Abstract
Objective
Systemic inflammatory response syndrome (SIRS) and sepsis remain the leading cause of death in the critically ill. A reduction in the antioxidant capacity, including selenoenzymes that are dependent on selenium (Se), could be a contributing factor. Se supplementation in septic patients have yielded conflicting results. We hypothesized that a high-dose Se supplementation would (1) improve markers of inflammation, nutrition and antioxidant defence, and (2) decrease mortality.
Methods
This prospective, randomized, open-label, single-centre clinical trial included 150 patients with SIRS/sepsis and a SOFA score of >5. Patients in the Se+ group (n = 75) received Se for 14 days (1,000 μg on day 1,500 μg/day on days 2–14). Patients in both the control (Se−) group (n = 75) and the Se+ group received a standard Se dose (<75 μg/day). Plasma Se, whole-blood glutathione peroxidase (GPx) activity, C-reactive protein (CRP), procalcitonin (PCT), albumin, prealbumin and cholesterol levels, along with APACHE II and SOFA scores, were determined at baseline and on days 1–7 and day 14. Mortality was assessed at day 28.
Results
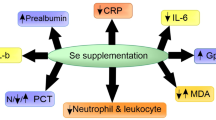
Plasma Se and GPx activity were increased in the Se+ group from day 1 onwards. Negative correlations were demonstrated between plasma Se, CRP (P = 0.035), PCT (P = 0.022) and SOFA (P = 0.001) at admission but not on days 7 or 14. Prealbumin and cholesterol increased in the Se+ group versus the respective baselines. Mortality was similar between groups, with no gender differences.
Conclusion
High-dose Se substitution in patients with SIRS/sepsis increased plasma Se and GPx levels, but did not reduce mortality. Markers of inflammation were reduced similarly in both groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is the leading cause of death in critically ill patients, with mortality rates approximately 45–55% [1]. Tissue hypoperfusion, oxidative stress and dysregulation of immune responses lead to multiorgan failure (MOF). The exact mechanisms are still poorly understood. Patients with systemic inflammatory response syndrome (SIRS) and sepsis exhibit an early precipitous decrease in plasma selenium (Se) levels by about 40% [2, 3], which correlates with the severity of the disease and mortality [4, 5]. Selenoenzymes, including glutathione peroxidase (GPx), play a major role in the regulation of inflammatory processes, including protection against reactive oxygen species. Therefore, Se substitution in these patients might be effective in restoring antioxidant capacities, thus preventing MOF.
Together with others [3], we have shown previously that the recommended replacement dose of Se (0.4–0.9 μmol, 30–75 μg daily) is inadequate to restore normal plasma Se levels (0.58–1.82 μmol/l, 46–143 μg/l) in patients with sepsis. Despite the supplementation, the observed plasma Se levels remain low (0.28–0.42 μmol/l) [6].
Previous studies of Se administration have yielded conflicting results [7, 8]. Earlier studies suggested promising results in terms of improvement in clinical outcome [9, 10]. In contrast, the most recent clinical trials have shown no effect [11–13] or a borderline positive effect on respective outcome parameters [14]. A recent review concluded that despite some promising results, no definitive answers regarding the effects of Se supplementation on the mortality or morbidity in critically ill patients exist [15]. Administration of Se or selenocompounds could lead to toxicity, most likely related to their prooxidant properties [16]. However, previous studies in septic patients have not indicated adverse effects even with high-dose regimens [12, 14].
In regard to the above-mentioned pathophysiological importance of Se and the repeatedly observed low plasma Se levels in our septic patients receiving normal Se substitution, in a prospective, randomized, open-label, single-centre clinical trial in a tertiary care centre we tested the hypotheses that high-dose Se supplementation, in the form of sodium selenite (Na-selenite), would (1) increase plasma Se levels, (2) decrease the severity of the disease, (3) reduce markers of inflammation, and improve nutrition and antioxidant defence, and (4) improve mortality.
