Abstract
Rationale
Cholesteryl ester deficiency which results in adrenal lipid store depletion has been proposed as a potential mechanism of sepsis associated adrenal insufficiency.
Objective
We investigated histological abnormalities associated with sepsis in human and mice adrenals.
Methods
From January 2006 to 2008, seven patients who died of septic shock and seven patients with rapidly fatal nonseptic illness were included. Adrenals were sampled within 12 h from death. Adrenals were also taken from 13 lipopolysaccharide (LPS)-challenged mice, 5 cecal ligation and puncture (CLP) mice and 5 controls. We semi-quantitatively analysed intensity of inflammation, necrosis, haemorrhage and lipid depletion.
Measurements and main results
In patients, lipid depletion scores were significantly higher in septic shock than in controls (p = 0.011). In animals, lipid depletion was higher following LPS or CLP than in controls (p = 0.003). In adrenal cortex, in patients and not in animals, global scores for inflammation (p = 0.002), necrosis (p = 0.009) and haemorrhage (p = 0.009) were significantly higher in septic shock than in controls. Similarly, in zona fasciculata, in patients and not in animals, scores for inflammation (p = 0.007), necrosis (p = 0.023) and haemorrhage (p = 0.023) were significantly higher in septic shock than in controls.
Conclusions
This study shows that diffuse lipid depletion in zona fasciculata is a hallmark of human septic shock, experimental endotoxaemia and sepsis. In patients, sepsis was associated with inflammation, necrosis and haemorrhage predominantly in zona fasciculata.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
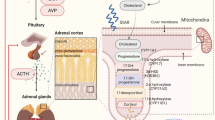
The hypothalamic–pituitary–adrenal (HPA) axis is a major factor of the host response to critical illness [1, 2]. About half of patients with septic shock are unable to mount an appropriate cortisol response [2, 3]. This so-called critical-illness-related corticosteroid insufficiency (CIRCI) may result in multiple organ dysfunction and ultimately in death [2, 3]. The causes of CIRCI remain unclear, and may involve anatomic damage, inflammatory processes and drug interference with metabolism of hormones at the level of the hypothalamus, the pituitary or the adrenals [4].
Adrenals storage is very limited, and cortisol response to stress depends upon its synthesis [4]. Cortisol is synthesized from cholesterol, and the rate-limiting step in the cortisol synthesis process is transport of free cholesterol into mitochondria. Free cholesterol is obtained via de novo synthesis regulated by 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase [5], or via catabolism of cholesteryl esters from intra-cellular stores or from circulating low-density lipoprotein (LDL) and high-density lipoprotein (HDL) [6, 7]. Interestingly, low circulating levels of total cholesterol as well as of LDL and HDL have been found in patients with severe sepsis [8]. The scavenger receptor B-I (SR-BI), a HDL receptor, is highly expressed in the adrenals and provides cholesterol for steroid synthesis via selective cholesteryl ester uptake from HDL and LDL [9]. Cai et al. [10] showed that SR-BI prevented death following endotoxin challenge in mice, mainly by facilitating glucocorticoids synthesis and hepatic clearance of LPS. In addition, SR-BI-deficient mice failed to mount an appropriate glucocorticoid synthesis response to overnight fasting and showed a 40% reduction in adrenal cholesterol content with depleted lipid stores [11]. These experiments suggest that cholesteryl ester deficiency impairs adrenal function.
Loss of cholesteryl esters in the adrenal glands results in diffuse lipid depletion. We hypothesized that septic shock could decrease lipid storage in adrenal gland and may be explain relative adrenal failure. We investigated prevalence of sepsis-associated lipid depletion in adrenal cortex in patients and in animals by conducting a morphologic study of human and mice adrenals to study sepsis-associated diffuse lipid depletion.
Methods
Animal model
All procedures were conducted in accordance with French law on animal experimentation, and the protocol was approved by our Institutional Animal Care and Use Committee.
Adult male C57BL/6 mice (Janvier, Le Genest Saint Isle, France; 26–31 g body weight) were housed at constant temperature (22°C) and exposed to a 12–12 h light–dark cycle for ≥1 week before use in the experimental protocols.
Cecal ligation and puncture model
Sepsis was induced by cecal ligation and puncture (CLP) as previously described [13] with minor modifications. Briefly, mice were anaesthetized with isoflurane, and a 1.5- to 2-cm abdominal incision was made to expose the cecum. It was ligated and punctured once with a 21-gauge needle, and a small amount of faeces was extruded. The bowel was repositioned into the abdomen, and the abdominal cavity was closed in two layers. For sham mice, laparotomy was performed and the cecum was manipulated but was neither ligated nor punctured. All animals were resuscitated with 30 ml/kg body weight normal saline subcutaneously at completion of surgery. All mice were sacrificed at day 5. Adrenal glands were obtained within 10 min from death.
