Abstract
Purpose
To investigate if femoral venous pressure (FVP) measurement can be used as a surrogate measure for intra-abdominal pressure (IAP) via the bladder.
Methods
This was a prospective, multicenter observational study. IAP and FVP were simultaneously measured in 149 patients. The effect of BMI on IAP was investigated.
Results
The incidences of intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS) were 58 and 7% respectively. The mean APACHE II score was 22 ± 10, SAPS 2 score 42 ± 20, and SOFA score 9 ± 4. The mean IAP was 11.2 ± 4.5 mmHg versus 12.7 ± 4.7 mmHg for FVP. The bias and precision for all measurements were −1.5 and 3.6 mmHg respectively with the lower and upper limits of agreement being −8.6 and 5.7. When IAP was above 20 mmHg, the bias between IAP and FVP was 0.7 with a precision of 2.0 mmHg (lower and upper limits of agreement −3 and 4.6 respectively). Excluding those with ACS, according to the receiver operating curve analysis FVP = 11.5 mmHg predicted IAH with a sensitivity and specificity of 84.8 and 67.0% (AUC of 0.83 (95% CI 0.81–0.86) with P < 0.001). FVP = 14.5 mmHg predicted IAP above 20 mmHg with a sensitivity of 91.3% and specificity of 68.1% (AUC 0.85 (95% CI 0.79–0.91), P < 0.001). Finally, at study entry, the mean IAP in patients with a BMI less then 30 kg/m2 was 10.6 ± 4.0 mmHg versus 13.8 ± 3.8 mmHg in patients with a BMI ≥ 30 kg/m2 (P < 0.001).
Conclusions
FVP cannot be used as a surrogate measure of IAP unless IAP is above 20 mmHg.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS) are associated with increased morbidity and mortality [1, 2]. Recently Cheatham et al. [3] showed that the implementation of a revised IAH/ACS management algorithm increased their patient survival to hospital discharge from 50 to 72%. Their main strategy was to use serial intra-abdominal pressure (IAP) measurements to detect IAH, clear medical management policies to reduce IAP and restore organ perfusion, and early surgical decompression when ACS developed. The World Society of Abdominal Compartment Syndrome (WSACS, www.wsacs.org) recommends to measure IAPs every 4–6 h in critically ill patients with risk factors for the development of IAH or ACS [4, 5]. The gold standard for the measurement of IAP is via the bladder in the supine position [5] but other routes (nasogastric, rectal, uterus, intraperitoneal) have also been used and validated [6–8].
One of the main problems of the IAP measurement via the bladder is its intermittent nature which varies between 4 and 6 hourly measurements in most intensive care units (ICU). In patients with IAH of grade 2 (IAP between 15 and 20 mmHg), the risk of developing ACS (IAP > 20 mmHg with new onset organ failure) is high and early diagnosis is essential [3, 9]. Therefore, a continuous IAP measurement might be more appropriate. IAP can be measured continuously either via the bladder with a 18-Fr standard three-way catheter or via the stomach with a balloon-tipped catheter [7, 10]. Femoral venous access is commonly used in the ICU setting, especially in emergency situations where rapid intravenous access is warranted or in cases where the more traditional sites like subclavian and internal jugular veins are at a higher risk of complications (pre-existing coagulopathy, injury to major nerves, thoracic duct, carotid artery, and pleura) [11]. There has been some controversy regarding the use of femoral venous pressure (FVP) as a surrogate measure of IAP [6]. We recently found, in an animal experimental study (13 pigs), that FVP correlated well with bladder pressure [12]. This correlation has been found by others [13–15].
The main aim of this study was to determine whether IAP assessed by the urinary bladder pressure corresponded well with FVP in critically ill patients. Furthermore, we examined if the measured FVP could predict IAH and if patients with an increased body mass index (BMI) had higher IAP.
Materials and methods
Study design
The WSACS Clinical Trials Working Group (CTWG) initiated a prospective multicenter observational trial comparing IAP measurements via the bladder with FVP. This study was approved by Human Research Ethics Committee of Fremantle Hospital.
Study sites were recruited via the WSACS CTWG and provided with the study protocol. Patient screening and enrolment were initiated after approval by the institutional review board or ethics committee at each study site.
