Abstract
Purpose
The aim of this experimental study is to investigate cardiovascular tolerance of blockade of beta-adrenergic receptors in an endotoxin model.
Design
Prospective, randomized, controlled study.
Setting
Animal laboratory in a university medical center.
Methods
Ten anesthetized, mechanically ventilated pigs were challenged with intravenous lipopolysaccharide (LPS) to achieve a status of profound hypodynamic shock. Systemic and pulmonary hemodynamics and cardiac output were continuously monitored throughout the 5-h study period, and blood samples were taken at baseline (T − 30 min), 1 h from the beginning of LPS infusion (T + 60 min), and every 2 h (T + 180 min and T + 300 min). Animals were randomly assigned to continuous intravenous esmolol infusion titrated to decrease heart rate by 20% or isotonic saline.
Results
Esmolol decreased heart rate by 20%, while in the saline group, heart rate increased by 7% and 22% at T + 180 min and T + 300 min, respectively (p < 0.001). In esmolol-treated animals, cardiac index decreased by 9% at T + 180 min and by 2% at T + 300 min, and in controls by 14% at T + 180 min and by 27% at T + 300 min (p = 0.870). In esmolol-treated animals, median (interquartile range, IQR) stroke index was 31 (6) and 47 (11) ml/min/m2 at T + 180 min and T + 300 min, respectively, and decreased steadily from 45 (20) to 18 (13) ml/min/m2 in controls (p = 0.030). There were no significant differences between groups for any other hemodynamics variables, except for systemic vascular resistance (SVR) (p = 0.017).
Conclusions
In large animals with endotoxemic shock, continuous infusion of esmolol, a selective beta-1 adrenergic blocker, titrated to decrease heart rate by 20%, was well tolerated and may offset LPS-induced cardiac dysfunction by a preload positive effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mechanisms of cardiovascular failure in septic shock remain unclear [1]. Proinflammatory mediators that are abundantly released in blood and tissues may decrease vessel tone, responsiveness to catecholamines [1, 2], and myocardial contractility [2–4]. In parallel, the counterregulatory neurohormonal response includes a rapid and important release of cortisol and catecholamines in blood and tissues [5]. In both septic animals [6] and patients [7], loss of cardiovascular variability precedes hypotension and multiple organ dysfunction. These findings mimic those observed in heart failure [8] and suggest that autonomic control of vessel tone and cardiac function may no longer be able to adjust the cardiovascular response to the intensity of the inflammatory stress, thereby contributing to the pathophysiology of septic shock. The underlying mechanisms may include neuronal apoptosis within autonomic nuclei in the brain stem [9]. Sustained systemic adrenergic activation may also have direct detrimental cardiac effects, particularly via the β1-adrenergic pathway [10]. Harmful effects included fibroblast hyperplasia, myocyte necrosis and apoptosis, and increased risk of arrhythmia [8]. Theoretically, modulating the adrenergic system may be a new approach in the treatment of sepsis [11, 12]. In high-risk surgical patients with ischemic heart disease, β-blocking drugs improved myocardial oxygen balance and function [13], and may improve survival [14, 15]. In severely burned children, oral propranolol improved peripheral metabolism, skeletal muscle energetics, and clinical outcome [16]. In rats with peritonitis, β1 blockade decreased circulating levels [17] and hepatic and cardiac expression [18] of proinflammatory cytokines, and improved cardiac function and hemodynamics [19]. While these preliminary findings on small animals are promising, the risk of severe bradycardia and cardiovascular collapse following infusion of a beta-blocking drug has not been investigated so far.
Therefore, we evaluated hemodynamic tolerance of continuous infusion of a selective β1 blocker in endotoxemic pigs.
Method
Preparation of animals
The study was approved by the Institutional Review Board for Animal Research and Care, and handling of the animals was in accordance with National Institutes of Health guidelines. Ten female piglets weighing 22–27 kg were anesthetized by intramuscular injection of 2.5 mg/kg body weight ketamine (Ketalar; Parke-Davis, Courbevoie, France), followed by sodium pentobarbital (10 mg/kg body weight) and cisatracurium, and maintained anesthetized by continuous intravenous infusion of midazolam (0.5 mg/kg/min) (Hypnovel; Produits Roche, Neuilly sur Seine, France) and cisatracurium (0.5 mg/kg/min) (Nimbex; GlaxoSmithKline, Brentford, Middlesex, UK). The animals were intubated using a cuffed tube and mechanically ventilated (Evita 2 Dura; Luebeck, Germany). After dissection of neck vessels, a catheter was inserted into the left carotid artery and a pulmonary artery catheter was inserted via the right external jugular vein (Swan-Ganz continuous cardiac output, mixed venous oxygen saturation monitoring; Edwards Lifesciences, Irvine, CA, USA). An esophageal temperature probe allowed continuous monitoring of body temperature, which was kept at 38°C by use of heat lamps suspended above the operating table. Heart rate and systemic and pulmonary arterial pressures were continuously monitored (7758B cardiac monitor; Hewlett Packard, Palo Alto, CA, USA) as well as cardiac output and SvO2 (Vigilance monitor; Baxter Edwards, Irvine, CA, USA) [20].