Methods
Study design
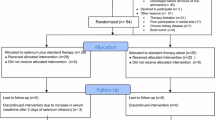
The study was approved by the Joint Institutional Ethics Committee of the First Faculty of Medicine, Charles University in Prague, and the General University Hospital in Prague, Czech Republic. Informed consent was obtained prior to the randomization from eligible patients or from legal representatives (next-of-kin or with a power of attorney). The study enrolled patients older than 18 years admitted for SIRS or sepsis with an initial sequential organ failure assessment (SOFA) [17] score of >5 (Fig. 1, Electronic supplementary material). We chose a priori to exclude patients who originally met the enrolment criteria but then were hospitalized for less than 5 days, to ensure that there was a sufficient time period for the drug to elicit its effects.
The study was designed as a prospective, randomized, open-label trial. The study took place in a single centre, University Hospital mixed Intensive Care Unit (ICU) in Prague from March 2004 until April 2009. Eligible patients with SIRS, sepsis or septic shock were randomized into two groups according to their unique identification number. The study group (Se+, n = 75) received the high-dose Se supplementation (1,000 μg of Se on the first day, 500 μg/day on subsequent days). Se was administered in the form of Na-selenite pentahydrate (Na2SeO3·5H2O), in which 100 μg of Se corresponds to 333 μg of Na-selenite (Selenase T; Biosyn Arzneimittel, Fellbach, Germany). Se was administered as a 30-min infusion in 100 ml of 0.9% NaCl via a central venous catheter daily for 5–14 days, according to the length of hospitalization. The infusion was started at 9:00 a.m. The control group (Se−, n = 75) did not receive any extra Se. Both groups received Se supplementation, as Na-selenite, added to the parenteral nutrition (<75 μg/day, or 0.38–0.95 μmol/day, respectively). The details of the laboratory test methods, clinical assessment, and statistical methods are shown in the Electronic supplementary material.
Results
Patients characteristics
There were no significant differences in baseline characteristics of the patients between groups in terms of age, gender, acute physiology and chronic health evaluation II (APACHE II) score, SOFA score, sepsis severity and admission diagnoses (Table 1).
Plasma Se levels
In the Se+ group, the plasma Se level was significantly higher than in the Se− group at all time points except at baseline (Fig. 1).
There was a trend for higher plasma Se levels in pooled data from all measurements in 28-day survivors from both groups compared to the non-survivors (0.75 ± 0.59 vs. 0.71 ± 0.56 μmol/l, respectively; P = 0.068).
During the administration of Na-selenite there were no specific adverse/toxic effects observed (seizures, refractory shock, acute lung injury). Of the 799 plasma Se samples, only 17 from 9 patients in the Se+ group showed levels above normal (2.07 ± 0.18 μmol/l; normal range, 0.58–1.82 μmol/l). The highest level was 2.56 μmol/l. All these nine patients survived (Table 2).
Glutathione peroxidase
Whole-blood GPx activity was higher in the Se+ group than in the Se− group at all time points except at baseline (Fig. 2). There were no differences in whole-blood GPx activity in pooled data from all patients between survivors and non-survivors.
Cholesterol, albumin, prealbumin
Cholesterol increased significantly in the Se+ group between baseline and day 7, and between baseline and day 14. Cholesterol was higher on day 14 in the Se+ group than in the Se− group, but no differences between groups were observed at either baseline or on day 7. There were no differences in albumin levels between groups at baseline or on day 7. Prealbumin increased significantly between baseline and day 7 and between baseline and day 14 in the Se+ group, but there were no differences between the Se+ group and the Se− groups at baseline or on day 7 (Table 3).
CRP, PCT, SOFA score
Plasma Se levels showed significant negative correlations with markers of inflammation (Se × CRP, r = −0.172, P = 0.035; Se × PCT, r = −0.187, P = 0.022) and severity of the disease (Se × SOFA, r = −0.277, P = 0.001) at admission (Fig. 2a in ESM). These correlations were not observed on day 7 or day 14 for any of these parameters (Fig. 2b, c in ESM).