Patient selection
Patients were enrolled into the trial from each study site between January 2009 and February 2010. Patients were included if they were aged at least 18 years, sedated, mechanically ventilated, and had a femoral venous catheter and an indwelling bladder catheter already in place. Patients were excluded if they were unable to tolerate changes in body position (supine position) due to spinal injuries, intracranial hypertension, or any other reason, if they were moribund (death perceived to be imminent or inevitable during the next month) or pregnant, or when intrabladder pressure (IBP) measurements were contraindicated (such as recent bladder surgery or injury). Patients were sedated to a Richmond Agitation and Sedation Scale (RASS) of −5 to ensure that abdominal muscle contractions were absent. Written informed consent or a waiver of informed consent was required as determined by each study site’s ethics committee.
Protocol
Once the patient was enrolled, IAP was measured according to the WSACS consensus recommendations using the standard bladder technique [4]. The IAP was measured through the patient’s indwelling catheter, according to the modified Kron technique using an AbVisor 300 or 611 kit (AbViser 300 kit, Wolfe Tory Medical, Salt Lake City, UT) or a FoleyManometer (Holtech Medical, Charlottenlund, Denmark) [16, 17]. The transducer was zeroed on the mid-axillary line at the level of the superior iliac crest. After 20 ml of normal saline (or 10 ml of urine with FoleyManometer) was injected through the indwelling urine catheter the IAP was measured at end-expiration in millimeters of Hg. The pressure transducer was connected to the electronic monitoring equipment available in the ICU of each study site.
After the IAP measurement, FVP measurements were obtained using a mid-chest reference point (phlebostatic axis) defined as 5 cm caudal to the sternal notch in the mid-axillary line [18, 19]. After zeroing, the pressure was measured approximately 30 s later at end-expiration in millimeters of Hg.
Data collection
Data on patient demographics, including weight, height, BMI, age, gender, ICU admission diagnosis, co-morbidities, the type (number of lumens) of central venous catheter used, and the venous access site (left or right femoral) of insertion, were collected. Severity of illness during the 24-h period before the first IAP measurement was documented through calculation of the Acute Physiology and Chronic Health Evaluation (APACHE) score, Simplified Acute Physiology score (SAPS), and Sequential Organ Failure Assessment (SOFA) scores. With each set of measurements, IAP, FVP, temperature, mean arterial pressure, Richmond Agitation Sedation score (RASS) score, and positive end-expiratory pressure were recorded. Pressures were measured in each patient three times a day at least 2 h apart for 48 h to reduce the potential for confounding that would be introduced by a change in clinical status. At the conclusion of the study, data on patient survival to intensive care discharge were collected.
IAH was defined by a sustained or repeated pathological elevation in IAP ≥ 12 mmHg, whereas ACS was defined as a sustained IAP > 20 mmHg that is associated with new organ dysfunction/failure [5].
All data were entered onto a case report form and subsequently entered into a Microsoft Access database especially designed for the study.
Statistical analysis
Statistical analysis was performed using Medcalc (Medcalc version 9.3.5.0, Mariakerke, Belgium) and SPSS for Windows (version 17.0). Data are presented as proportions or mean ± SD. Paired t tests were used to test for statistical significance between IAP and FVP measures on the same patients at the same times. General linear modelling (GLM) for repeated measures was used to test whether differences between IAP and FVP measures on the same patients changed significantly over time. A significance level of P < 0.05 was used throughout. For assessing agreement between the two methods of measurement of IAP we used Bland–Altman plots [20]. The ability of FVP to predict IAH was gauged from identification of those at high risk (discrimination) as assessed from the area under the receiver operating characteristic (ROC) curve (AUC) with discrimination considered perfect if AUC = 1, good if AUC > 0.8, moderate if 0.6–0.8, poor if less than 0.6, and no better than chance if AUC = 0.5 [21]. The Youden Index [22] was used to define the “optimal” threshold value of FVP for which (sensitivity + specificity − 1) was maximized [23].
