Abstract
Purpose
A prospective multicentre cohort study was conducted to determine the prevalence of hypovitaminosis D in adult critically ill patients, to characterize alterations in the parathyroid hormone (PTH)–vitamin D–calcium axis and to explore associations between hypovitaminosis D and adverse clinical outcomes.
Methods
Demographic, disease severity scores and clinical outcome data were collected in 100 consecutive patients with expected intensive care unit (ICU) admission of at least 2 days. Levels of 25-hydroxyvitamin D (25-OH-D), 1,25-dihydroxyvitamin D (1,25-(OH)2-D), PTH and ionized calcium were measured on days 1, 3 and on day 7 or ICU discharge.
Results
The prevalence of vitamin D insufficiency (25 nmol/L ≤ 25-OH-D ≤ 50 nmol/L) and deficiency (25-OH-D < 25 nmol/L) were 54 and 24 %, respectively, and levels did not recover during ICU stay. Admission 25-OH-D levels correlated with 1,25-(OH)2-D (R = 0.61, p = 0.001), Simplified Acute Physiology Score (SAPS-II) (R = −0.3, p = 0.01), Acute Physiology and Chronic Health Evaluation (APACHE-II) scores (R = −0.2, p = 0.05), but not calcium (R = 0.16, p = 0.11) or PTH (R = −0.11, p = 0.31) levels. Vitamin D deficiency was associated with fewer hospital-free days, OR 3.15 (1.18–8.43) in univariate analysis. Secondary hyperparathyroidism (PTH > 7 pmol/L) was observed in 37.5 % of hypocalcaemic and 32.5 % of vitamin D insufficient/deficient patients, and was associated with higher SAPS-II [43 (31.3–60) vs. 36 (30–43), p = 0.03].
Conclusions
Hypovitaminosis D and secondary hyperparathyroidism are highly prevalent in critically ill patients. Low vitamin D status persists during ICU stay and is associated with worse disease severity and fewer hospital-free days.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In addition to its important endocrine role in the regulation of calcium metabolism and bone health, vitamin D is now recognized to have multiple pleiotropic functions [1]. These occur via the local production of the active form of vitamin D, 1,25-dihydroxyvitamin D, through the activity of a tissue form of 1-alpha-hydroxylase. This enzyme is found in a large number of mammalian tissues along with the vitamin D receptor. This has led to the recognition of important roles of vitamin D in the regulation of hormone secretion, immune function, cellular proliferation and differentiation [1, 2].
Despite the abundance of natural sunlight in Australia, a high prevalence of vitamin D deficiency has been noted, ranging from 67 to 86 % in high risk groups such as the elderly, especially if institutionalized, dark-skinned and veiled women, geriatric admissions to hospital and patients with skin cancer or malabsorption [3–6]. There is also a significant prevalence of mild vitamin D deficiency, observed in more than 20 % of healthy, younger adults, particularly during winter in the more southern latitudes of Australia [7]. Despite this knowledge, vitamin D deficiency is seldom considered and rarely replaced adequately, if at all, in critically ill patients.
Our group [8] provided the first evidence of a high prevalence of hypovitaminosis D among critically ill patients and that this was associated with higher mortality as predicted by the Simplified Acute Physiology Score (SAPS-II). Several international studies [9–13] have since replicated our findings. Despite the recognition of interplay between vitamin D, calcium and PTH, a systematic characterization of the entire PTH–vitamin D–calcium axis during critical illness has not been undertaken. It remains unclear whether vitamin D deficiency/insufficiency plays a causal role in disease severity among critically ill patients and the role of correcting vitamin D deficiency in critically ill patients has not been established.
The importance of obtaining prospective observational data on the evolution of the PTH–vitamin D–calcium axis in a diverse spectrum of critically ill patients to guide the design of further interventional studies therefore becomes imperative. The aims of the current study were to determine the prevalence of hypovitaminosis D in a wide spectrum of critical illness in adults, to evaluate changes in PTH–vitamin D–calcium axis during critical illness and to explore associations between low vitamin D levels, disease severity and clinical outcomes.
Methods
Study design
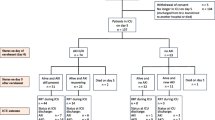
We conducted a prospective, multicentre inception cohort study of 100 consecutive patients from 3 tertiary referral ICUs in Sydney, Australia (33° latitude) who were expected to stay in the ICU for more than 2 days. Patients on renal replacement therapy were excluded. The inception period was conducted over an 8-month period (July 2010–February 2011 to include cooler and warmer months). Human research ethics committee approval was obtained and informed consent was obtained from the patient or their surrogate.
