohort were trauma patients, known to be mostly males [25]. We think that this particularity does not reasonably prevent the generalization of our results. Also, the definition of an “at-risk stomach” was based on experimental data on primates: pulmonary instillation of 0.4 and 0.6 mL/kg of hydrochloric acid did not lead to any significant clinical or radiological changes, whereas 0.8 mL/kg led to severe pulmonary symptoms and death of 50 % of the animals [2]. A gastric volume of 0.8 mL/kg is usually chosen as the cutoff volume [9, 10] to determine an “at-risk stomach”, considering as a precautionary principle the risk of aspiration of the whole gastric content.
Another limitation could be that aspiration of residual gastric volume through an enteral feeding tube was not part of our protocol. We chose to retain ordinary care, and to have no intervention apart from US measurements on our patients. Moreover, the size of enteral feeding tube has been shown to influence the measurement of residual gastric volume [26]; our patients had different types and different sizes of enteral feeding tubes (12F–18FR). Most were silicon gastric tubes, in which the lumen can easily collapse when suction is applied. Nevertheless we could see the nasogastric tubes in the stomachs of 92 % of the cases, suggesting that positioning of the nasogastric tube with US could possibly replace the standard abdominal radiographic technique. This would considerably gain time, decrease irradiation, and reduce costs. So, in the era of developing whole body ultrasonography [27], gastric US could add to other essential visceral assessments such as focused assessment with sonography for trauma (FAST) [28], bowel and colon [29], intraperitoneal free air [30], or vascular access [31].
Another noteworthy point is that no learning curve was assessed in our study. US examinations were performed by intensivists after they had 4 h of training. The intensivists were already experienced in US practice within the ICU (especially abdominal and pleural sonography [32]). The skill level required to be reliable enough to perform usCSA is, as yet, unknown [33].
Lastly, we could not position all patients in the ideal semi-upright position because of orthopedic contraindications (pelvic or spinal fracture) (n = 18). These patients were, however, placed in a head-up inclined position that favored antral filling. Moreover, measurements were not performed in the right lateral decubitus position, whereas Perlas et al. [9] showed that this position allowed better correlation with gastric volume. However, we deliberately chose not to add any extra constraint when evaluating our technique, which remained simple, feasible, and quick to perform in the daily care in ICU.
Conclusion
Antral cross-sectional area measured by ultrasound is feasible in the majority of critically ill patients. Antral CSA is positively correlated with gastric volume and allows qualitative assessment of gastric volume with clinically acceptable accuracy.
Even though obtaining a usCSA is sometimes impossible in critically ill patients, the technique is still promising. It may help to assess gastric status before an emergency airway procedure with aspiration risks or trigger appropriate medications when enteral feeding is not well tolerated.
Further studies and a higher number of patients are needed to confirm the results of this pilot experience.
References
Marik PE (2001) Aspiration pneumonitis and aspiration pneumonia. N Engl J Med 344:665–671. doi:10.1056/NEJM200103013440908
Raidoo DM, Rocke DA, Brock-Utne JG et al (1990) Critical volume for pulmonary acid aspiration: reappraisal in a primate model. Br J Anaesth 65:248–250
(1999) Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: a report by the American Society of Anesthesiologist Task Force on Preoperative Fasting. Anesthesiology 90:896–905
Hsu C-W, Sun S-F, Lee DL et al (2011) Impact of disease severity on gastric residual volume in critical patients. World J Gastroenterol 17:2007–2012. doi:10.3748/wjg.v17.i15.2007
Nguyen NQ, Ng MP, Chapman M et al (2007) The impact of admission diagnosis on gastric emptying in critically ill patients. Crit Care 11:R16. doi:10.1186/cc5685
Nimmo WS, Heading RC, Wilson J et al (1975) Inhibition of gastric emptying and drug absorption by narcotic analgesics. Br J Clin Pharmacol 2:509–513
Steyn PF, Twedt D, Toombs W (1997) The effect of intravenous diazepam on solid phase gastric emptying in normal cats. Vet Radiol Ultrasound Off 38:469–473
Mutlu GM, Mutlu EA, Factor P (2001) GI complications in patients receiving mechanical ventilation. Chest 119:1222–1241
Perlas A, Chan VWS, Lupu CM et al (2009) Ultrasound assessment of gastric content and volume. Anesthesiology 111:82–89. doi:10.1097/ALN.0b013e3181a97250
Bouvet L, Miquel A, Chassard D et al (2009) Could a single standardized ultrasonographic measurement of antral area be of interest for assessing gastric contents? A preliminary report. Eur J Anaesthesiol 26:1015–1019. doi:10.1097/EJA.0b013e32833161fd
Bouvet L, Mazoit J-X, Chassard D et al (2011) Clinical assessment of the ultrasonographic measurement of antral area for estimating preoperative gastric content and volume. Anesthesiology 114:1086–1092. doi:10.1097/ALN.0b013e31820dee48
Chapman MJ, Nguyen NQ, Fraser RJL (2007) Gastrointestinal motility and prokinetics in the critically ill. Curr Opin Crit Care 13:187–194. doi:10.1097/MCC.0b013e3280523a88
Ko JP, Berman EJ, Kaur M et al (2012) Pulmonary nodules: growth rate assessment in patients by using serial CT and three-dimensional volumetry. Radiology 262:662–671. doi:10.1148/radiol.11100878