Results
A total of 149 patients were enrolled from 8 sites (see Table 1 in the ESM) with a total of 866 paired IAP/FVP measurements. The patients’ demographics and severity of illness are presented in Table 1. Predominantly medical patients (57%) were included in the study with similar proportions enrolled from surgical (21%) and trauma patients (20%) but only 2% being burns patients. IAH was present at one or more times during observation in 87 patients (58%), whereas ACS defined as an IAP > 20 mmHg with new organ failure was seen in only 11 patients (7.4%) [5]. The overall mortality was 24.8% (37/149) of which 26 (70.3%) had IAH and 4 (10.8%) had ACS. Mortality rates in patients with IAH versus non-IAH (excluding those with ACS) were 29.9% (26/87; 95% CI 20.8–40.8%) and 13.7% (7/51; 95% CI 6.2–26.9%), respectively, P = 0.039. Mortality in patients with ACS was 36.4% (4/11; 95% CI 12.4–68.4%) and without ACS 23.9% (33/138; 95% CI 17.2–32.1%), P = 0.47.
Nine out of 149 (6.0%) patients did not have IAP measurements on day 2 due to removal of urinary catheter (2), withdrawal of therapy (4), and death (3) but all patients managed a full set of measurements on day 1.
IBP and FVP comparison
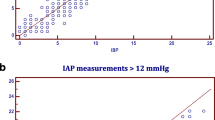
The bias, precision, limits of agreement (LOA), Spearman’s rank correlation coefficient, and coefficient of determination (R 2) for all measurements at different time intervals are shown in Table 2 in the ESM. GLM for repeated measures showed that the difference between IAP and FVP did not change significantly over time (Huyn–Feldt test for within-subjects effects P = 0.55; Bonferroni-corrected pairwise comparisons, all P = 1.000). Therefore we pooled the results for all 866 pairs. The pooled mean IAP was 11.2 ± 4.5 mmHg versus 12.7 ± 4.7 mmHg for FVP (P < 0.001) and the bias was −1.5 (LOA −8.7, 5.7) (see Fig. 1). When IAP values were above 20 mmHg, the bias between IAP and FVP was 0.7 with a precision of 2.0 mmHg (lower and upper limits of agreement −3 and 4.6, respectively). When IAP was equal to or above 12 mmHg, the bias was 0.4 with a precision of 3.9 mmHg (LOA −8.1, 7.3). (see Fig. 2).
FVP to predict IAH
Excluding those with ACS, to predict IAH the ROC curve showed an AUC of 0.83 (95% CI 0.81–0.86) with P < 0.001; the best threshold was an FVP = 11.5 mmHg with an 84.8% sensitivity and a 67.0% specificity to detect an IAP ≥ 12 mmHg (see Fig. 3 in the ESM). To predict a grade 3 IAH, the ROC curve showed an AUC of 0.85 (95% CI 0.79–0.91) with P < 0.001. The best threshold was an FVP of 14.5 mmHg with a 91.3% sensitivity and a 68.1.0% specificity to detect an IAP > 20 mmHg (see Fig. 3).
IBP versus BMI
The mean IAP in patients with a BMI < 30 kg/m2 was 10.6 ± 4.0 mmHg versus 13.8 ± 3.8 mmHg in patients with a BMI > 30 kg/m2 (P < 0.001) on the first measurement of day 1 and this was confirmed across the range of measurements on day 1 and day 2 (Tables 3 and 4 in the ESM). The bias and precision for patients with a BMI < 30 kg/m2 were 1.4 and 3.63 mmHg with the lower and upper limits of agreement being −5.7 and 8.5. The bias and precision for patients with a BMI ≥ 30 kg/m2 were 1.9 and 3.75 mmHg with the lower and upper limits of agreement being −9.3 and 5.4 mmHg respectively (see Figs. 1 and 2 in ESM). There was no difference in mortality between the two groups (see Table 2).
Discussion
The main finding of our study was that FVP cannot be used as a surrogate measure for IAP because of large limits of agreement. However, in patients with IBP above 20 mmHg, FVP can offer opportunities in the management of IAH and ACS patients. An FVP of 11.5 mmHg was able to predict IAH.
