Abstract
Purpose
Studies into the preferences of patients and relatives regarding informed consent for intensive care unit (ICU) research are ongoing. We investigated the impact of a study’s invasiveness on the choice of who should give consent and on the modalities of informed consent.
Methods
At ICU discharge, randomized pairs of patients and relatives were asked to answer a questionnaire about informed consent for research. One group received a vignette of a noninvasive study; the other, of an invasive study. Each study comprised two scenarios, featuring either a conscious or unconscious patient. Multivariate models assessed independent factors related to their preferences.
Results
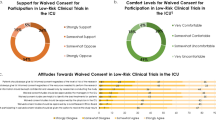
A total of 185 patients (40 %) and 125 relatives (68 %) responded. The invasiveness of a study had no impact on which people were chosen to give consent. This increased the desire to get more than one person to give consent and decreased the acceptance of deferred or two-step consent. Up to 31 % of both patients and relatives chose people other than the patient himself to give consent, even when the patient was conscious. A range of 3 to 17 % of the respondents reported that they would accept a waiving of consent. Younger respondents and individuals feeling coerced into study participation wanted to be the decision makers.
Conclusions
Study invasiveness had no impact on patients’ and relatives’ preferences about who should give consent. Many patients and relatives were reluctant to give consent alone. Deferred and two-step consent were less acceptable for the invasive study. Further work should investigate whether sharing the burden of informed consent with a second person facilitates participation in ICU research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Research is essential to further medical knowledge and eventually improve patient care [1]. Informed consent—respectful of a patient’s autonomy—is a key ethical requirement in human research. It implies a competent patient, complete information about the study (including all invasive procedures) in plain language, and sufficient time to decide upon participation.
Since revelations about scandalous human studies carried out without subject consent [2, 3], developed countries have enacted laws that bind the investigators [4, 5] to standards aimed at protecting patients. Application of these laws differs depending on the nature of the research and the study population. Most laws recognize vulnerable populations in need of enhanced protection, such as patients who are incompetent or critically ill [6–8].
Patients in intensive care units (ICU) face acute clinical situations, with short therapeutic windows [9]. Sedation, pain, anxiety and fear can alter consciousness and impede decision-making [10, 11], and the best practices for respecting patient autonomy in this context are still being debated [12–14]. If the patient lacks competency to give consent, the Good Clinical Practice and most national laws refer investigators to a surrogate—a legal representative, relative, or close friend, depending on the country [8, 11]. However, finding available surrogates in emergency settings and obtaining their informed consent for research is difficult [15, 16]. Moreover, their ability or willingness to take on such a responsibility has not been demonstrated, a challenge in which emotional burden plays a role [17–21]. To our knowledge, patients’ and relatives’ preferences regarding informed consent in ICU research have been investigated separately [22, 23], but only once together [24]. The present study investigated the preferences of both patients and relatives as to who should give informed consent and the modalities of that consent, as well as the impact of study invasiveness. Such research is very important to the design of future studies and to help clarify the best approaches for improving medicine for critically ill patients.
Methods
We screened all adult patients that were discharged alive after ICU stays longer than 24 h between October 2006 and August 2007 (CC), and January 2008 and April 2008 (FG). Patients with cerebral impairment or psychiatric disorders according to their ICU clinical charts, aged below 18 years, and/or non French-speaking, were excluded. The patient, and relative when available, were met on wards 2–10 days after ICU discharge and asked to fill in the questionnaire individually. The investigator remained on hand to answer questions.
Since overly long questionnaires increase the risk of refusal, each patient–relative pair was randomly assigned one of two vignettes about a clinical study. Randomization was performed without stratification, using sealed, opaque envelopes to reduce bias (www.randomizer.org).
One vignette described a noninvasive study using retrospective data extraction from patient charts, without benefit or risk; the second vignette described an invasive study consisting of a prospective, randomized controlled trial, with a small risk and potential benefit to the patient (see online data supplement E1).
The questionnaire was developed using the saturation technique (see online data supplement E2 and [24]). Participants chose among different people who might give consent and among different modalities of informed consent. Two scenarios were proposed: a conscious or unconscious patient. Willingness to participate in the study was purposely not investigated. Participants filled out questionnaires in the hospital; a small number returned them by prepaid envelope after discharge. When necessary, a telephone reminder was made within 2 weeks.
Ethical issues
The protocol was approved by the Geneva University Hospital Ethics Committee. A signed, returned questionnaire was considered as informed consent.
