Abstract
Purpose
To describe changes in hemodynamic variables, sedation, and pain score after discontinuation of prolonged infusions of dexmedetomidine in a pediatric population of critically ill cardiac patients.
Methods
Retrospective case series of patients who received continuous infusions of dexmedetomidine for longer than 3 days in a pediatric cardiac intensive care unit from 2008 to 2010.
Results
Sixty-two patients, age 5.2 months (range 0.3 months–17 years) and weight 5.1 kg (range 2.2–84 kg), were included. Thirty-nine patients (63%) were younger than 1 year of age. Median duration of dexmedetomidine infusion was 5.8 days (range 4–26 days) and median infusion dose was 0.71 μg/kg/h (range 0.2–2.1 μg/kg/h). Median weaning time and dose at discontinuation were 43 h (range 0–189 h) and 0.2 μg/kg/h (range 0.1–1.3 μg/kg/h). Tachycardia, transient hypertension and agitation were observed in 27, 35 and 27% of patients. Episodes of tachycardia were more frequent in children older than 1 year of age (61 vs. 8%, p < 0.001), patients who received dexmedetomidine for 4 days when compared to those who received 5 days or longer (48 vs. 17%, p = 0.011), and patients whose infusion was discontinued abruptly (42 vs. 14%, p = 0.045). Tachyarrhythmias were seen in nine patients (15%) after discontinuation of the dexmedetomidine infusion. Adequate sedation and analgesia scores at the moment of infusion discontinuation were seen in 90 and 88% of patients, respectively.
Conclusions
Our study suggests that tachycardia, transient hypertension, and agitation are frequently observed in pediatric cardiac intensive care unit patients after discontinuing prolonged dexmedetomidine infusions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dexmedetomidine, a selective α2-adrenoreceptor agonist, is currently approved by the Food and Drug Administration for adult sedation for up to 24 h during mechanical ventilation [1] and for monitored anesthesia care. Nevertheless, it is used extensively in the pediatric population in general critical care [2–5], cardiac critical care [6–10], cardiac anesthesia [11], general anesthesia [12], and ambulatory anesthesia [13] due its ability to produce sedation, anxiolysis, and analgesia while minimally affecting respiratory function [14–17]. Despite the widespread use of dexmedetomidine, current literature contains little information regarding experience with prolonged infusions and few case reports describe possible rebound or withdrawal symptoms after its discontinuation [18–21].
Driven by unpublished and observational clinical data from our Cardiac Intensive Care Unit (CICU) at Children’s Hospital of Pittsburgh of University of Pittsburgh Medical Center (UPMC) regarding episodes of hypertension, tachycardia, and agitation after discontinuing infusions of dexmedetomidine, we designed this study to describe changes in hemodynamic variables, sedation, and pain score within the first 12 h after suspending prolonged infusions of dexmedetomidine in a population of pediatric cardiac intensive care patients.
Materials and methods
The study protocol was approved by the Institutional Review Board of the University of Pittsburgh/Children’s Hospital of Pittsburgh of UPMC, which waived the need for written, informed consent. However, parental informed consent for dexmedetomidine use was obtained for all patients younger than 1 year of age, infusions longer than 72 h, and infusion dose greater than 1.5 μg/kg/h. We performed a retrospective, case series study of pediatric patients in the CICU at Children’s Hospital of Pittsburgh of UPMC between 1 January 2008 and 1 January 2010. The inclusion criteria were age less than 18 years and having received a continuous infusion of dexmedetomidine lasting longer than 3 days. Exclusion criteria were age greater than 18 years, death, inability to obtain clinical information, and any administration of clonidine from 48 h before to 12 h after dexmedetomidine discontinuation.
Although there is no standard protocol for discontinuing dexmedetomidine in our unit, patients are typically weaned at a rate of 0.1–0.4 μg/kg/h every 8–24 h based on duration and dose of the infusion at the moment of discontinuation. If an episode of agitation is observed during weaning, the infusion is returned to the previous dose and kept there for at least 6 h before attempting to continue weaning. If sedation remains inadequate after 20–30 min following the increment, a rescue agent is also administered.