One of the main problems of the IAP measurement via the bladder is its intermittent nature which varies between 4 and 6 hourly measurements in most ICUs. An alternative is the use of an 18-Fr standard three-way catheter which allows continuous bladder pressure measurement. However, most ICUs would find it unjustifiable to replace the existing Foley catheter by this larger and more expensive 18-Fr catheter. This technique also raises concerns because the issue of draining urine and measuring IAP at the same time is unsolved and once a urine column is formed from the bladder over the Foley into the collection bag a negative suction force can be created causing underestimation of the real IAP.
Until now there were conflicting data in the literature (see Table 3) with regards to the usefulness of FVP as an alternative for IAP measured via the bladder [6]. Markou et al. [24] showed that in mechanically ventilated patients pressures measured in the inferior vena cava reflect IAP measured via the bladder well when bladder pressures exceed 15 mmHg (18.8 ± 0.69 vs. 19.18 ± 0.63 mmHg, P < 0.01) but not when they are below 15 mmHg (14.6 ± 0.58 vs. 11.4 ± 02, P < 0.05). Later, Arfvidsson et al. [13] showed that in morbidly obese patients elevated IAP assessed by urinary bladder pressure (14.5 ± 2.7 mmHg) corresponded well with an increased directly measured ileo-femoral venous pressure (14 ± 3 mmHg) and concluded that raised IAP and the concomitant reduced venous flow found in morbidly obese patients explain why venous congestion including deep vein thrombosis and pulmonary embolism are found in this group of patients.
In animal studies these correlations are less clear. Barnes et al. [25] found that by increasing the IAP in dogs, the increase in IAP was reflected in the FVP. Harman et al. [26] demonstrated that inferior vena cava pressure (IVCP) equaled IAP measured directly via the peritoneum in seven mongrel dogs and that renal blood flow was reduced by 25% when IAPs were increased to 20 mmHg. Lacey et al. [27] found a good correlation between bladder and inferior vena cava pressures in rabbits and a proportional increase in both with rising IAP, unlike gastric, rectal, superior vena cava, and brachial pressures. And finally, Gudmundsson et al. [28] reported a good correlation between inferior vena cava, bladder, and femoral venous pressure in 8 pigs. However, this was questioned by others. Bloomfield et al. [29] could not find a positive correlation between FVP and IAP intraperitoneal in a swine model and concluded that FVP overestimated IAP. Similar findings were published by Ishizaki et al. [30] in 21 dogs where the IVCPs were much higher than the corresponding insufflation pressures and by Jakob et al. [31] in a pig model. Finally, Lee et al. [14] demonstrated good correlation between IBP and IVCP but not between vena cava pressures and IAP measured via the peritoneum.
The WSACS recommends a bias of less than 1 mmHg with a precision of 2 mmHg and/or limits of agreement between −4 and 4 mmHg, for two IAP techniques to be considered interchangeable [32]. Our study is the largest human trial comparing IBP with FVP and although there was good correlation and a reasonable bias, the limits of agreement were too large to consider both techniques equivalent. However, FVP and IBP can be used interchangeably when IBP is above 20 mmHg (bias of 0.7 mmHg with a precision of 2.0 mmHg). Markou et al. [24] found similar results in a subgroup of mechanically ventilated patients (7 in total with IAP above 20 mmHg) with good agreement between IVCP and IBP. This is an important finding as the timing of surgical intervention in patients with impending ACS (grade 2 or 3 IAH) is still subject to debate and a more rapid surgical intervention might translate into an improved outcome. Although the clinical awareness of IAH and ACS has generally improved, many ICU departments do not routinely measure IAP in patients at risk of developing IAH and ACS. Patients with grade 2 or 3 IAH may develop ACS and organ failure earlier then anticipated. We therefore suggest that in patients with IAP above 20 mmHg and thus at risk of developing ACS, FVP could be used as a surrogate and continuous measure of IAP when a femoral line is in place.
An FVP of 11.5 mmHg was able to predict IAH and a threshold of 14.5 mmHg was able to predict ACS. Although the difference in threshold was relatively small (only 3 mmHg), FVP above 14.5 mmHg is an important parameter to consider for the monitoring and management of patients with impending ACS. The potential benefit of continuous IAP measurement in these high-risk patients should be weighed against the risk of a potential catheter-related infection. The use of femoral venous access has been discouraged in the ICU on the basis of some evidence that this site is more prone to infection and increased risk of thromboembolic complications [33]. This is why femoral venous access will mainly be used in emergency situations. However, this has been challenged by others [34–37].