In the Se+ group, CRP and PCT decreased between baseline and day 7 and between baseline and day 14. In the Se− group, CRP decreased between baseline and day 7 but not between baseline and day 14. In contrast, PCT decreased between baseline and day 14, but not between baseline and day 7 (Table 3).
Mortality
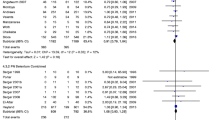
The overall mortality among the patients in the study was 28.7% (43 out of 150 patients). There was no difference in mortality between the Se+ group (25.3%, 19/75) and the Se− group (32.0%, 24/75) group (P = 0.367; Fig. 3). There were no differences in mortality between men and women in either group. In the Se− group, 55% of men (28/51) and 75% of women (18/24) died (P = 0.095). In the Se+ group, 65% of men (30/46) and 66% of women (19/29) died (P = 0.979).
In the post hoc subgroup analysis we dichotomized the patients into those above and those below the median admission SOFA scores of the respective subgroups at admission (Se+ vs. Se−) to evaluate the effect of Se supplementation on mortality according to the severity of the disease. Similarly, we divided the patients according to the median APACHE II score at admission. None of the subgroup analyses showed a difference in mortality between the Se+ and Se− groups. There was a trend towards a reduction in mortality in the Se+ group in relation to the Se− group in the subgroup of patients with an APACHE II score of >28 (P = 0.100) (Fig. 3).
Discussion
In our moderate size, prospective, open-label randomized study in critically ill patients, we showed that high-dose Se supplementation effectively restored plasma Se levels and increased whole-blood GPx activity. The markers of inflammation (CRP, PCT) decreased over time irrespective of Se supplementation. However, the decrease was more pronounced in the Se+ group. Prealbumin and cholesterol levels were more readily restored in the Se+ group. Despite the improvements in these biochemical markers, Se supplementation did not affect 28-day mortality either between the Se+ and Se− groups, or between any of the subgroups divided with respect to severity according to the SOFA or APACHE scores. Thus, Se supplementation had no effect on the major endpoints in this study.
The observation that early sepsis is associated with a decrease in plasma Se levels sparked enthusiasm for Se supplementation. Se is an essential trace element representing a key component of selenoenzymes that provide a defence against reactive oxygen species. From among the multiple selenoenzymes described, GPx and selenoprotein P have been the most frequently studied in plasma [18].
The assessment of Se status and its requirements is difficult because of its complex distribution in the body. In healthy volunteers, selenoprotein P accounts for 40–70% of plasma Se, followed by GPx (20–40%) and albumin-bound Se (6–10%). Free Se represents <1% [19]. This distribution could be altered in critically ill patients with increased capillary leakage, decreased albumin and prealbumin levels, and GPx depletion [2]. Hence, plasma Se levels may not represent the actual Se status. Decreased plasma Se levels in sepsis could result from translocation of selenoenzymes due to increased vascular permeability, in a similar manner to albumin [20].
Whole-blood GPx is one of many assays used to estimate the activity of GPx. Plasma GPx represents a rapid turnover pool, while other blood components that contain GPx (erythrocytes, platelets) may be more indicative of prolonged intake [21]. According to a recent meta-analysis, plasma, platelet and whole-blood GPx activity were shown to adequately reflect the intake of Se [22]. Plasma Se is a generally recognized method of assessment of Se deficiency despite the fact that the plasma compartment corresponds to only a small part (about 0.3 mg) of the body Se content (about 20 mg) and may not adequately reflect an acute Se status in tissues, where Se is redistributed and preferentially incorporated into different selenoenzymes.
While Se is a cornerstone in antioxidant defence, Na-selenite, used in our study in a rapid infusion, has a biphasic effect. The initial effect is prooxidant, and only after incorporation of Se into selenoenzymes does the effect become antioxidant. Both phases could be used as potent therapeutic strategies [7, 23]. In our study, we demonstrated a sustained increase in plasma Se levels with high-dose Se supplementation (Se+ group) but not with standard Se supplementation (Se− group). Whole-blood GPx activity was restored readily with Se supplementation, in accordance with the findings of previous studies [10, 11, 24].