Data collection
Baseline demographic and diagnostic data were collected on admission to ICU. Source of admission (emergency department, hospital ward or other domiciliary care facility) was recorded. Severity of illness scores collected were the Acute Physiology and Chronic Health Evaluation (APACHE-II) (range 0–71, with higher scores indicating more severe illness) [14], SAPS-II (range 0–146, with higher scores indicating more severe illness) [15] on admission and Sequential Organ Failure (SOFA) scores (range 0–20, with higher scores indicating more severe illness) on day 1, day 3 and either day 7 or discharge from ICU, whichever was earlier. (The cardiovascular, respiratory, renal, hepatic and haematological components of the SOFA score were recorded, for which scores can range from 0 to 4 for each organ system, with higher scores indicating more severe dysfunction [16].)
In addition to routine blood investigations, levels of 25-OH-D (Diasorin® radioimmunoassay, CV 10 %), 1,25-(OH)2-D (Diasorin® radioimmunoassay, CV 10 %), intact PTH (two-site immunoassay for intact PTH, Siemens diagnostics, CV 10 %) and ionized calcium (ion-sensitive electrode–Radiometer ABL 720 blood gas analyser, CV 1.3 %) were measured on day 1 (approximately 24 h after the resuscitation phase was complete) and in the morning hours of day 3 and either at day 7 or at ICU discharge if this was prior to day 3 or 7.
None of the patients received vitamin D supplementation during their ICU stay, other than that provided in enteral or parenteral feeds (280 IU of vitamin D3 per litre of iso-caloric enteral feed, 400 IU ergocalciferol in each 3-in-1 premix bag of parenteral feed). The first levels were sampled within 24 h of admission, prior to commencement of feeding.
As vitamin D is highly protein-bound to D-binding protein (DBP), the free (unbound) fraction of vitamin D might be a more physiologically appropriate measurement [17]. As an assay for DBP was not available at the time, a “protein correction” was performed for vitamin D. As DBP is a globulin, we “corrected” for serum globulin by dividing the 25-OH-D by the globulin level. As a result, patients with higher globulin levels (and consequently potentially lower free vitamin D levels) would have a lower “corrected” vitamin D level.
To assess the significance of an intact PTH–vitamin D–calcium axis, patients were classified as being PTH-responders or non-responders. PTH-responders were defined as patients who had an elevated PTH level (>7 pmol/L) in the presence of a low 25-OH-D (<50 nmol/L) and/or a low ionized calcium (iCa < 1.15 mmol/L) on admission to ICU. Patients with impaired renal function on day 1, (eGFR < 30 ml/min, n = 17) were excluded from the PTH analysis. Severe hepatic dysfunction was not seen in this cohort.
Outcome measures collected were ICU and hospital length of stay, ICU and hospital-free days (HFD), ICU and hospital mortality. ICU or HFDs were defined as the number of days the patient spent alive outside the ICU or hospital, respectively, in the 28-day period starting from the day of ICU admission.
Statistical analysis
Continuous variables with a normal distribution were expressed as mean (standard deviation) while all others were expressed as median (interquartile range). Coefficients of correlation between variables were calculated using Pearson’s or Spearman’s pairwise correlation, depending on the distribution of variables. Longitudinal changes in vitamin D metabolites and PTH were analysed using mixed models, considering both linear and polynomial models. Differences in variables between groups were tested by using unpaired Student’s t test or Wilcoxon’s ranked sum test, depending on the distribution of variables. Bivariate and multivariate logistic regression models were used to assess the associations between outcomes (i.e. mortality, ICU and HFD, PTH response) and risk factors. 25-OH-D levels were analysed as a continuous and an ordinal factor (sufficiency >50 nmol/L, insufficiency 25–50 nmol/L and deficiency <25 nmol/L) [3, 18]. All analyses were performed using R language (version 2.14.2) [19].
Results
Patient characteristics
The patient cohort studied was a mixed medical/surgical group with a spread of clinical diagnoses. Mean age was 52 ± 17 years with mean APACHE-II and SAPS-II of 21 ± 8 and 42 ± 15, respectively (Table 1). Fifty-four patients (54 %) were admitted to the ICU in the cooler months, while the remaining were admitted in the warmer months of the year.