This study also confirms that patients with a higher BMI have higher IAP which is consistent with previous publications (see Table 2 in the ESM). This is important when interpreting IAP in the obese patient. A previous multicenter study also identified BMI as an independent predictor for IAH. Femoral venous catheterization should not be encouraged, however, as a recent study showed an increased risk of catheter-related infection in this particular group of patients [37].
The main limitation in our trial is that measuring IAP via the bladder or via FVP are both indirect methods. Comparing two different indirect techniques might explain the moderate bias and precision found in our study. The fact that this study was a multicenter observational trial might also have contributed to errors. The strict adherence to the protocol could not be guaranteed. Indeed, there were large differences between the level of agreement between some of the study centers. But overall, adjustments for study site did not alter the study’s conclusions.
Another limitation of the trial is that although the bias and precision in patients with high IAP are within the current guidelines to consider both techniques as equivalent, it only represented a small group of patients. Further measurements of potential influencing factors such as the measurement of intrathoracic pressures (e.g., COPD) or right heart function (e.g., tricuspid incompetence) might have helped explain the differences found between the FVP and the bladder pressure.
Conclusion
Currently, FVP cannot be recommended as a surrogate measure for IAP measurements via the bladder. However, when the IAP is above 20 mmHg, FVP can be used to measure IAP continuously. As the incidence of catheter-related sepsis for femoral catherization is relatively low, especially when used for a relatively short period (i.e., less than 5 days), we encourage the use of femoral venous catheters in patients at risk of IAH of grade 3 and 4. The use of FVP as a continuous measure of IAP is important in the prevention of early ACS.
FVP monitoring might also be useful to predict IAH and therefore can be used as a screening tool for IAH. Further studies are warranted to confirm these findings.
References
Malbrain ML, Chiumello D, Pelosi P, Wilmer A, Brienza N, Malcangi V, Bihari D, Innes R, Cohen J, Singer P, Japiassu A, Kurtop E, De Keulenaer BL, Daelemans R, Del Turco M, Cosimini P, Ranieri M, Jacquet L, Laterre PF, Gattinoni L, Malbrain MLNG, Chiumello D, Pelosi P, Wilmer A, Brienza N, Malcangi V, Bihari D, Innes R, Cohen J, Singer P, Japiassu A, Kurtop E, De Keulenaer BL, Daelemans R, Del Turco M, Ranieri M, Jacquet L, Laterre P-F, Gattinoni L (2004) Prevalence of intra-abdominal hypertension in critically ill patients: a multicentre epidemiological study. Intensive Care Med 30:822–829
Malbrain ML, Chiumello D, Pelosi P, Bihari D, Innes R, Ranieri VM, Del Turco M, Wilmer A, Brienza N, Malcangi V, Cohen J, Japiassu A, De Keulenaer BL, Daelemans R, Jacquet L, Laterre PF, Frank G, de Souza P, Cesana B, Gattinoni L, Malbrain MLNG, Chiumello D, Pelosi P, Bihari D, Innes R, Ranieri VM, Del Turco M, Wilmer A, Brienza N, Malcangi V, Cohen J, Japiassu A, De Keulenaer BL, Daelemans R, Jacquet L, Laterre P-F, Frank G, de Souza P, Cesana B, Gattinoni L (2005) Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: a multiple-center epidemiological study. Crit Care Med 33:315–322
Cheatham ML, Safcsak K, Cheatham ML, Safcsak K (2010) Is the evolving management of intra-abdominal hypertension and abdominal compartment syndrome improving survival? Crit Care Med 38:402–407
Cheatham ML, Malbrain ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, Balogh Z, Leppaniemi A, Olvera C, Ivatury R, D’Amours S, Wendon J, Hillman K, Wilmer A (2007) Results from the international conference of experts on intra-abdominal hypertension and abdominal compartment syndrome. II. recommendations. Intensive Care Med 33:951–962