Statistical analysis
We used Stata Statistical Software, Release 11.0® (Stata Corporation, College Station, TX, USA). All tests were two-tailed and a p value of <0.05 was considered statistically significant.
Two-tailed Fisher’s exact tests, unpaired t tests, χ2 and Pearson χ2 tests were used, as appropriate, to compare different respondent subgroups (all patients [not shown], patients with relative, unaccompanied patients, and relatives: definitions in Table 1) and to analyze answers regarding consciousness and study invasiveness. We performed agreements and Kappa tests for the concordance of answers between pairs. Agreement tends to an overestimation due to the contribution of hazard. The Kappa assesses effects unrelated to hazard, but is imprecise and tends to underestimate concordance in the presence of multiple items with an uneven distribution, and if the most frequent response exceeds 80 % of answers, the Kappa is unusable [25].
To identify factors independently associated with outcomes, multivariate logistic regression models were calculated considering all participants, with odds ratios and 95 % confidence intervals. Potential interactions between the different factors in all the multivariate models were also assessed. All predictors at a 0.05 alpha level in the univariate analysis were entered (factors: age, feeling coerced, invasiveness, and respondent groups).
Results
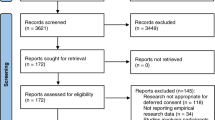
Of 462 eligible patients, 185 participated (40 %). The consort diagram (Fig. 1) summarizes the distribution of patients and relatives, as well as self-reported reasons for refusal. Table 1 shows their characteristics. Patient age and gender distributions were similar to our general ICU population [age (mean ± SD): 64 ± 14 vs. 61 ± 18 years, male: 71 vs. 60 %, respectively]. Relatives were younger and more frequently female. Seventy-six relatives were life partners (62 %), 34 were children (28 %), 3 were parents (2 %) and 10 were described as 'other' (8 %). Compared to the general Swiss population, matched by age (23 % primary, 46 % secondary and 31 % higher education), more respondents had a higher education [26].
Table 2 summarizes participant preferences regarding the person who should give consent, according to study invasiveness and the patient’s state of consciousness. Study invasiveness had no impact on patient or relative preferences. Only the unaccompanied patient subgroup showed a difference between invasive and noninvasive study: more respondents wanted their family doctor to give consent for the invasive study—even more so when the patient was unconscious. More unaccompanied patients than patients with relative wanted their family doctor to give consent in the invasive study only. Significantly more patients and relatives wanted relatives to give consent on behalf of unconscious patients, whereas both patients and relatives preferred the patient to give consent if conscious. Up to 31 % of both patients and relatives considered that somebody other than the patient should give informed consent even when the patient was conscious. Up to 48 % of patients and 41 % of relatives considered that somebody other than the relative should give informed consent when the patient was unconscious. In such cases, the family or ICU doctors were the people designated, by up to 37 and 11 %, respectively. Independently of study invasiveness and the patient’s state of consciousness, none of the patients or relatives chose a lay representative, judge or hospital doctor outside the ICU to give consent, and up to 17 % of patients and 10 % of relatives considered that giving informed consent was not necessary at all. The concordance of preferences between patients and relatives was good, and was not influenced by educational levels, relationship type or previous participation in clinical studies (results not shown).
Table 3 summarizes results regarding the desire for more than one person to give consent and preferences about the type of informed consent, depending on study invasiveness and the patient’s state of consciousness. State of consciousness made no difference and there was no difference between subgroups. There was good concordance between patients and relatives. More relatives wanted “more than one person to give consent” for conscious patients, and more patients with relative and more relatives preferred “more than one person to give consent” for unconscious patients in the invasive study vignette than in the noninvasive study one. Again, family or ICU doctors were often designated as the second person (results not shown). Fewer unaccompanied patients accepted deferred or two-step consent in the invasive study.
The number of participants desiring a signature and/or a witness did not differ according to study invasiveness or between subgroups. Up to 37/62 (70 %) patients with a relative and up to 44/63 (77 %) relatives desired a signature, while up to 32/63 (53 %) patients with a relative and up to 30/62 (55 %) relatives desired the presence of a witness (see online data supplement E3).