The exact time of dexmedetomidine discontinuation was determined for each patient and information regarding dexmedetomidine administration was documented (duration of administration, mean dose, cumulative dose, dose at discontinuation, and weaning time). Since the elimination half-life of dexmedetomidine is approximately 2 h, it is expected that after six half-lives (12 h), the majority of the drug has been eliminated and the effects of discontinuation become evident [22–25]. Therefore, we decided to observe the variables of interest [heart rate (HR), systolic, diastolic and mean blood pressure, respiratory rate, sedation score, and pain score] for each patient at baseline and hourly for 12 h following discontinuation of the infusion. Baseline values were defined as the values noted 1 h prior to discontinuation. Hypertensive or hypotensive episodes, tachycardia, and bradycardia were defined as any value above or below the 95th and 5th percentiles for age. Presence of cardiac arrhythmias and need for inotropes, vasopressors, vasodilators, and antiarrhythmics were also recorded. When several values for vital signs were found within the same hourly record, the most abnormal value was used. All information for this study was extracted from the patients’ electronic medical records.
The quality of sedation was assessed using the following ICU sedation scale: 0—none, alert or normal sleep, easy to arouse; 1—mild sedation, occasionally drowsy, easy to arouse; 2—moderate sedation, frequently drowsy but easy to arouse; and 3—severe sedation, somnolent, difficult to arouse. Analgesia was assessed using three 0–10 pain score scales: the crying, requires increased oxygen administration, increased vital signs, expression, and sleeplessness (CRIES) for neonates; the facial expression, leg movement, activity, cry, and consolability (FLACC) for children 2 months–7 years; and the numeric visual analog scale (NVAS) for children older than 7 years [26–28]. A score of 0 was considered no pain, 1–3 as mild pain, 4–7 as moderate pain, and 8–10 as severe pain. Targeted levels of sedation and analgesia were 0–2 and 0–3, respectively. Episodes of agitation reported by the attending physician or nursing staff and the use of other sedatives and analgesics were also recorded.
SPSS Statistic version 18 for Mac was used for statistical analysis. The continuous variables—blood pressure, heart rate, and respiratory rate—were reported as mean ± SD and 95% confidence interval. These results were categorized into five age groups: group 1, 1 day to 1 month; group 2, 2 months to 2 years; group 3, 2–5 years; group 4, 5–13 years; and group 5, 13–18 years. Categorical variables were reported as frequencies and percentages. Differences in the frequency of hypertension, hypotension, tachycardia, and bradycardia were compared using a chi-square test. Differences in dexmedetomidine dose between children younger than 1 year and older children were compared using an unpaired Student’s t test. The relationship between duration of dexmedetomidine infusion and weaning time was evaluated using Spearman correlation. Differences in weaning time between patients who received dexmedetomidine for 4 days versus 5 days or longer were evaluated using the independent-samples Mann–Whitney U test. A p value of less than 0.05 was considered significant.
Results
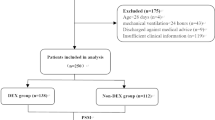
From 1 January 2008 to 1 January 2010, 87 of 505 patients who received dexmedetomidine in the CICU received it for longer than 3 days. After excluding 25 patients (17 received clonidine during the 48 h prior to discontinuing dexmedetomidine to decrease risk of withdrawal symptoms, three received clonidine to treat suspected withdrawal syndrome, two were older than 18 years, two died, and one for whom it was impossible to obtain the clinical information), 62 remained for analysis.