The necessity for nutritional enhancement with antioxidants has been a matter of recent debate. In 2009, guidelines for nutritional support recommended that a combination of antioxidant vitamins and trace minerals (specifically including Se) should be provided to all critically ill patients receiving specialized nutritional therapy [25, 26]. Several studies in patients with trauma and burns exploring the effect of a “cocktail” of micronutrients (Zn, Cu, Se, vitamins E and C) have indicated a decrease in the frequency of MOF and infectious complications [27, 28]. Na-selenite has been used most frequently in recent and currently ongoing studies, despite its potential toxicity. The therapeutic regimen used in our study was based on previous studies that used Na-selenite in doses up to 1,000 μg/day (with one study using a loading dose of 4,000 μg via continuous infusion over 24 h) without adverse effects [7, 14, 23, 29]. A 30-min infusion was used daily instead of a continuous infusion, to induce both phases of the action of Se.
Early studies exploring Se supplementation were extremely successful. Kuklinski et al. [9] reported a “drastic” decrease in mortality among patients with acute pancreatitis with Se supplementation. Zimmerman et al. [30] found a decrease in the frequency and severity of MOF, and a decrease in mortality from 40% to 15%. However, these effects were not reproduced in a later study by Lindner et al. [31], also in patients with pancreatitis. Angstwurm et al. [10], in a small study in SIRS patients, used Na-selenite administered intravenously for 9 days (535, 285, 155 μg each for 3 days and thereafter 35 μg per day). The control group (n = 21) received 35 μg of Na-selenite throughout the treatment period. Rapid normalization of initially low plasma Se and GPx levels was observed. The reduction in mortality in the supplemented compared with the control group did not reach significance (33.5 vs. 52%, respectively; P = 0.13).
Forceville et al. [12] administered Na-selenite for 10 days (4,000 μg on the first day, 1,000 μg/day on the subsequent 9 days) by continuous infusion in septic patients. No significant differences were found in the duration of vasopressor treatment, mechanical lung ventilation, mortality, number of days without dialysis or the frequency of nosocomial pneumonia. The continuous infusion of the drug probably did not induce the initial prooxidant surge that may be necessary to achieve the benefits observed with bolus administration [32]. This speculation is corroborated by an experimental study by Wang et al. [33] that showed an increase in survival time in sheep with septic shock only with large bolus administration of Na-selenite, but not with continuous infusion.
Mishra et al. [11] prospectively studied 40 patients with severe sepsis randomized into groups. One group received high Se supplementation (474, 316 and 158 μg/day, each dose for three consecutive days), followed by a standard dose of 31.6 μg/day. The control group received only the standard dose. A negative correlation was found between plasma Se levels and SOFA score, but no difference was observed in 28-day mortality or in the need for renal replacement therapy. As in our study, GPx activity increased.
Angstwurm et al. [14] enrolled patients with sepsis (APACHE III score >70) in a large study (Selenium in Intensive Care, SIC study). The intervention group (n = 92) was given a 1,000 μg of Na-selenite on the first day as a 30-min intravenous bolus, and then for 2 weeks 1,000 μg daily as a continuous infusion. The control group received placebo (n = 97). All patients received a standard dose of trace elements including <100 μg of Se per day. The overall intention-to-treat analysis did not show a significant reduction in mortality with Se supplementation in comparison with placebo (39.7% vs. 50.0%, respectively; P = 0.109; OR 0.66; CI 0.39–1.1). After exclusion of patients with protocol violations, 28-day mortality in the intervention and placebo groups were 42.4% and 56.7%, respectively (P = 0.049, OR 0.56; CI 0.32–1.00). Mortality was also significantly reduced in defined subgroups of the intervention group (those with septic shock and disseminated intravascular coagulation), in patients with an APACHE III score of >101, and in patients with failure of more than three organs.