Hemodynamic and oxygenation parameters
Heart rate, and systemic and pulmonary systolic (SAP and PASP), diastolic (DBP and PADP), and mean (MBP and PAMP) arterial pressures (mmHg) were recorded at 0.2 Hz. Cardiac output (CO, L/min) and mixed venous oxygen saturation (SvO2, %) (Baxter 130 H 7.5F; Baxter Edwards Critical Care, Irvine, CA) were recorded at 0.5 Hz. Using standard formulas, we computed cardiac index (CI, L/min/m2), stroke index (SI, ml/min/m2), cardiac power index (CPI) [21], systemic vascular resistance (SVR) and pulmonary vascular resistance (PVR) (dyne s/cm5), and left (LVSW) and right (RVSW) ventricular stroke work (g m/m2). Body surface area was 0.6 ± 0.03 m2, as calculated by Kelley’s formula [22]. Arterial and venous blood gas tensions and hemoglobin saturation were measured in an acid–base and co-oxymeter analyzer at 37°C (ABL-520; Radiometer, Copenhagen, Denmark). After the recording, before data analysis, all signals were synchronized in relation to the different experimental phases.
Experimental protocol
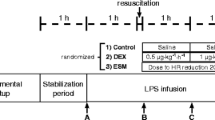
Figure 1 summarizes the experimental procedure. Briefly, after a 30-min period of stabilization (T − 30 min), animals received 30-min intravenous infusion of 150 μg/kg/min Escherichia coli lipopolysaccharide (LPS, serotype 055:B5; Sigma Chemical Co., St. Louis, MO, USA), diluted in 50 ml sterile isotonic saline [20]. Thirty minutes after the end of LPS infusion (T + 60 min), pigs were randomized to receive continuous infusion of isotonic saline solution or esmolol. Esmolol was titrated to decrease heart rate by 20% in keeping with previous experiments in small animals showing favorable hemodynamic and immune effects [18, 19]. In both groups, animals were infused intravenously with 16 ml/kg/h isotonic saline throughout the 5-h study period. None of the animals received vasopressors or inotropic drugs. Arterial and venous blood was sampled at baseline (T − 30 min), 1 h from the beginning of LPS infusion (T + 60 min), and every 2 h (T + 180 min and T + 300 min).
Study design. The first A-VBG was sampled after 30 min of stabilization. Then, animals received 30-min intravenous infusion of 150 μg/kg/min LPS. After 30 min (post LPS phase), at T + 60 min, animals were randomized to receive esmolol or isotonic saline (IS). Before beginning the infusion, the second A-VBG was sampled. At T + 180 min and T + 300 min, i.e., 2 and 4 h after beginning treatment with beta blockers or IS, the third and fourth A-VBG were sampled. Throughout the duration of the experiment, HR, BP, and PBP were recorded at 0.2 Hz, and cardiac output and SvO2 at 0.5 Hz. HR heart rate, CI cardiac index, SvO 2 mixed venous oxygen saturation, PBP pulmonary blood pressure, BP blood pressure, A-VBG arteriovenous blood gas, LPS Escherichia coli lipopolysaccharide, IS isotonic saline
Data analysis
The analyses were mainly exploratory, the aim being to try to explain and describe variability in the data. Formal sample size calculation was difficult to perform because of the longitudinal design of the study. Indeed, in this case we need several pieces of a priori information, such as the correlation between measurements as well as their variances at each time in addition to the expected effect size, which are often not easy to obtain precisely. However, the sample size was consistent with previous studies [20]. Median (interquartile range, IQR) is reported for all variables. Wilcoxon’s rank-sum test was used for comparison of measurements between two different times. Changes in the measured variables over time and across treatment groups were examined using linear mixed models [23] to account for correlation between measurements from the same animal. Effects of time and treatment as well as their interaction were included and tested as appropriate (using the Kenward and Roger adjustment for degrees-of-freedom calculation [24]) in the model along with corresponding baseline measurements. Model selection was performed using the Akaike information criterion and Schwarz Bayesian criterion [25, 26]. As data were not normal, all measurements were transformed by calculating the natural logarithm. Statistical analyses were performed using SAS 9.1 statistical software (SAS Institute, Cary, NC, USA). p-Values <0.05 were considered to be statistically significant. The statistician remained blinded to the interventions.