We performed multiple multivariate analyses of the whole population studied in order to investigate the potential factors associated with preferences, types and procedures of informed consent. Eight multivariate models are shown in the online data supplement, as well as submodels of the logistic regression analyzing conscious and unconscious patients separately (see online data supplement E4). Among the five factors identified in the univariate analysis and included in the final model, four had a significant impact. Study invasiveness and feelings of coercion had no impact on preferences for who should give consent, but they did increase the desire to have more than one person to provide consent and did decrease the rate of acceptance for deferred or two-step consent. Younger individuals were associated with lower acceptance of two-step consent and increased desire for signed or witnessed consent. We did not identify any significant interaction between the independent factors in any of the multivariate models.
Discussion
The impact of study invasiveness on the preferences of potential ICU research participants regarding informed consent has never been investigated. The strengths and originality of our study are the parallel evaluation of patients and relatives, and their greater appreciation of critical illness than the general population.
Our major finding was that study invasiveness had no impact on patient or relative preferences with regard to who should give consent. However, it did increase the desire to have that decision-making responsibility shared with a second person, especially when the patient was unconscious. Family and ICU doctors were often designated in such cases. Likewise, when the patient was unaccompanied, family doctors were considered the person best qualified to give consent. Study invasiveness tended to decrease the acceptance rate of deferred or two-step consent. Procedures for giving consent by signature, with or without witnesses, were neither influenced by invasiveness, nor, as our pilot study showed, by the patient’s state of consciousness [24]. These findings add to previous data reporting that risks related to a trial do not change patients’ preferences [23].
The second relevant finding was that up to a third of patients and relatives would not wish to designate the patient as the consent-giver, even if he were conscious. This contrasts strongly with international and national rules advocating that the patient himself should give consent. When the patient was unconscious the majority of respondents chose relatives, but as many as 45 % preferred other people. This is in line with previous reports that only 26 % of respondents considered decision making by relatives to be acceptable in research situations [27]. These findings raise the important question of whether it is always judicious to confer the entire burden of responsibility for giving consent to relatives, or even to patients, in critical care research. The idea of obtaining informed consent from more than one person is noteworthy. This is in line with previous findings suggesting that relatives would like treating or ICU clinicians to be responsible for giving consent [22]. Moreover, in our study, none of the respondents would have turned to a lay person, judge or another hospital physician. The contribution of family or ICU doctors seems comforting to ICU patients and relatives, more so than from other unknown professionals. This contrasts with propositions by some lawyers who would like to see an attorney automatically attributed to any unconscious patient to be included in a study [28].
The third surprising finding was that as many as 10 % of respondents considered informed consent unnecessary for an invasive study whereas as few as 17 % of respondents would waive consent for a noninvasive study, regardless of the patient’s state of consciousness. Taken together, these results show that people want to be informed, consulted and allowed to give consent to a study, whatever its nature. This is in contradiction with some current rules that would allow a waiving of consent for observational studies [29]. The suggested requirement for dual consent in which one person should be the family or ICU doctor might be a solution regarding the exceptional and specific circumstances of emergencies [30, 31]. Our results were also consistent with literature in which between 50 and 86 % of respondents accepted deferred consent [22, 32, 33]. This suggests that, depending on the importance of the study and provided there has been a scrupulous examination by an IRB or an Ethics Committee, critical care studies with short therapeutic windows may be started without consent [34]. Information about the trial and informed consent to continue the study should be provided as quickly as possible.
Unlike recent publications, our study showed close parallels between patients and relatives with regard to who should give consent [35].
Younger respondents seemed to have a greater desire to decide for themselves than older ones, as expressed by a higher rate of self-designation as consent-giver, and a decreased willingness to use two-step consent or the requirement of consent with a signature or a witness. Only a small proportion of participants reported feeling coerced. However, this feeling increased the desire to have more than one person to give consent and decreased the rate of acceptance of deferred or two-step consent. These results should remind researchers that their attitude during the procedure of seeking consent can have an impact on inclusion rates [15].
Overall, a high rate of patients and relatives did desire a signature and the presence of a witness for informed consent, independently of study invasiveness. Our results confirm that seeking out relatives to give consent when the patient is unconscious is the right thing to do, as is currently the practice for ICU research in most countries.