The 28-day mortality rates in the Se+ and Se− groups in our study were similar. The trend towards a lower mortality with Se supplementation in the most critically ill patients with a higher APACHE II score in our study is consistent with the results of the SIC study [14]. However, we recognize that our study was not powered for this subgroup analysis, and therefore no firm conclusion can be drawn. It could be hypothesized that plasma Se levels and selenoenzymes, including GPx and selenoprotein P, are inversely related to sepsis severity and oxidative stress, and could be viewed as novel markers of sepsis [34].
Cholesterol has previously been shown to correlate with the severity of organ failure [35]. In our study, cholesterol levels increased only in the Se+ group. Prealbumin, another acute phase reactant [36], displayed a similar pattern. In contrast, albumin levels remained unchanged in both groups, perhaps due to the effect of albumin administration, and its longer half-life.
High-dose Se supplementation in the Se+ group rapidly induced higher plasma Se levels and whole-blood GPx activity, compared to the steady levels in the Se− group. This suggests increased Se requirements for the synthesis of GPx in sepsis [37]. We found a significant inverse correlation between plasma Se levels and PCT, CRP and SOFA score. Similar relationships between plasma Se levels and CRP and SOFA score have been reported previously in septic patients [11]. Our results are in line with a proposed delicate interplay between plasma Se levels, selenoenzymes, and the severity of sepsis and MOF [38].
The lack of a difference in 28-day mortality between the Se+ and Se groups could have several explanations. The overall mortality in our study was lower than reported previously in large studies [1, 39], but correlates well with a recent global survey of hospitals that adopted the Surviving Sepsis Campaign [40]. We recognize that we also included patients with SIRS and excluded patients treated for less than 5 days. Our patient population was also heterogeneous, with a broad spectrum of admission diagnoses, and a wide range of admission APACHE II scores. A subgroup analysis showed a weak trend to improving mortality with Se supplementation in the most critically ill patients. We recognize that the number of patients in the septic shock subgroup was small, limiting the conclusion. A similar pattern has also been observed by others, reaching clinical significance in some studies [14]. It could also be suggested that such high-dose Se is of limited value, except in very specific patient groups, the characteristics of which need to be clearly identified.
Our study had several limitations. The study was not blinded to the clinicians. However, the statistician was masked to the treatment assignment. The study period was relatively long in order to enrol the projected number of participants. This could have had an effect on the treatment. We recognize that our study was not powered for subgroup analysis and the results should be interpreted with caution.
Conclusion
High-dose Se supplementation resulted in a significant increase in the plasma Se levels and whole-blood GPx activity. Inflammatory markers decreased in both groups irrespective of Se supplementation. Prealbumin and cholesterol levels increased more rapidly, compared to baseline, in the Se+ group. However, these beneficial effects did not translate into a reduction in 28-day mortality. In accordance with the findings of other studies, we observed a trend towards improved mortality in a subgroup analysis of patients with severe sepsis or septic shock.
References
Engel C, Brunkhorst FM, Bone HG, Brunkhorst R, Gerlach H, Grond S, Gruendling M, Huhle G, Jaschinski U, John S, Mayer K, Oppert M, Olthoff D, Quintel M, Ragaller M, Rossaint R, Stuber F, Weiler N, Welte T, Bogatsch H, Hartog C, Loeffler M, Reinhart K (2007) Epidemiology of sepsis in Germany: results from a national prospective multicenter study. Intensive Care Med 33:606–618