Demographics are shown in Table 1 and surgical procedures performed are shown in Table 2. Information regarding dexmedetomidine administration is shown in Table 3. Thirty-nine of 62 patients (63%) were younger than 1 year old. At the moment of dexmedetomidine discontinuation, 41 patients (66%) were spontaneously breathing and 21 patients (34%) remained on mechanical ventilation. Twenty-eight patients (45%) received a mean infusion dose of dexmedetomidine greater than 0.75 μg/kg/h and one patient received a mean dose greater than 1.3 μg/kg/h. Mean dexmedetomidine infusion dose was 0.76 μg/kg/h for children younger than 1 year of age and 0.70 μg/kg/h for older patients (p = 0.513). Twenty-three patients (37%) received dexmedetomidine infusions for longer than 7 days. In 44 patients (71%), dexmedetomidine was weaned over a period greater than 12 h, while in 18 patients (29%) it was discontinued abruptly (14 patients, 23%) or weaned over less than 12 h (four patients, 6%). There was a medium strength correlation between duration of infusion and weaning time [r = 0.492, p (2-tailed) <0.001], with patients who received dexmedetomidine for 4 days having shorter weaning times than those who received it for 5 days or longer (medians 24 vs. 48 h, p = 0.012). For all patients, the infusion was discontinued in the CICU.
Within the first 12 h after discontinuation, tachycardia was observed in 17 patients (27%). Of these, six experienced tachycardia at baseline and 11 were experiencing a new episode of tachycardia. Of the new episodes, 90% began during the first 6 h after discontinuing dexmedetomidine. Episodes were more frequent in patients whose dexmedetomidine was discontinued abruptly compared to those who were weaned (42 vs. 14%, p = 0.045), and tachycardia was more frequent among patients who received dexmedetomidine for 4 days compared to those who received it for 5 days or longer (48 vs. 17%, p = 0.011). It was also more frequently observed in children older than 1 year of age (61 vs. 8%, p < 0.001). Type of tachycardia varied among the 17 patients as follows: eight sinus tachycardia, four atrial tachycardia, two ectopic junctional tachycardia, two atrial flutters, and one supraventricular tachycardia. One patient had atrial fibrillation with fast ventricular response and became hemodynamically unstable, requiring cardioversion. Bradycardia was observed in five patients (8%), all of whom experienced bradycardia at baseline (three patients with complete atrioventricular block requiring pacing and two patients with sinus bradycardia).
Transient hypertension was seen in 22 patients (35%), with a mean of 3.3 hypertensive episodes per patient. Nine of these patients exhibited hypertension at baseline and the other 13 showed new hypertensive episodes after discontinuing dexmedetomidine. Of the nine new episodes, 46% occurred in patients whose dexmedetomidine was discontinued abruptly and 19% in patients who were weaned (p = 0.112). Arterial hypotension was seen in 9 patients (14%), with no episodes of hypotension at baseline. Ten patients (16%) had episodes of both tachycardia and hypertension. The most used cardiovascular medications during the study period were milrinone infusion (45%), angiotensin converting enzyme inhibitor (42%), calcium chloride or calcium gluconate intermittent infusions (29%), digoxin (18%), and amiodarone (11%). Table 4 illustrates a complete analysis of the hemodynamic and respiratory data.
Figures 1and 2 show the percentage of patients with a sedation score less than 2 and an analgesia less than 4 during the 12 h following discontinuation of the dexmedetomidine infusion. Sedation scores less than 2 were seen in 90% or more of patients at any time point after discontinuation. Episodes of agitation were seen in 17 patients (27%) during the first 12 h after discontinuation of dexmedetomidine. Pain scores between 0 and 3 were seen in 88% of patients at the moment of infusion discontinuation and 98% after 12 h without dexmedetomidine. The most frequently administered sedatives were lorazepam (21%), chloral hydrate (14%), and midazolam (11%). Rescue doses of fentanyl were administered to 7 patients (11%) and morphine to three patients (5%).
One extubated patient required reintubation during the 12 h immediately following discontinuation of dexmedetomidine. Fifteen of the 21 patients (71%) who were still on mechanical ventilation at the time of discontinuation were successfully extubated within this time frame.
Discussion
In a previous publication from our CICU [7], we reported our general experience with the use of dexmedetomidine in children following cardiothoracic surgery. In the present study, we describe the immediate changes in hemodynamic, sedation, and pain states after suspending prolonged infusions of dexmedetomidine in the same population of patients. We decided to exclude patients who received clonidine during the study period due to the fact that its α2-adrenoceptor agonist effect may impact expected changes in the variables of interest after discontinuing the dexmedetomidine infusion.