Malbrain ML, Cheatham ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, Balogh Z, Leppaniemi A, Olvera C, Ivatury R, D’Amours S, Wendon J, Hillman K, Johansson K, Kolkman K, Wilmer A, Malbrain MLNG, Cheatham ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, Balogh Z, Leppaniemi A, Olvera C, Ivatury R, D’Amours S, Wendon J, Hillman K, Johansson K, Kolkman K, Wilmer A (2006) Results from the international conference of experts on intra-abdominal hypertension and abdominal compartment syndrome. I. definitions. Intensive Care Med 32:1722–1732
Malbrain ML (2004) Different techniques to measure intra-abdominal pressure (IAP): time for a critical re-appraisal. Intensive Care Med 30:357–371
Balogh Z, De Waele JJ, Malbrain ML, Malbrain MLNG (2007) Continuous intra-abdominal pressure monitoring. Acta Clin Belg 62(Suppl):26–32
De Waele JJ, De laet I, Malbrain ML, Malbrain MLNG (2007) Rational intraabdominal pressure monitoring: how to do it? Acta Clin Belg 62(Suppl):16–25
De Keulenaer BL, De Waele JJ, Powell B, Malbrain ML, Malbrain MLNG (2009) What is normal intra-abdominal pressure and how is it affected by positioning, body mass and positive end-expiratory pressure? Intensive Care Med 35:969–976
Balogh Z, Jones F, D’Amours S, Parr M, Sugrue M (2004) Continuous intra-abdominal pressure measurement technique. Am J Surg 188:679–684
Williams JF, Seneff MG, Friedman BC, McGrath BJ, Gregg R, Sunner J, Zimmerman JE (1991) Use of femoral venous catheters in critically ill adults: prospective study. Crit Care Med 19:550–553
Regli A, De Keulenaer BL, Hockings LE, Musk GC, Roberts B, van Heerden PV (2011) The role of femoral venous pressure and femoral venous oxygen saturation in the setting of intra-abdominal hypertension—a pig model. Shock 35:422–427
Arfvidsson B, Eklof B, Balfour J (2005) Iliofemoral venous pressure correlates with intraabdominal pressure in morbidly obese patients. Vasc Endovascular Surg 39:505–509
Lee S, Anderson J, Kraut E, Wisner D, Wolfe B (2002) A simplified approach to the diagnosis of elevated intra-abdominal pressure. J Trauma 52:1169–1172
Gudmundsson FF, Viste A, Gislason H, Svanes K (2002) Comparison of different methods for measuring intra-abdominal pressure. Intensive Care Med 28:509–514
Kron IL, Harman PK, Nolan SP (1984) The measurement of intra-abdominal pressure as a criterion for abdominal re-exploration. Ann Surg 199:28–30
Malbrain ML, De laet I, Viaene D, Schoonheydt K, Dits H, Malbrain MLNG, De laet I, Viaene D, Schoonheydt K, Dits H (2008) In vitro validation of a novel method for continuous intra-abdominal pressure monitoring. Intensive Care Med 34:740–745
Magder S, Magder S (2006) Central venous pressure monitoring. Curr Opin Crit Care 12:219–227
Ball C, Westhorpe RN (2009) Central venous pressure monitoring. Anaesth Intensive Care 37:689
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Guzder RN, Gatling W, Mullee MA, Mehta RL, Byrne CD (2005) Prognostic value of the Framingham cardiovascular risk equation and the UKPDS risk engine for coronary heart disease in newly diagnosed type 2 diabetes: results from a United Kingdom study. Diabet Med 22:554–562
Youden WJ (1950) Index for rating diagnostic tests. Cancer 3:32–35
Greiner M, Pfeiffer D, Smith RD (2000) Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med 45:23–41
Markou N, Grigorakos L, Myrianthefs P, Boutzouka E, Rizos M, Evagelopoulou P, Apostolakos H, Baltopoulos G (2004) Venous pressure measurements in the superior and inferior vena cava: the influence of intra-abdominal pressure. Hepatogastroenterology 51:51–55
Barnes GE, Laine GA, Giam PY, Smith EE, Granger HJ (1985) Cardiovascular responses to elevation of intra-abdominal hydrostatic pressure. Am J Physiol 248:R208–R213