Vitamin D status on ICU admission
The prevalence of vitamin D insufficiency and deficiency was 55 % (95 % CI 45.2–64.3) and 24 % (95 % CI 16.7–33.2), respectively. 25-OH-D levels were undetectable (<15 nmol/L) in 8 % of the patients. Using a more stringent definition of sufficiency (>75 nmol/L) as suggested by some recent data, only 3 % of patients had sufficient levels on day 1 with the prevalence of insufficiency (50–75 nmol/L) and deficiency (<50 nmol/L) being 22 and 75 %, respectively. Characteristics of the patients are showed in Table 2. Briefly, in patients with lower vitamin D, there appeared to be a trend towards older age, higher APACHE-II and SAPS and longer lengths of stay in ICU and hospital compared to their counterparts, although this was not statistically significant. Only 17 % of the cohort had low 1,25-(OH)2-D levels (<40 pmol/L) on day 1.
There was a trend towards lower vitamin D levels in patients admitted in cooler months compared to those admitted in warmer months (35.3 ± 16.3 vs. 41.9 ± 18.6 nmol/L, p = 0.06). Significantly lower levels were seen in patients who spent at least 7 days in hospital or an institution prior to ICU admission (32.0 ± 21.2 vs. 40.1 ± 16.9 nmol/L, p = 0.01).
Relationship between parameters of the vitamin D axis
Admission levels of 25-OH-D were highly correlated with 1,25-(OH)2-D (R = 0.61, p = 0.001) but there was no correlation between 25-OH-D and calcium (R = 0.16, p = 0.11) or PTH (R = −0.11, p = 0.31) levels (Table 3).
PTH–vitamin D–calcium axis
The prevalence of ionized hypocalcaemia (calcium <1.15 mmol/L) was 83 % at admission to ICU.
Secondary hyperparathyroidism (as defined by PTH >7 pmol/L) was seen in 37.5 % of hypocalcaemic and 32.5 % of vitamin D insufficient/deficient patients. PTH-responders were found to have higher SAPS-II [43 (31.3–60) vs. 36 (30–43), p = 0.03] on admission to ICU, but there was no mortality difference between PTH-responders and non-responders.
Variation during critical illness
There was no significant difference in 25-OH-D or 1,25-(OH)2-D levels between days 1, 3 and 7 of ICU stay (Table 3). There was, however, a significant drop in PTH levels and rise in calcium levels between days 1 and 7 (Table 4).
Associations with severity of illness and outcome
Admission 25-OH-D levels showed a modest correlation with the SAPS-II (R = −0.3, p = 0.01) and APACHE-II scores (R = −0.2, p = 0.05) (Fig. 1). On the other hand there was no relationship between admission levels of calcium, PTH or 1,25-(OH)2-D and SAPS-II or APACHE-II scores. However, there was a significant correlation between admission calcium levels and SOFA (R = −0.22, p = 0.03).
When a globulin concentration correction was performed for 25-OH-D, the correlation was somewhat strengthened (R = −0.32, p < 0.0001 and R = −0.24, p = 0.02 for SAPS-II and APACHE-II, respectively).
Higher SAPS-II and APACHE-II scores in this cohort indicated increased risk of mortality OR 1.77 (1.01–3.09) and 3.3 (1.41–7.7), respectively. There was no relationship between 25-OH-D levels or any of the other parameters and mortality in this cohort (Supplementary Table 1).
However, vitamin D deficiency (<25 nmol/L) was associated with significantly fewer hospital-free days, OR 3.15 (1.18–8.43) with a similar, albeit non-significant, association with vitamin D insufficiency, OR 2.30 (0.86–6.15). None of the other biochemical parameters were associated with mortality, hospital or ICU-free days (Supplementary Table 1).
Multivariate analyses were constructed using two models including age, SAPS, 25-OH-D and PTH in one model and age, APACHE-II, 25-OH-D and PTH in the second model (Supplementary Table 2). Age and APACHE-II were the only independent predictors of mortality.
Discussion
Principal findings
This prospective study characterizes expressions of the vitamin D, calcium and PTH status in adult patients with diverse diagnoses during the course of critical illness. It confirms a high prevalence of hypovitaminosis D, with vitamin D sufficiency observed in only 22 % of patients. None of these patients were on vitamin D supplementation in hospital prior to ICU admission. Data prior to hospitalization were not collected in this study, but were previously collected by our group in a point prevalence study in 2010. Of 506 ICU patients studied, only 2 % were on oral vitamin D supplements of 400–600 Units/day. In this context, we consider vitamin D supplements on admission unlikely to contribute to our current study findings.