The results of the present study indicate that episodes of tachycardia, hypertension, and agitation are frequently observed when pediatric CICU patients are subject to discontinuation of prolonged infusions of dexmedetomidine. Although there are several potential explanations for the hemodynamic changes seen in our patients (primary cardiac disease, pain, inadequate sedation, bleeding, fever, and cardiac medications among others), we speculate that these changes may be directly related to the discontinuation of dexmedetomidine. Analgesia seemed adequate based on the low pain scores and the minimal need for rescue doses of narcotics (16%), there were no significant changes in the infusion of cardiac drugs for the majority of patients during the occurrence of these events, and although episodes of agitation were frequent, they did not occur in the same patients who presented episodes of tachycardia and hypertension (only two patients in each group experienced agitation simultaneously with tachycardia or hypertension).
The typical hemodynamic profile of dexmedetomidine administration at the usual clinical dose is characterized by reduction of HR and blood pressure (BP) [29, 30]. This decrease in HR and BP is the result of dexmedetomidine acting on α2A-adrenoceptors in the vasomotor centers of the brainstem, specifically the rostral ventrolateral medulla (RVLM) [30] and the presynaptic postganglionic sympathetic fibers, which results in central and peripheral sympatholysis [11, 29, 31–34]. Although some degree of increase in HR and BP should be normally expected after discontinuation of a dexmedetomidine infusion, the magnitude and clinical impact of such an increase has not yet been described. The increase in HR and BP that we observed may be explained by the hyperadrenergic state that follows dexmedetomidine discontinuation. Ten patients experienced combined episodes of tachycardia and hypertension. The combined effects of increased HR and systemic vascular resistance (SVR) may not be well tolerated by patients with limited cardiovascular reserve, as in the case of pediatric cardiac patients who are especially susceptible to the secondary effects of tachycardia, including increase in myocardial oxygen consumption, decrease of coronary reserve, predisposition to arrhythmias, decrease in diastolic filling, and decrease in myocardial reserve [7].
There are several interesting findings in this retrospective case series that we would like to highlight. First, the mean infusion dose of dexmedetomidine used in this cohort of patients was higher than that reported in similar studies (0.74 vs. 0.35 μg/kg/h in our previous study) [7]. The aforementioned difference may be due to the extended period of administration of dexmedetomidine, which may have caused patients to develop some degree of tolerance to the drug, therefore requiring a higher dose. It is also important to note that the high infusion dose of dexmedetomidine used in this population of patients may have contributed to the relatively high frequency of hypertension, tachycardia, and agitation found after discontinuation of dexmedetomidine.
Second, we found that episodes of tachycardia were more frequent in children older than 1 year of age. Although the exact cause of this finding is not totally understood, it is known that there are important differences in the autonomic nervous system during infancy as compared to childhood [35–37]. When the sympathetic nervous system of older children or adults is blocked (by spinal anesthesia or dexmedetomidine), heart rate and blood pressure are primarily controlled by the “unopposed” parasympathetic nervous system, which results in a significant decrease in both values. However, since infants and younger children do not have a fully developed parasympathetic system, they do not show the same magnitude of decrease in heart rate and blood pressure. Thus, when the blockage over the sympathetic system is released, older children go from a lower value (parasympathetic regulated) of heart rate and blood pressure to a basal one (dually regulated—sympathetic and parasympathetic), showing an important change in the value. In contrast, younger children, who never experienced a large drop in heart rate or blood pressure, do not have a large increase in these values when the sympathetic nervous system returns to normal. At the molecular level, it has been found that response to catecholamines is not equal in neonates and older children or adults. The high sympathetic tone present in the neonatal age produces an upregulation of the most active forms of stimulating G proteins in the cardiac myocytes, in contrast to the downregulating effect seen during adulthood. This may be a protective mechanism to ensure adequate sympathetic stimulation in situations where there are low levels of circulating catecholamines and may also explain the maintenance of near normal heart rate and blood pressure values in spite of dexmedetomidine infusion.