Our study presents some limitations. Firstly, the 40 % patient response rate is low. Nonetheless, this was an increase on our pilot study. Such participation rates are not rare in ICU research [20, 23, 24, 36, 37], especially for complex questionnaires on vignettes. This is undoubtedly due to patients’ considerable residual fatigue and other impairments after recent ICU stays (Fig. 1). The 68 % response rate among relatives is acceptable. Secondly, we cannot exclude the bias that respondents may have been favorably predisposed towards research [38]. However, the equal participants’ distribution in the two study groups (invasive and noninvasive) and the fact that the patient characteristics were similar to usual ICU populations, suggests that our data is representative. Thirdly, the study design precludes direct comparisons of how any individual would feel about both the invasive and the noninvasive study. We considered it too confusing to ask the same participant to compare the two study types in conscious and unconscious patients. As for randomized controlled trials we assume that the groups are similar at baseline. Also, our vignettes may not represent the most frequent research situations in critical care. Fourthly, the results come from a single center in Switzerland and might not be applicable to other centers or countries. Cultural differences might play a role. However, our findings are in accordance with previous US or Canadian publications [22, 23]. This might enhance the credibility of our study’s original findings.
Conclusion
Our study provides helpful information on informed consent procedures. The surprising finding was that study invasiveness had no impact on the choice of who should give consent. Invasiveness increased the desire that consent be given by more than one person and decreased the acceptance rate of deferred or two-step consent. Our results confirm that current research rules comply with people's wishes, i.e. that consent be given by themselves when patients are conscious and by the relative when unconscious. When patients are unaccompanied, they prefer that someone they know is asked to give consent. The original idea to have a second person available—for example, family or ICU doctors—who would give consent together with the patient or the relative could be helpful and might also reduce feelings of coercion expressed by some participants. Further studies should test such new possibilities for informed consent in ICU research.
References
Schwetz BA (2007) Protecting subjects without hampering research progress: guidance from the office for human research protections. Cleve Clin J Med 74(Suppl 2):S60–S62 discussion S68–9
Department of Health and Human Services (1991) Common Rule (45 CFR 46). Federal policy for the protection of human subjects; notices and rules
Schmidt TA, Salo D, Hughes JA, Abbott JT, Geiderman JM, Johnson CX, McClure KB, McKay MP, Razzak JA, Schears RM, Solomon RC (2004) Confronting the ethical challenges to informed consent in emergency medicine research. Acad Emerg Med 11(10):1082–1089
Swiss Federal Office of Public Health (2006) Loi relative à la recherche sur l’être humain, LRH; RS 101, FF 2009 7259, Available via http://www.bag.admin.ch/themen/medizin/00701/00702/07558/index.html?lang=fr. Accessed 13 March 2013
Lemaire F, Bion J, Blanco J, Damas P, Druml C, Falke K, Kesecioglu J, Larsson A, Mancebo J, Matamis D, Pesenti A, Pimentel J, Ranieri M (2005) The European union directive on clinical research: present status of implementation in EU member states’ legislations with regard to the incompetent patient. Intensive Care Med 31(3):476–479
Lemaire F (2006) The inability to consent in critical care research: emergency or impairment of cognitive function? Intensive Care Med 32(12):1930–1932
Baren JM, Fish SS (2005) Resuscitation research involving vulnerable populations: are additional protections needed for emergency exception from informed consent? Acad Emerg Med 12(11):1071–1077
Flanagan BM, Philpott S, Strosberg MA (2011) Protecting participants of clinical trials conducted in the intensive care unit. J Intensive Care Med 26(4):237–249
Luce JM, White DB (2009) A history of ethics and law in the intensive care unit. Crit Care Clin 25(1):221–237
Chenaud C, Merlani P, Ricou B (2006) Informed consent for research in ICU obtained before ICU admission. Intensive Care Med 32(3):439–444
Silverman H (2011) Protecting vulnerable research subjects in critical care trials: enhancing the informed consent process and recommendations for safeguards. Ann Intensive Care 1(1):8
Roberts I, Prieto-Merino D, Shakur H, Chalmers I, Nicholl J (2011) Effect of consent rituals on mortality in emergency care research. Lancet 377(9771):1071–1072
Luce JM (2003) Is the concept of informed consent applicable to clinical research involving critically ill patients? Crit Care Med 31(3 Suppl):S153–S160