Hawker FH, Stewart PM, Snitch PJ (1990) Effects of acute illness on selenium homeostasis. Crit Care Med 18:442–446
Forceville X, Vitoux D, Gauzit R, Combes A, Lahilaire P, Chappuis P (1998) Selenium, systemic immune response syndrome, sepsis, and outcome in critically ill patients. Crit Care Med 26:1536–1544
Manzanares W, Biestro A, Galusso F, Torre MH, Manay N, Pittini G, Facchin G, Hardy G (2009) Serum selenium and glutathione peroxidase-3 activity: biomarkers of systemic inflammation in the critically ill? Intensive Care Med 35:882–889
Sakr Y, Reinhart K, Bloos F, Marx G, Russwurm S, Bauer M, Brunkhorst F (2007) Time course and relationship between plasma selenium concentrations, systemic inflammatory response, sepsis, and multiorgan failure. Br J Anaesth 98:775–784
Kazda A, Brodska H, Vinglerova H, Blaha J, Valenta J, Stritesky M, Zima T, Urban M (2004) Selenium and zinc plasmatic levels in intensive care patients. Crit Care 8(Suppl 1):P265
Vincent JL, Forceville X (2008) Critically elucidating the role of selenium. Curr Opin Anaesthesiol 21:148–154
Manzanares W, Hardy G (2009) Selenium supplementation in the critically ill: posology and pharmacokinetics. Curr Opin Clin Nutr Metab Care 12:273–280
Kuklinski B, Zimmermann T, Schweder R (1995) Decreasing mortality in acute pancreatitis with sodium selenite. Clinical results of 4 years antioxidant therapy. Med Klin (Munich) 90(Suppl 1):36–41
Angstwurm MW, Schottdorf J, Schopohl J, Gaertner R (1999) Selenium replacement in patients with severe systemic inflammatory response syndrome improves clinical outcome. Crit Care Med 27:1807–1813
Mishra V, Baines M, Perry SE, McLaughlin PJ, Carson J, Wenstone R, Shenkin A (2007) Effect of selenium supplementation on biochemical markers and outcome in critically ill patients. Clin Nutr 26:41–50
Forceville X, Laviolle B, Annane D, Vitoux D, Bleichner G, Korach JM, Cantais E, Georges H, Soubirou JL, Combes A, Bellissant E (2007) Effects of high doses of selenium, as sodium selenite, in septic shock: a placebo-controlled, randomized, double-blind, phase II study. Crit Care 11:R73
Berger MM, Soguel L, Shenkin A, Revelly JP, Pinget C, Baines M, Chiolero RL (2008) Influence of early antioxidant supplements on clinical evolution and organ function in critically ill cardiac surgery, major trauma, and subarachnoid hemorrhage patients. Crit Care 12:R101
Angstwurm MW, Engelmann L, Zimmermann T, Lehmann C, Spes CH, Abel P, Strauss R, Meier-Hellmann A, Insel R, Radke J, Schuttler J, Gartner R (2007) Selenium in Intensive Care (SIC): results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock. Crit Care Med 35:118–126
Stawicki SP, Lyons M, Aloupis M, Sarani B (2007) Current evidence from phase III clinical trials of selenium supplementation in critically Ill patients: why should we bother? Mini Rev Med Chem 7:693–699
Heyland DK (2007) Selenium supplementation in critically ill patients: can too much of a good thing be a bad thing? Crit Care 11:153
Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S (1998) Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 26:1793–1800
Burk RF, Hill KE (2005) Selenoprotein P: an extracellular protein with unique physical characteristics and a role in selenium homeostasis. Annu Rev Nutr 25:215–235
Harrison I, Littlejohn D, Fell GS (1996) Distribution of selenium in human blood plasma and serum. Analyst 121:189–194
Fleck A, Raines G, Hawker F, Trotter J, Wallace PI, Ledingham IM, Calman KC (1985) Increased vascular permeability: a major cause of hypoalbuminemia in disease and injury. Lancet 1:781–784
Finley JW, Duffield A, Ha P, Vanderpool RA, Thomson CD (1999) Selenium supplementation affects the retention of stable isotopes of selenium in human subjects consuming diets low in selenium. Br J Nutr 82:357–360
Ashton K, Hooper L, Harvey LJ, Hurst R, Casgrain A, Fairweather-Tait SJ (2009) Methods of assessment of selenium status in humans: a systematic review. Am J Clin Nutr 89:2025S–2039S