Third, the higher frequency of tachycardia seen in patients with 4-day dexmedetomidine infusions compared to longer duration infusions was totally unexpected. However, based on the positive correlation found between the duration of the infusion and the weaning time, and the fact that patients who received dexmedetomidine infusions for 4 days had shorter weaning times, it is likely that they were not weaned as cautiously as those who received longer infusions.
Finally, arrhythmia was another frequently observed outcome upon discontinuation of dexmedetomidine. In a recent study of children after cardiac surgery, Chrysostomou et al. [9] showed that dexmedetomidine has a potential antiarrhythmic role in the treatment of atrial and junctional tachyarrhythmias. Moreover, Hammer et al. [38] described how dexmedetomidine depressed sinus and atrioventricular nodal function in children undergoing electrophysiologic study and ablation of supraventricular accessory pathways. It is not yet clear if these potential antiarrhythmic effects of dexmedetomidine are due to a direct action on the heart’s conduction system, the result of decreasing plasma concentration of catecholamines, the effect on the central nervous system through its sympatholytic and sympathomimetic effects, or all the aforementioned [39].
With regard to prolonged infusions of dexmedetomidine, Walker et al. [40] studied the quality of sedation and glycemic profile of dexmedetomidine in 65 pediatric burn patients in the ICU, reporting an average length of infusion as 11 days and mean dose as 0.5 μg/kg/h. They did not, however, find rebound hypertension or withdrawal after weaning the infusion over the course of 12–24 h. To our knowledge there have not been any studies published describing discontinuation of prolonged infusions of dexmedetomidine in pediatric cardiac patients.
Study limitations
-
1.
Its retrospective nature does not allow us to affirm that the observed changes were due to the discontinuation of dexmedetomidine.
-
2.
There are several potential confounding variables in this population of pediatric cardiac patients that might account for the changes observed.
-
3.
Since there is no standardized protocol for discontinuing dexmedetomidine in our CICU, it was impossible to exactly describe the weaning process from dexmedetomidine in a retrospective fashion.
-
4.
The study included a relatively small number of patients; a larger subject population may have shown more pronounced hemodynamic changes.
-
5.
As a result of the limited data points captured by the electronic medical record in our unit, the exact duration of episodes of tachycardia, bradycardia, hypertension, and hypotension were not possible to determine.
Conclusions
Our study suggests that tachycardia, transient hypertension, and agitation are frequently observed in pediatric cardiac patients after discontinuing prolonged infusions of dexmedetomidine. Tachycardia was more frequent among children older than 1 year of age, those who received infusions lasting 4 days versus longer durations, and those whose infusion was abruptly discontinued. Although there are several possible causes for the observed changes, they could be related to the hyperadrenergic state experienced upon discontinuation of dexmedetomidine infusions. Prospective and randomized pediatric studies should be conducted to evaluate tolerance of and withdrawal from prolonged infusions of dexmedetomidine.