Harman PK, Kron IL, McLachlan HD, Freedlender AE, Nolan SP (1982) Elevated intra-abdominal pressure and renal function. Ann Surg 196:594–597
Lacey SR, Bruce J, Brooks SP, Griswald J, Ferguson W, Allen JE, Jewett TC Jr, Karp MP, Cooney DR (1987) The relative merits of various methods of indirect measurement of intraabdominal pressure as a guide to closure of abdominal wall defects. J Pediatr Surg 22:1207–1211
Gudmundsson FF, Viste A, Gislason H, Svanes K (2002) Comparison of different methods for measuring intra-abdominal pressure. Intensive Care Med 28:509–514
Bloomfield G, Saggi B, Blocher C, Sugerman H (1999) Physiologic effects of externally applied continuous negative abdominal pressure for intra-abdominal hypertension. J Trauma 46:1009–1014; discussion 1014–1006
Ishizaki Y, Bandai Y, Shimomura K, Abe H, Ohtomo Y, Idezuki Y (1993) Safe intraabdominal pressure of carbon dioxide pneumoperitoneum during laparoscopic surgery. Surgery 114:549–554
Jakob SMKR, Tenhunen JJ, Pradl R, Takala J (2010) Increasing abdominal pressure with and without PEEP: effects on intra-peritoneal, intra-organ and intra-vascular pressures. BMC Gastroenterol 10:70
De Waele JJ, Cheatham ML, Malbrain ML, Kirkpatrick AW, Sugrue M, Balogh Z, Ivatury R, De Keulenaer B, Kimball EJ, Malbrain MLNG (2009) Recommendations for research from the international conference of experts on intra-abdominal hypertension and abdominal compartment syndrome. Acta Clin Belg 64:203–209
Merrer J, De Jonghe B, Golliot F, Lefrant JY, Raffy B, Barre E, Rigaud JP, Casciani D, Misset B, Bosquet C, Outin H, Brun-Buisson C, Nitenberg G, French Catheter Study Group in Intensive Care (2001) Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA 286:700–707
Deshpande KS, Hatem C, Ulrich HL, Currie BP, Aldrich TK, Bryan-Brown CW, Kvetan V, Deshpande KS, Hatem C, Ulrich HL, Currie BP, Aldrich TK, Bryan-Brown CW, Kvetan V (2005) The incidence of infectious complications of central venous catheters at the subclavian, internal jugular, and femoral sites in an intensive care unit population. Crit Care Med 33:13–20; discussion 234–235
Lorente L, Henry C, Martin MM, Jimenez A, Mora ML, Lorente L, Henry C, Martin MM, Jimenez A, Mora ML (2005) Central venous catheter-related infection in a prospective and observational study of 2, 595 catheters. Crit Care 9:R631–R635
Durbec O, Viviand X, Potie F, Vialet R, Albanese J, Martin C (1997) A prospective evaluation of the use of femoral venous catheters in critically ill adults. Crit Care Med 25:1986–1989
Parienti JJ, Thirion M, Megarbane B, Souweine B, Ouchikhe A, Polito A, Forel JM, Marque S, Misset B, Airapetian N, Daurel C, Mira JP, Ramakers M, du Cheyron D, Le Coutour X, Daubin C, Charbonneau P, Members of the Cathedia Study G, Parienti J-J, Thirion M, Megarbane B, Souweine B, Ouchikhe A, Polito A, Forel J-M, Marque S, Misset B, Airapetian N, Daurel C, Mira J-P, Ramakers M, du Cheyron D, Le Coutour X, Daubin C, Charbonneau P (2008) Femoral vs jugular venous catheterization and risk of nosocomial events in adults requiring acute renal replacement therapy: a randomized controlled trial. JAMA 299:2413–2422
Richardson JD, Trinkle JK (1976) Hemodynamic and respiratory alterations with increased intra-abdominal pressure. J Surg Res 20:401–404
Acknowledgments
To standardize the IAP measurement technique, Wolfe-Tory provided the AbViser bladder monitoring kits to study sites that did not currently use this device, free of charge.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
De Keulenaer, B.L., Regli, A., Dabrowski, W. et al. Does femoral venous pressure measurement correlate well with intrabladder pressure measurement? A multicenter observational trial. Intensive Care Med 37, 1620–1627 (2011). https://doi.org/10.1007/s00134-011-2298-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2298-x