Forceville X, Van Antwerpen P (2008) Selenocompounds and selenium. A biochemical approach to sepsis. In: Vincent JL (ed) Yearbook of intensive care and emergency medicine. Springer, Heidelberg, pp 454–469
Cohen HJ, Chovaniec ME, Mistretta D, Baker SS (1985) Selenium repletion and glutathione peroxidase—differential effects on plasma and red blood cell enzyme activity. Am J Clin Nutr 41:735–747
Martindale RG, McClave SA, Vanek VW, McCarthy M, Roberts P, Taylor B, Ochoa JB, Napolitano L, Cresci G (2009) Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition: Executive Summary. Crit Care Med 37:1757–1761
Singer P, Berger MM, Van den Berghe G, Biolo G, Calder P, Forbes A, Griffiths R, Kreyman G, Leverve X, Pichard C, Espen (2009) ESPEN guidelines on parenteral nutrition: intensive care. Clin Nutr 28:387–400
Berger MM, Baines M, Raffoul W, Benathan M, Chiolero RL, Reeves C, Revelly JP, Cayeux MC, Senechaud I, Shenkin A (2007) Trace element supplementation after major burns modulates antioxidant status and clinical course by way of increased tissue trace element concentrations. Am J Clin Nutr 85:1293–1300
Berger MM, Cavadini C, Chiolero R, Guinchard S, Krupp S, Dirren H (1994) Influence of large intakes of trace elements on recovery after major burns. Nutrition 10:327–334; discussion 352
Avenell A, Noble DW, Barr J, Engelhardt T (2004) Selenium supplementation for critically ill adults. Cochrane Database of Systematic Reviews, Issue 4. Art. No.: CD003703. doi:10.1002/14651858.CD003703.pub2
Zimmermann T, Albrecht S, Kuhne H, Vogelsang U, Grutzmann R, Kopprasch S (1997) Selenium administration in patients with sepsis syndrome. A prospective randomized study. Med Klin Munich 92(Suppl 3):3–4
Lindner D, Lindner J, Baumann G, Dawczynski H, Bauch K (2004) Investigation of antioxidant therapy with sodium selenite in acute pancreatitis. A prospective randomized blind trial. Med Klin Munich 99:708–712
Forceville X (2007) Effects of high doses of selenium, as sodium selenite, in septic shock patients a placebo-controlled, randomized, double-blind, multi-center phase II study—selenium and sepsis. J Trace Elem Med Biol 21(Suppl 1):62–65
Wang Z, Forceville X, Van Antwerpen P, Piagnerelli M, Ahishakiye D, Macours P, De Backer D, Neve J, Vincent JL (2009) A large-bolus injection, but not continuous infusion of sodium selenite improves outcome in peritonitis. Shock 32:140–146
Forceville X, Mostert V, Pierantoni A, Vitoux D, Le Toumelin P, Plouvier E, Dehoux M, Thuillier F, Combes A (2009) Selenoprotein P, rather than glutathione peroxidase, as a potential marker of septic shock and related syndromes. Eur Surg Res 43:338–347
Dunham CM, Fealk MH, Sever WE 3rd (2003) Following severe injury, hypocholesterolemia improves with convalescence but persists with organ failure or onset of infection. Crit Care 7:R145–R153
Devakonda A, George L, Raoof S, Esan A, Saleh A, Bernstein LH (2008) Transthyretin as a marker to predict outcome in critically ill patients. Clin Biochem 41:1126–1130
Rinaldi S, Landucci F, De Gaudio AR (2009) Antioxidant therapy in critically septic patients. Curr Drug Targets 10:872–880
Gartner R, Albrich W, Angstwurm MW (2001) The effect of a selenium supplementation on the outcome of patients with severe systemic inflammation, burn and trauma. Biofactors 14:199–204
Friedman G, Silva E, Vincent JL (1998) Has the mortality of septic shock changed with time. Crit Care Med 26:2078–2086
Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, Schorr C, Artigas A, Ramsay G, Beale R, Parker MM, Gerlach H, Reinhart K, Silva E, Harvey M, Regan S, Angus DC (2010) The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 36:222–231
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Valenta, J., Brodska, H., Drabek, T. et al. High-dose selenium substitution in sepsis: a prospective randomized clinical trial. Intensive Care Med 37, 808–815 (2011). https://doi.org/10.1007/s00134-011-2153-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2153-0