Secondary hyperparathyroidism was seen in a third of vitamin D insufficient/deficient patients and was associated with worse disease severity scores; however, in this cohort it was not associated with mortality. Vitamin D deficient patients had fewer hospital-free days, compared to those who were sufficient. Collectively, the current findings reveal profound changes in the PTH–vitamin D–calcium axis in critical illness that appear to be associated with worse disease severity and fewer hospital-free days.
Comparison with other studies
We conducted an initial dedicated evaluation of vitamin D status in critically ill patients in 2009 which revealed a previously under-recognized high prevalence of vitamin D insufficiency/deficiency in ICU patients [8]. Only 7 % of patients were sufficient and predicted mortality was three times higher in deficient patients. Subsequently other studies confirmed a similarly high prevalence [9–13]. Low vitamin D state was associated with worse outcomes in all but one study [13].
There has been concern about the accuracy of vitamin D measurements in critical illness, prompted by a recent study revealing a 35 % reduction in 25-OH-D concentrations secondary to 3-L fluid loading in patients undergoing cardiopulmonary bypass [20]. The applicability of this finding to current published reports is uncertain. It is highly unlikely that the low vitamin D levels observed are entirely due to a dilutional effect of intravenous volume resuscitation. Up to 20 % of patients had undetectable levels [8, 10] and the current study showed persistently low concentrations over 7 days, thus arguing against an acute dilutional effect. Another study [21] demonstrated variability in 25-OH-D levels over a 24-h period. The authors therefore recommend more than one measurement to more accurately determine vitamin D status, as has been undertaken in the current study.
Strengths and weaknesses
The study population was taken as a consecutive collection of critically ill adults in three tertiary centres, therefore demonstrating that vitamin D deficient states in critically ill patients are not the result of selection bias. Metabolites of vitamin D, PTH and ionized calcium were sampled at three time periods per patient, thereby providing information about levels during the hyper acute, acute and more chronic or recovery phases of critical illness. However, owing to the heterogeneity of the patient population, the numbers of patients per diagnostic group were small, making it difficult to draw conclusions about particular patient subgroups. As patients had an overall low hospital mortality rate, the relationship between vitamin D deficiency and mortality could not be determined. Therefore surrogate measures such as ICU- and hospital-free days had to be utilized. Although the association of vitamin D deficiency with severity of illness and hospital length of stay has been demonstrated, a causative link cannot be made.
Clinical implications
Causes of low vitamin D states are multifactorial in critically ill patients, stemming from limited sunlight exposure, poor intake and vitamin D wastage secondary to loss of transport proteins [22]. D-binding protein (DBP) is the major carrier protein for circulating 25-OH-D. DBP concentrations have been found to be 30 % lower in critically ill patients, especially among those with sepsis [23, 24], trauma [25] and renal failure [26, 27]. Although DBP measurements were not available during the course of the current study, globulin was used as a surrogate marker. This ‘corrected’ protein-bound vitamin D was more strongly correlated with illness severity than the uncorrected level, highlighting the need to consider this aspect in future studies.
It is well known that vitamin D plays a critical role in the calcium–PTH axis. However, there are a paucity of data in the systematic characterization of the relationship between vitamin D, calcium and PTH in critical illness. Hypocalcaemia is common in critically ill patients [28, 29], leading to secondary hyperparathyroidism, as observed in over a third of our cohort (37.5 %). This, however, does not correct calcium malabsorption from the intestine, which appears to require both calcitriol and 25-OH-D. Furthermore, calcitriol produced at tissue level, which is responsible for the non-skeletal functions of vitamin D, cannot be measured clinically. We speculate that the rise in PTH heightens the conversion of 25-OH-D to 1,25-(OH)2-D, thereby maintaining 1,25-(OH)2-D levels, but stimulating bone resorption and intestinal calcium absorption, thus maintaining calcium homeostasis. However, secondary hyperparathyroidism may persist in these patients because of tissue level vitamin D deficiency [30]. Therefore, the lack of a PTH response may in fact confer better tissue vitamin D utilization, indicative of tissue vitamin D sufficiency, and therefore be associated with better recovery as evidenced by lower severity of illness scores seen in our cohort of PTH non-responders. In other words, inconsistent correlative data between low circulating vitamin D levels and disease outcome in the literature [31] may reflect the imprecise portrayal of tissue vitamin D status by circulating levels. This is consistent with a study of PTH response in the elderly, which showed that the absence of secondary hyperparathyroidism in the presence of hypovitaminosis D was associated with longer survival [32].