References
Tobias J (2007) Dexmedetomidine: applications in pediatric critical care and pediatric anesthesiology. Pediatr Crit Care Med 8:115–131
Tobias JD, Berkenbosch JW (2008) Initial experience with dexmedetomidine in paediatric-aged patients. Paediatr Anaesth 12:171–175
Tobias JD, Berkenbosch JD, Russo P (2003) Additional experience with dexmedetomidine in pediatric patients. South Med J 96:871–875
Tobias JD, Berkenbosch JW (2004) Sedation during mechanical ventilation in infants and children: dexmedetomidine versus midazolam. South Med J 97:451–455
Tobias JD (2006) Dexmedetomidine to treat opioid withdrawal in infants following prolonged sedation in the pediatric ICU. J Opioid Manag 2:201–205
Chrysostomou C, Sanchez De Toledo J, Avolio T, Motoa MV, Berry D, Morell VO, Orr R, Munoz R (2009) Dexmedetomidine use in a pediatric cardiac intensive care unit: can we use it in infants after cardiac surgery? Pediatr Crit Care Med 10:654–660
Chrysostomou C, Di Filippo S, Manrique AM, Schmitt CG, Orr RA, Casta A, Suchoza E, Janosky J, Davis PJ, Munoz R (2006) Use of dexmedetomidine in children after cardiac surgery. Pediatr Crit Care Med 7:126–131
Phan H, Nahata MC (2008) Clinical uses of dexmedetomidine in pediatric patients. Pediatr Drugs 10:49–69
Chrysostomou C, Beerman L, Shiderly D, Berry D, Morell VO, Munoz R (2008) Dexmedetomidine: A novel drug for the treatment of atrial and junctional tachyarrhythmias during the perioperative period for congenital cardiac surgery: a preliminary study. Anesth Analg 107:1514–1522
Barton KP, Munoz R, Morell VO, Chrysostomou C (2008) Dexmedetomidine as the primary sedative during invasive procedures in infants and toddlers with congenital heart disease. Pediatr Crit Care Med 9:612–615
Mukhtar AM, Obayah EM, Hassona AM (2006) The use of dexmedetomidine in pediatric cardiac surgery. Anesth Analg 103:52–56
Shukry M, Clyde MC, Kalarickal PL, Ramadhyani U (2005) Does dexmedetomidine prevent emergence delirium in children after sevoflurane-based general anesthesia? Paediatr Anaesth 15:1098–1104
Mahmoud M, Gunter J, Donnelly LF, Wang Y, Nick TG, Sadhasivam S (2009) A comparison of dexmedetomidine with propofol for magnetic resonance imaging sleep studies in children. Anesth Analg 109:745–753
Antonelli M, Azoulay E, Bonten M, Chastre J, Citerio G, Conti G, De Backer D, Lemaire F, Gerlach H, Hedenstierna G, Joannidis M, Macrae D, Mancebo J, Maggiore SM, Mebazaa A, Preiser JC, Pugin J, Wernerman J, Zhang H (2010) Year in review in intensive care medicine 2009. Part III: mechanical ventilation, acute lung injury and respiratory distress syndrome, pediatrics, ethics, and miscellanea. Intensive Care Med 36:567–584
Chrysostomou C, Schmitt CG (2008) Dexmedetomidine: sedation, analgesia and beyond. Expert Opin Drug Metab Toxicol 4:619–627
Carroll CL, Krieger D, Campbell M, Fisher DG, Comeau LL, Zucker AR (2008) Use of dexmedetomidine for sedation of children hospitalized in the intensive care unit. J Hosp Med 3:142–147
Tokuhira N, Atagi K, Shimaoka H, Ujiro A, Otsuka Y, Ramsay M (2009) Dexmedetomidine sedation for pediatric post-Fontan procedure patients. Pediatr Crit Care Med 10:207–212
Weber D, Thammasitboon S, Rosen D (2007) Acute discontinuation syndrome from dexmedetomidine after protracted use in a pediatric patient. Pediatr Anesth 18:87–88
Darnell C, Steiner J, Szmuk P, Sheeran P (2010) Withdrawal from multiple sedative agent therapy in an infant: is dexmedetomidine the cause or the cure? Pediatr Crit Care Med 11:e1–e3
Tobias J (2010) Dexmedetomidine: are tolerance and withdrawal going to be an issue with long-term infusions? Pediatr Crit Care Med 11:158–160
Bejian S, Valasek C, Nigro JJ, Cleveland DC, Willis BC (2009) Prolonged use of dexmedetomidine in the paediatric cardiothoracic intensive care unit. Cardiol Young 19:98–104
Kamibayashi T, Maze M (2000) Clinical uses of alpha2-adrenergic agonists. Anesthesiology 93:1345–1349