Truog RD (2005) Will ethical requirements bring critical care research to a halt? Intensive Care Med 31(3):338–344
Smith OM, McDonald E, Zytaruk N et al (2012) Rates and determinants of informed consent: a case study of an international thromboprophylaxis trial. J Crit Care. doi:10.1016/j.jcrc.2012.08.005
Siegel MD (2006) Alone at life’s end: trying to protect the autonomy of patients without surrogates or decision-making capacity. Crit Care Med 34(8):2238–2239
Barrett KA, Ferguson ND, Athaide V et al (2012) Surrogate decision makers’ attitudes towards research decision making for critically ill patients. Intensive Care Med 38(10):1616–1623
Barrett KA, Scales DC (2012) Considering the vulnerabilities of surrogate decision-makers when obtaining consent for critical care research. Intensive Care Med 38(1):4–6
Iverson E, Celious A, Kennedy CR et al (2012) Real-time perspectives of surrogate decision makers regarding critical illness research—findings of focus group participants. Chest 142(6):1433–1439
Ciroldi M, Cariou A, Adrie C, Annane D, Castelain V, Cohen Y, Delahaye A, Joly LM, Galliot R, Garrouste-Orgeas M, Papazian L, Michel F, Barnes NK, Schlemmer B, Pochard F, Azoulay E (2007) Ability of family members to predict patient’s consent to critical care research. Intensive Care Med 33(5):807–813
Coppolino M, Ackerson L (2001) Do surrogate decision makers provide accurate consent for intensive care research? Chest 119(2):603–612
Perner A, Ibsen M, Bonde J (2010) Attitudes to drug trials among relatives of unconscious intensive care patients. BMC Anesthesiol 10:6
Scales DC, Smith OM, Pinto R, Barrett KA, Friedrich JO, Lazar NM, Cook DJ, Ferguson ND (2009) Patients’ preferences for enrolment into critical-care trials. Intensive Care Med 35(10):1703–1712
Chenaud C, Merlani P, Verdon M, Ricou B (2009) Who should consent for research in adult intensive care? Preferences of patients and their relatives: a pilot study. J Med Ethics 35(11):709–712
Sim J, Wright CC (2005) The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys Ther 85(3):257–268
Swiss Federal Office of Statistics (2011) http://www.bfs.admin.ch/bfs/portal/fr/index.html
Stephenson AC, Baker S, Zeps N (2007) Attitudes of relatives of patients in intensive care and emergency departments to surrogate consent to research on incapacitated participants. Crit Care Resusc 9(1):40–50
Lautrette A, Peigne V, Watts J, Souweine B, Azoulay E (2008) Surrogate decision makers for incompetent ICU patients: a European perspective. Curr Opin Crit Care 14(6):714–719
Claudot F, Alla F, Fresson J, Calvez T, Coudane H, Bonaiti-Pellie C (2009) Ethics and observational studies in medical research: various rules in a common framework. Int J Epidemiol 38(4):1104–1108
Largent EA, Wendler D, Emanuel E, Miller FG (2010) Is emergency research without initial consent justified?: the consent substitute model. Arch Intern Med 170(8):668–674
Silbergleit R, Biros MH, Harney D, Dickert N, Baren J (2012) Implementation of the exception from informed consent regulations in a large multicenter emergency clinical trials network: the RAMPART experience. Acad Emerg Med 19(4):448–454
Potter JE, McKinley S, Delaney A (2012) Research participants’ opinions of delayed consent for a randomised controlled trial of glucose control in intensive care. Intensive Care Med 39(3):472–480
Jansen TC, Kompanje EJ, Bakker J (2009) Deferred proxy consent in emergency critical care research: ethically valid and practically feasible. Crit Care Med 37(1 Suppl):S65–S68
Annane D, Outin H, Fisch C, Bellissant E (2004) The effect of waiving consent on enrollment in a sepsis trial. Intensive Care Med 30(2):321–324
Newman JT, Smart A, Reese TR, Williams A, Moss M (2012) Surrogate and patient discrepancy regarding consent for critical care research. Crit Care Med 40(9):2590–2594
Mason S, Barrow H, Phillips A, Eddison G, Nelson A, Cullum N, Nixon J (2006) Brief report on the experience of using proxy consent for incapacitated adults. J Med Ethics 32(1):61–62
Mehta S, Quittnat Pelletier F, Brown M, Ethier C, Wells D, Burry L, MacDonald R (2012) Why substitute decision makers provide or decline consent for ICU research studies: a questionnaire study. Intensive Care Med 38(1):47–54
Lim DA, Chan MF, Childs C (2013) Surrogate consent for critical care research: exploratory study on public perception and influences on recruitment. Crit Care 17(1):R5
Acknowledgments
This study was supported by institutional funds only.
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gigon, F., Merlani, P., Chenaud, C. et al. ICU research: the impact of invasiveness on informed consent. Intensive Care Med 39, 1282–1289 (2013). https://doi.org/10.1007/s00134-013-2908-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-013-2908-x