These data and complex health outcome relationships suggest the need for interpreting low vitamin D status in critically ill patients in conjunction with parathyroid status.
Future directions
The main question arising from the current study is whether vitamin D deficiency is causally linked to adverse outcomes. While currently no data support a therapeutic role of vitamin D in critical illness, the association of low vitamin D status with severity of illness and several surrogate markers of outcome suggests that further interventional studies of vitamin D supplementation are worthwhile. Our findings provide novel observational data revealing the complex interplay between low vitamin D states and parathyroid status, which should assist with the design of intervention studies.
Although vitamin D has a wide safety margin and therefore a low potential for toxicity, vitamin D supplementation in critical illness may not result in improved outcome. Previous experiences with manipulation to correct endocrine perturbations in critical illness have shown inconsistent results, and in some cases have resulted in adverse outcomes. Examples include growth hormone supplementation [33], tight glycemic control [34] and steroid supplementation in adrenal insufficiency [35]. By contrast with these hormonal variations, the serum levels of 25-OH-D are not thought to be under any humoral control. Rather, low vitamin D levels in critical illness could represent a longer duration of underlying illness or high utilization during acute illness. Several small studies have evaluated the safety and efficacy of high dose oral vitamin D in ICU patients [9, 36].
Conclusion
In summary, low vitamin D levels are highly prevalent in critically adults with 78 % of patients having either insufficient or deficient levels. 25-OH-D and 1,25-(OH)2-D levels did not vary significantly during ICU stay. Low levels of vitamin D and hyperparathyroidism were associated with increased severity of illness on ICU admission. Furthermore, low vitamin D levels were associated with fewer hospital-free days in univariate but not multivariate analyses after accounting for severity of illness. While it remains to be determined whether low vitamin D status is causally implicated in the pathogenesis of critical illness co-morbidities, findings from this study highlight the imperative for future evaluation of dosing and safety regimens followed by randomized control trials of vitamin D in the ICU.
References
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357:266–281
Bikle D (2009) Nonclassic actions of vitamin D. J Clin Endocrinol Metab 94:26–34
Diamond TH, Eisman JA, Mason RS, Nowson CA, Pasco JA, Sambrook PN, Wark JD, Working Group of the Australian and New Zealand Bone and Mineral Society, Endocrine Society of Australia, Osteoporosis Australia (2005) Vitamin D and adult bone health in Australia and New Zealand: a position statement. Med J Aust 182:281–285
Grover SR, Morley R (2001) Vitamin D deficiency in veiled or dark-skinned pregnant women. Med J Aust 175:251–252
Inderjeeth CA, Nicklason F, Al-Lahham Y, Greenaway TM, Jones G, Parameswaran VV, David R (2000) Vitamin D deficiency and secondary hyperparathyroidism: clinical and biochemical associations in older non-institutionalised Southern Tasmanians. Aust N Z J Med 30:209–214
Sambrook PN, Cameron ID, Cumming RG, Lord SR, Schwarz JM, Trube A, March LM (2002) Vitamin D deficiency is common in frail institutionalised older people in northern Sydney. Med J Aust 176:560
Pasco JA, Henry MJ, Nicholson GC, Sanders KM, Kotowicz MA (2001) Vitamin D status of women in the Geelong osteoporosis study: association with diet and casual exposure to sunlight. Med J Aust 175:401–405
Lee P, Eisman JA, Center JR (2009) Vitamin D deficiency in critically ill patients. N Engl J Med 360:1912–1914
Mata-Granados JM, Vargas-Vasserot J, Ferreiro-Vera C, Luque de Castro MD, Pavon RG, Quesada Gomez JM (2010) Evaluation of vitamin D endocrine system (VDES) status and response to treatment of patients in intensive care units (ICUs) using an on-line SPE-LC-MS/MS method. J Steroid Biochem Mol Biol 121:452–455
Lucidarme O, Messai E, Mazzoni T, Arcade M, du Cheyron D (2010) Incidence and risk factors of vitamin D deficiency in critically ill patients: results from a prospective observational study. Intensive Care Med 36:1609–1611
McKinney JD, Bailey BA, Garrett LH, Peiris P, Manning T, Peiris AN (2011) Relationship between vitamin D status and ICU outcomes in veterans. J Am Med Dir Assoc 12:208–211