Le Gall JR, Lemeshow S, Saulnier F (1993) A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Baker SP, O’Neill B, Haddon W Jr, Long WB (1974) The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma 14:187–196
Carp H, Jayaram A, Stoll M (1992) Ultrasound examination of the stomach contents of parturients. Anesth Analg 74:683–687
Koenig SJ, Lakticova V, Mayo PH (2011) Utility of ultrasonography for detection of gastric fluid during urgent endotracheal intubation. Intensive Care Med 37:627–631. doi:10.1007/s00134-010-2125-9
Podczeck F, Mitchell CL, Newton JM et al (2007) The gastric emptying of food as measured by gamma-scintigraphy and electrical impedance tomography (EIT) and its influence on the gastric emptying of tablets of different dimensions. J Pharm Pharmacol 59:1527–1536. doi:10.1211/jpp.59.11.0010
Willems M, Quartero AO, Numans ME (2001) How useful is paracetamol absorption as a marker of gastric emptying? A systematic literature study. Dig Dis Sci 46:2256–2262
Jordi J, Verrey F, Lutz TA (2013) Simultaneous assessment of gastric emptying and secretion in rats by a novel computed tomography based method. Am J Physiol Gastrointest Liver Physiol. doi:10.1152/ajpgi.00230.2013
Jordi J, Herzog B, Camargo SMR et al (2013) Specific amino acids inhibit food intake via the area postrema or vagal afferents. J Physiol 591:5611–5621. doi:10.1113/jphysiol.2013.258947
Karcz WK, Kuesters S, Marjanovic G et al (2008) 3D-MSCT gastric pouch volumetry in bariatric surgery—preliminary clinical results. Obes Surg 19:508–516. doi:10.1007/s11695-008-9776-4
Wormanns D, Kohl G, Klotz E et al (2004) Volumetric measurements of pulmonary nodules at multi-row detector CT: in vivo reproducibility. Eur Radiol 14:86–92. doi:10.1007/s00330-003-2132-0
Bolte H, Riedel C, Jahnke T et al (2006) Reproducibility of computer-aided volumetry of artificial small pulmonary nodules in ex vivo porcine lungs. Invest Radiol 41:28–35
Yeguiayan J-M, Garrigue D, Binquet C et al (2011) Medical pre-hospital management reduces mortality in severe blunt trauma: a prospective epidemiological study. Crit Care 15:R34. doi:10.1186/cc9982
Metheny NA, Stewart J, Nuetzel G et al (2005) Effect of feeding-tube properties on residual volume measurements in tube-fed patients. JPEN J Parenter Enter Nutr 29:192–197
Mayo PH (2013) Critical care ultrasonography: the Italian approach. Intensive Care Med 39:1849–1850. doi:10.1007/s00134-013-3011-z
Scalea TM, Rodriguez A, Chiu WC et al (1999) Focused assessment with sonography for trauma (FAST): results from an international consensus conference. J Trauma 46:466–472
Carrie C, Gisbert-Mora C, Quinart A et al (2012) Non-occlusive mesenteric ischemia detected by ultrasound. Intensive Care Med 38:333–334. doi:10.1007/s00134-011-2424-9
Moriwaki Y, Sugiyama M, Toyoda H et al (2009) Ultrasonography for the diagnosis of intraperitoneal free air in chest-abdominal-pelvic blunt trauma and critical acute abdominal pain. Arch Surg 144:137–141. doi:10.1001/archsurg.2008.553 discussion 142
Lamperti M, Bodenham AR, Pittiruti M et al (2012) International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med 38:1105–1117. doi:10.1007/s00134-012-2597-x
Volpicelli G, Elbarbary M, Blaivas M et al (2012) International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 38:577–591. doi:10.1007/s00134-012-2513-4
Expert Round Table on Ultrasound in ICU (2011) International expert statement on training standards for critical care ultrasonography. Intensive Care Med 37:1077–1083. doi:10.1007/s00134-011-2246-9
Acknowledgments
The authors declare that the study has been approved by the appropriate ethics committee (Comité d’Evaluation de l’Ethique des projets pour la Recherche Biomédicale, Paris Nord, France N°10-060) and was therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Specific national laws have also been observed.
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Take-home message: Gastric emptying is commonly disturbed in critically ill patients, and a simple, quick, reliable, and non-invasive bedside test to assess gastric volume would be of great interest. Ultrasound assessment of gastric cross-sectional area is feasible in critically ill patients and allows accurate discrimination of “at-risk stomachs”.
Electronic supplementary material
Below is the link to the electronic supplementary material.
134_2014_3320_MOESM1_ESM.doc
Electronic supplement 1: Correlation between US and CT measurements. usCSA ultrasonography of antral cross-sectional area, ctCSA computed tomography measurement of antral cross-sectional area, GV gastric volume measured on the CT scan. (DOC 37 kb)
134_2014_3320_MOESM2_ESM.tif
Electronic supplement 2. Bland–Altman diagram showing agreement between the radiologist and intensivists regarding the ultrasonographic measurements (n = 29). Results expressed in cm2. Mean = –0.12 cm2; mean + 2SD = 1.96 cm2; Mean – 2SD = –2.21 cm2. (TIFF 4563 kb)
134_2014_3320_MOESM3_ESM.tif
Electronic supplement 3. Receiver operating characteristic curve of antral cross-sectional area measured by ultrasound (usCSA) for a total gastric volume of ≥ 0.8 mL/kg. AUC area under the curve. (TIFF 4563 kb)
Rights and permissions
About this article
Cite this article
Hamada, S.R., Garcon, P., Ronot, M. et al. Ultrasound assessment of gastric volume in critically ill patients. Intensive Care Med 40, 965–972 (2014). https://doi.org/10.1007/s00134-014-3320-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-014-3320-x