Results
Effects of LPS
During the 30-min period of LPS infusion, heart rate, systemic mean arterial pressure, systemic vascular resistance, and pulmonary mean arterial pressure increased steadily [HR: on average +8% (p = 0.065); MBP: on average +14% (p = 0.002); SVR: on average +37% (p = 0.002); PAMP: on average >+100% (p = 0.002)] (Table 1, see also Supplementary Fig. S1). Simultaneously, cardiac index and stroke index decreased by on average 8% (p = 0.037) and 18% (p = 0.006), respectively.
Following LPS (T + 60 min), HR continued to increase up to 130 (39) bpm, corresponding to an increase of 15% in comparison with the previous 30-min period and of 24% in comparison with baseline (Table 1 see also Supplementary Fig. S1). Systemic MAP and SVR decreased significantly, by 15% (p = 0.008) and 7% (p = 0.004), respectively. Similarly, PAMP increased by 25% (p = 0.008). Cardiac index and stroke index remained almost unchanged [CI: 4.3 (1.0) versus 4.3 (1.1) L/min/m2, p = 0.57; SI: 34 (14) versus 29 (20) ml/min/m2, p = 0.426].
As compared with baseline, at T + 60 min, SvO2 and DO2 decreased by on average 9% (p = 0.004) and 30% (p = 0.002), respectively. Meanwhile, avDO2 and OER (oxygen extraction ratio) increased by on average 37% (p = 0.010) and 15% (p = 0.047), respectively (Table 1, see also Supplementary Fig. S2). PaO2/FiO2 ratio and thoracopulmonary compliance decreased significantly by on average 11% (p = 0.014) and 17% (p = 0.027), respectively. Airway resistance increased by +7% (p = 0.014).
Effects of esmolol
The median (IQR) dose of esmolol and the median time required to achieve a 20% decrease in heart rate were 347 (129) µg/kg/min and 30 (34) min, respectively. Comparisons between esmolol-treated and esmolol-free animals are presented in Table 2; Fig. 2 (see also Supplementary Figs. S3 and S4).
Hemodynamic effects of esmolol infusion in LPS model. Relative variations (mean and SEM) in hemodynamics parameters are given from the beginning of the treatment, i.e., T + 60 min, (esmolol or SI) to the end of the experimentation (4 h of recording). Linear mixed model analyses. Each point represents the mean value of the 5 pigs for the different hemodynamic parameters and the two groups (LPS and LPS-BB). Each hemodynamic signal was acquired at frequency of 0.2 Hz. One point represents the mean value of 10 min recording. In the LPS BB group, the HR was decreased by 20% as expected. This level was maintained during all the experimentation. This HR decrease was not associated with a cardiac index decrease. HR heart rate, CI cardiac index, SI stroke index, MBP mean blood pressure, SVR systemic vascular resistance, BB beta-blockers
In esmolol-treated animals, heart rate was kept constant over time at 20% below basal values. In the saline group, HR increased by 7% and 22%, respectively, at T + 180 min and T + 300 min (group difference: p < 0.001). In esmolol-treated animals, cardiac index decreased by 9% at T + 180 min and by 2% at T + 300 min. In controls, it decreased by 14% at T + 180 min and by 27% at T + 300 min (Table 2; group difference: p = 0.870). By contrast, in esmolol-treated animals, stroke index was 31 (6) and 47 (11) ml/min/m2 at T + 180 min and T + 300 min, respectively, and decreased in controls from 45 (20) to 18 (13) ml/min/m2 (group difference: p = 0.030). There were no significant difference between esmolol-treated and esmolol-free septic pigs for any other variables, except for SVR (group difference: p = 0.017) (Table 2; Fig. 2).
Discussion
This was the first time that intravenous β1-blockers were investigated in septic large animals. This study demonstrated that this treatment was well tolerated in terms of cardiovascular functions.