Braun A, Chang D, Mahadevappa K, Gibbons FK, Liu Y, Giovannucci E, Christopher KB (2011) Association of low serum 25-hydroxyvitamin D levels and mortality in the critically ill. Crit Care Med 39:671–677
Cecchi A, Bonizzoli M, Douar S, Mangini M, Paladini S, Gazzini B, Degl’Innocenti S, Linden M, Zagli G, Peris A (2011) Vitamin D deficiency in septic patients at ICU admission is not a mortality predictor. Minerva Anestesiol 77:1184–1189
Knaus WA, Zimmerman JE, Wagner DP, Draper EA, Lawrence DE (1981) APACHE-acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med 9:591–597
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the european society of intensive care medicine. Intensive Care Med 22:707–710
Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG (1986) Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab 63:954–959
Holick MF (2009) Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol 19:73–78
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, http://www.R-project.org/
Krishnan A, Ochola J, Mundy J, Jones M, Kruger P, Duncan E, Venkatesh B (2010) Acute fluid shifts influence the assessment of serum vitamin D status in critically ill patients. Crit Care 14:R216
Venkatesh B, Davidson B, Robinson K, Pascoe R, Appleton C, Jones M (2012) Do random estimations of vitamin D3 and parathyroid hormone reflect the 24-h profile in the critically ill? Intensive Care Med 38:177–179
Lee P (2011) Vitamin D metabolism and deficiency in critical illness. Best Pract Res Clin Endocrinol Metab 25:769–781
Van den Berghe G, Van Roosbroeck D, Vanhove P, Wouters PJ, De Pourcq L, Bouillon R (2003) Bone turnover in prolonged critical illness: effect of vitamin D. J Clin Endocrinol Metab 88:4623–4632
Jeng L, Yamshchikov AV, Judd SE, Blumberg HM, Martin GS, Ziegler TR, Tangpricha V (2009) Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med 7:28
Dahl B, Schiodt FV, Ott P, Wians F, Lee WM, Balko J, O’Keefe GE (2003) Plasma concentration of Gc-globulin is associated with organ dysfunction and sepsis after injury. Crit Care Med 31:152–156
Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE (1999) An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 96:507–515
Thrailkill KM, Jo CH, Cockrell GE, Moreau CS, Fowlkes JL (2011) Enhanced excretion of vitamin D binding protein in type 1 diabetes: a role in vitamin D deficiency? J Clin Endocrinol Metab 96:142–149
Hastbacka J, Pettila V (2003) Prevalence and predictive value of ionized hypocalcemia among critically ill patients. Acta Anaesthesiol Scand 47:1264–1269
Zaloga GP, Chernow B (1986) Hypocalcemia in critical illness. JAMA 256:1924–1929
Lee P, Nair P, Eisman JA, Center JR (2009) Vitamin D deficiency in the intensive care unit: an invisible accomplice to morbidity and mortality? Intensive Care Med 35:2028–2032
Thacher TD, Clarke BL (2011) Vitamin D insufficiency. Mayo Clin Proc 86:50–60
Chen JS, Sambrook PN, March L, Cameron ID, Cumming RG, Simpson JM, Seibel MJ (2008) Hypovitaminosis D and parathyroid hormone response in the elderly: effects on bone turnover and mortality. Clin Endocrinol (Oxf) 68:290–298
Takala J, Ruokonen E, Webster NR, Nielsen MS, Zandstra DF, Vundelinckx G, Hinds CJ (1999) Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med 341:785–792
Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hebert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ (2009) Intensive versus conventional glucose control in critically ill patients. N Engl J Med 360:1283–1297
Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, Weiss YG, Benbenishty J, Kalenka A, Forst H, Laterre PF, Reinhart K, Cuthbertson BH, Payen D, Briegel J (2008) Hydrocortisone therapy for patients with septic shock. N Engl J Med 358:111–124
Amrein K, Sourij H, Wagner G, Holl A, Pieber TR, Smolle KH, Stojakovic T, Schnedl C, Dobnig H (2011) Short-term effects of high-dose oral vitamin D3 in critically ill vitamin D deficient patients: a randomized, double-blind, placebo-controlled pilot study. Crit Care 15:R104
Acknowledgments
We gratefully acknowledge the contribution of David Gattas, Dorrilyn Rajabhandari, Heidi Buhr (Royal Prince Alfred Hospital, Sydney), Manoj Saxena, Rebecca Sidoli and Deborah Inskip (St George Hospital, Sydney) to patient enrolment and data collection.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nair, P., Lee, P., Reynolds, C. et al. Significant perturbation of vitamin D–parathyroid–calcium axis and adverse clinical outcomes in critically ill patients. Intensive Care Med 39, 267–274 (2013). https://doi.org/10.1007/s00134-012-2713-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2713-y