Petroz GC, Sikich N, James M, van Dyk H, Shafer SL, Schily M, Lerman J (2006) A phase I, two-center study of the pharmacokinetics and pharmacodynamics of dexmedetomidine in children. Anesthesiology 105:1098–1110
Potts AL, Anderson BJ, Warman GR, Lerman J, Diaz SM, Vilo S (2009) Dexmedetomidine pharmacokinetics in pediatric intensive care—a pooled analysis. Pediatric Anesthesia 19:1119–1129
Díaz SM, Rodarte A, Foley J, Capparelli EV (2007) Pharmacokinetics of dexmedetomidine in postsurgical pediatric intensive care unit patients: preliminary study. Pediatr Crit Care Med 8:419–424
Krechel SW, Bildner J (1995) CRIES: a new neonatal postoperative pain measurement score. Initial testing of validity and reliability. Paediatr Anaesth 5:53–61
Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S (1997) The FLAAC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs 23:293–297
Wong DL, Hockenberry-Eaton M, Wilson D et al (1999) Whaley and Wong’s nursing care of infants and children. Mosby, St. Louis
Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD (2000) The effects of increasing plasma concentration of dexmedetomidine in humans. Anesthesiology 93:382–394
Shehabi Y, Ruettimann U, Adamson H, Innes R, Ickeringill M (2004) Dexmedetomidine infusion for more than 24 hours in critically ill patients: sedative and cardiovascular effects. Intensive Care Med 30:2188–2196
Venn RM, Bryant A, Hall GM, Grounds RM (2001) Effects of dexmedetomidine on adrenocortical function, and the cardiovascular, endocrine and inflamatory responses in postoperative patients needing sedation in the intensive care unit. Br J Anaesth 86:650–656
Wilkins BW, Hesse C, Charkoudian N, Nicholson WT, Sviggum HP, Moyer TP, Joyner MJ, Eisenach JH (2008) Autonomic cardiovascular control during a novel pharmacologic alternative to ganglionic blockade. Clin Pharmacol Ther 83:692–701
Shirasaka T, Qiu DL, Kannan H, Takasaki M (2007) The effects of centrally administered dexmedetomidine on cardiovascular and sympathetic function in conscious rats. Anesth Analg 105:1722–1728
Doze VA, Chen BX, Mervyn M (1989) Dexmedetomidine produces a hypnotic-anesthetic action in rats via activation of central alpha-2 adrenoceptors. Anesthesiology 71:75–79
Chow LT, Chow SS, Anderson RH, Gosling JA (2001) Autonomic innervation of the human cardiac conduction system: Changes from infancy to senility—an immunohistochemical and histochemical analysis. Anat Rec 264:169–182
Oberlander TF, Berde CB, Lam KH, Rappaport LA, Saul JP (1995) Infants tolerate spinal anesthesia with minimal overall autonomic changes: analysis of heart rate variability in former premature infants undergoing hernia repair. Anesth Analg 80:20–27
Auman JT, Seidler FJ, Slotkin TA (2002) β-Adrenoceptor control of G protein function in the neonate: determinant of desensitization or sensitization. Am J Physiol Regul Integr Comp Physiol 283:R1236–R1244
Hammer GB, Drover DR, Cao H, Jackson E, Williams GD, Ramamoorthy C, Van Hare GF, Niksch A, Dubin AM (2008) The effects of dexmedetomidine on cardiac electrophysiology in children. Anesth Analg 106:79–83
Kamibayashi T, Hayashi Y, Mammoto T, Yamatodani A, Sumikawa K, Yoshiya I (1995) Role of the vagus nerve in the antidysrhythmic effect of dexmedetomidine on halothane/epinephrine dysrhythmias in dogs. Anesthesiology 83:992–999
Walker J, Maccallum M, Fischer C, Kopcha R, Saylors R, McCall J (2006) Sedation using dexmedetomidine in pediatric burn patients. J Burn Care Res 27:206–210
Acknowledgments
This publication was made possible by grant number 5UL1 RR024153-04 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burbano, N.H., Otero, A.V., Berry, D.E. et al. Discontinuation of prolonged infusions of dexmedetomidine in critically ill children with heart disease. Intensive Care Med 38, 300–307 (2012). https://doi.org/10.1007/s00134-011-2441-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2441-8