Pigs were chosen as a clinically relevant species, resembling humans in various functions as assessed by cardiovascular, respiratory, and biochemical parameters [27]. We deliberately investigated the cardiovascular tolerance of esmolol in the worst hemodynamic conditions associated with sepsis, to maximize the potential detrimental effects of slowing heart rate with β1-blockade. First, this porcine model of endotoxin shock was characterized, as previously reported [20], by hypotension, vasodilation, and decreased cardiac output, despite administration of 16 ml/kg/h fluid resuscitation. Second, animals were kept free of inotropes and vasopressor therapy. Third, we sedated the animals with sodium pentothal, which is known to have major cardiodepressive effects.
We selected esmolol as it is an ultrashort-acting β1-blocker to be administered intravenously. At therapeutic doses, it has no intrinsic sympathomimetic activity or membrane-stabilizing effect. Its half-life of distribution is very fast (approximately 2 min) [28]. These pharmacokinetic properties allowed gentle titration and rapid resolution of any potential negative effects after stopping treatment. The dose of esmolol was selected in individual animals to achieve a 20% decrease in heart rate, as previous experiments in septic rats have shown substantial favorable effects on cardiac and immune functions [18]. A 20% decrease in heat rate was also how propranolol was titrated to show benefit in severely burned children [16].
Esmolol infusion did not induce cardiovascular collapse in any of the septic animals. This treatment has no relevant effect on cardiac index or systemic arterial pressure, and the lower systemic vascular resistance observed at the end of the experiment depicted the profound preterminal vasoconstriction in controls. The prevention by esmolol of detrimental increase in systemic vascular resistance may further contribute to improved cardiac work during endotoxemia. The observed lack of esmolol-related alteration in systemic and pulmonary hemodynamic and in indices of tissue oxygenation is in agreement with findings reported in small animals [18, 19]. The improvement in stroke index following esmolol infusion argued in favor of cardioprotective effects of β-1 blockade in large animals with sepsis. These findings also support the hypothesis of a positive preload effect induced by heart rate reduction. Our findings are in line with those previously obtained in a sublethal endotoxemic model in dogs with propranolol, a nonselective beta-blocking agent [29]. In that model, infusion of propranolol 1 h after endotoxemia prevented the second phase of hypotension and improved animals’ survival. While these preclinical findings are promising, much additional work is needed to characterize the risk–benefit profile of this approach. Indeed, we have learned from the POISE trial that preliminary promising effects of beta-blockers in the perioperative setting [14, 15] may not translate into survival benefit [30].
In conclusion, in large animals with endotoxemic shock, selective β-1 blockade is well tolerated and offsets sepsis-induced cardiac dysfunction, confirming findings reported in small animals.
References
Annane D, Bellissant E, Cavaillon JM (2005) Septic shock. Lancet 365:63–78
Parrillo JE, Parker MM, Natanson C, Suffredini AF, Danner RL, Cunnion RE, Ognibene FP (1990) Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med 113:227–242
Gulick T, Chung MK, Pieper SJ (1989) Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte beta-adrenergic responsiveness. Proc Natl Acad Sci USA 86:6753–6757
Kumar A, Thota V, Dee L, Olson J, Uretz E, Parrillo JE (1996) Tumor necrosis factor alpha and interleukin 1 beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med 183:949–958
Benedict CR, Grahame-Smith DG (1978) Plasma noradrenaline and adrenaline concentrations and dopamine beta hydroxylase activity in patients with shock due to septicemia, trauma, and heamorrhage. Q J Med 185:1–20
Goldstein B, Kempski MH, Stair D, Tipton RB, De King D, De Long DJ, De Asla R, Cox C, Lund N, Woolf PD (1995) Autonomic modulation of heart rate variability during endotoxin shock in rabbits. Crit Care Med 23:1694–1702
Annane D, Trabold F, Sharshar T, Jarrin I, Blanc AS, Raphael JC, Gajdos P (1999) Inappropriate sympathetic activation at onset of septic shock: a spectral analysis approach. Am J Respir Crit Care Med 160:458–465
Mann DL, Bristow MR (2005) Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation 11:2837–2849
Sharshar T, Gray F, Lorin de la Grandmaison G, Hopkinson NS, Ross E, Dorandeu A, Orlikowski D, Raphael JC, Gajdos P, Annane D (2003) Apoptosis of neurons in cardiovascular autonomic centres triggered by inducible nitric oxide synthase after death from septic shock. Lancet 362:1799–1805
Bristow MR, Feldman AM, Adams KF Jr, Goldstein S (2003) Selective versus nonselective beta-blockade for heart failure therapy: are there lessons to be learned from the COMET trial? J Card Fail 9:444–453
de Montmollin E, Aboab J, Mansart A, Annane D (2009) Bench-to-bedside review: beta-adrenergic modulation in sepsis. Crit Care 13:230
Werdan K, Schmidt H, Ebelt H, Zorn-Pauly K, Koidl B, Hoke RS, Heinroth K, Müller-Werdan U (2009) Impaired regulation of cardiac function in sepsis, SIRS, MODS. Can J Physiol Pharmacol 87:266–274
Zaugg M, Schaub MC, Pasch T (2002) Modulation of beta-adrenergic receptor subtype activities in perioperative medicine: mechanisms and sites of action. Br J Anaesth 88:101–123
Mangano DT, Layug EL, Wallace A, Tateo I (1996) Effect of atenolol on mortality and cardiovascular morbidity after non cardiac surgery. N Engl J Med 335:1713–1720
Poldermans D, Boersma E, Bax JJ (1999) The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. N Engl J Med 341:1789–1794
Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR (2001) Reversal of catabolism by beta-blockade after severe burns. N Engl J Med 345:1223–1229
Gore DC, Wolfe RR (2006) Hemodynamic and metabolic effects of selective beta1 adrenergic blockade during sepsis. Surgery 139:686–694
Suzuki T, Morisaki H, Serita R, Yamamoto M, Kotate Y, Ishizaka A, Takeda J (2005) Infusion of the beta-adrenergic blocker esmolol attenuates myocardial dysfunction in septic rats. Crit Care Med 33:2294–2301
Ackland GL, Yao ST, Rudiger A, Dyson A, Stidwill R, Poputnikov D, Singer M, Gourine AV (2010) Cardioprotection, attenuated systemic inflammation, and survival benefit of beta1-adrenoceptor blockade in severe sepsis in rats. Crit Care Med 38:388–394
Jourdain M, Carrette O, Tournoys A, Fourrier F, Mizon C, Mangalaboyi J, Goudemand J, Mizon J, Chopin C (1997) Effects of inter-alpha-inhibitor in experimental endotoxic shock and disseminated intravascular coagulation. Am J Respir Crit Care Med 156:1825–1833
Fincke R, Hochman JS, Lowe AM, Menon V, Slater JN, Webb JG, Le Jemtel TH, Cotter G, Shock Investigators (2004) Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the shock trial registry. J Am Coll Cardiol 44:340–348
Kelley KW, Curtis SE, Marzan GT, Karara HM, Anderson CR (1973) Body surface area of female swine. J Anim Sci 36:927–930
Verbeke G, Molenberghs G (2000) Linear mixed models for longitudinal data. Springer, New York
Kenward MG, Roger JH (1997) Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53:983–997
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Automat Control AC-19:716–723
Schwarz G (1978) Estimating the dimension of a model. Ann Stat 6:461–464
Schmidt DA, Tumbleson ME (1986) Swine hematology. In: Tumbleson ME (ed) Swine in biomedical research. Plenum, New York, pp 767–782
Volz-Zang C, Eckrich B, Jahn P, Schneidrowski B, Schulte B, Palm D (1994) Esmolol, an ultrashort acting selective beta 1-adrenoreceptor antagonist: pharmacodynamic and pharmacokinetic properties. Eur J Clin Pharmacol 46:399–404
Berk JL, Hagen JF, Beyer WH, Gerber MJ, Dochat GR (1969) The treatment of endotoxin shock by beta adrenergic blockade. Ann Surg 169:74–81
POISE Study Group, Devereaux PJ, Yang H, Yusuf S, Guyatt G, Leslie K, Villar JC, Xavier D, Chrolavicius S, Greenspan L, Pogue J, Pais P, Liu L, Xu S, Málaga G, Avezum A, Chan M, Montori VM, Jacka M, Choi P (2008) Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 371:1839–1847
Acknowledgments
This study was funded by a grant from the Société de Réanimation de Langue Française.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aboab, J., Sebille, V., Jourdain, M. et al. Effects of esmolol on systemic and pulmonary hemodynamics and on oxygenation in pigs with hypodynamic endotoxin shock. Intensive Care Med 37, 1344–1351 (2011). https://doi.org/10.1007/s00134-011-2236-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2236-y