Endotoxaemia model
Endotoxin (Escherichia coli LPS 055:B5; Sigma-Aldrich) was dissolved in sterile NaCl immediately before use. In initial experiments testing the effects of LPS on mortality, mice were briefly anaesthetized with isoflurane and challenged by incremental doses of endotoxin given intraperitoneally, and producing no to high lethalities (1–100 mg/kg). A 100% lethal dose (LD100: 75 mg/kg) and a 50% lethal dose (LD50: 37.5 mg/kg) were determined. For the control group, mice did not undergo any surgical procedure. Mice were sacrificed at day 5. Adrenal glands were obtained within 10 min from death.
Patients
We investigated patients who died from septic shock [12] at Raymond Poincaré University Hospital, Garches, France (cases). Two investigators (A.P. and D.A.) analysed cause of death, and any discrepancy between them was resolved by consensus. Exclusion criteria were as follows: age younger than 18 years, pregnancy, evidence of an underlying adrenal disease at clinical or post mortem examination or any concomitant disease other than infection that might have accounted for shock and death. Controls were subjects who died outside the hospital and referred to the forensic department at our institution. Careful post mortem examination of controls ruled out any infection. We obtained informed consent from patients’ closest relatives. The protocol was approved by the Comité de Protection des Personnes de Saint Germain en Laye, France.
Data collection
We recorded demographic and anthropometric characteristics, any co-morbid conditions, vital signs and severity of illness using the Simplified Acute Physiology Score II (SAPS II) [14] and the Sepsis-related Organ Failure Assessment (SOFA) score [15]. Standard laboratory tests and any relevant microbiological data were recorded on a daily basis. Cortisol levels were obtained immediately before and 60 min after an intravenous bolus of 0.25 mg cosyntropin (Novartis or Alliance) in patients within 24 h of septic shock onset. After centrifugation, cortisol was immediately measured on serum samples with the use of the Elecsys cortisol assay (Roche Diagnostics).
Adrenals sampling
In 14 patients, adrenal glands were sampled within 12 h from death. The adrenals were fixed in formal-sublimate-acetic acid (FSA) [16] and in 10% neutral formalin. Paraffin sections cut at 4 μm were stained with haematoxylin and eosin and azan [17]. The alcian-blue periodic acid-Schiff reaction (AB/PAS) for glycoprotein [18] was also performed on paraffin-sectioned material. Frozen sectioned material was used for histochemical demonstration of total lipids [19] and of cholesterol and its esters [20].
Two investigators (A.P. and G.L. de la G.) noted double-blinded macroscopic changes, and any discrepancy between them was resolved by consensus. Representative samples were selected for microscopic examination. We discarded any adrenals that presented autolysis. We graded lipid depletion in zona fasciculata as follow: 0 for no lipid depletion, 1 for mild, 2 for marked, 3 for intense lipid depletion. Using the same grading system, we quantified inflammatory infiltrates, necrosis, haemorrhage, siderosis and calcifications within the medulla (junctional and non-junctional zones) and the cortex (zona reticularis, zona fasciculata, zona glomerulosa). Then, to better assess cortex lesions, we calculated an overall semi-quantitative score by adding up the scores for inflammatory infiltrates (range 0–9), necrosis (range 0–9), haemorrhage (range 0–9), siderosis (range 0–9) and calcifications (range 0–9) obtained in the three layers of the cortex using the same approach previously described [21].
Statistical analysis
We used Fisher’s exact test for comparison of categorical variables, and Kruskal–Wallis test (or Mann–Whitney test whenever appropriate) for continuous variables. Correlations were tested by Spearman’s rho. We used set correlations for studies of association between histological changes in the adrenals and (1) the severity of shock (lactate levels, worst mean blood pressure, cumulative dose of catecholamine and shock duration), (2) renal failure (blood urea nitrogen and creatinine levels), (3) coagulation disorders (prothrombin ratio and platelet counts, and use of anticoagulant) and (4) cortisol response to corticotrophin. A p value of 0.05 was considered statistically significant. All statistical analyses were done with STATA/IC version 10.0 (Stata Corp. LP, College Station, TX, USA).
Results
Animals
Lipid depletion scores differed between the three groups of animals (p = 0.003, Figs. 1, 2) and were significantly higher in the LPS-challenged mice than in controls (p = 0.0005), and in CLP mice than in controls (p = 0.003). Lipid depletion scores were also higher following LPS than CLP (p = 0.011).
Inflammation, necrosis and haemorrhagic scores within the medulla or the cortex were not different between the three groups of animals (Table 1).
Patients
From January 2006 to 2008, 68 of 155 septic shock patients died in the intensive care unit (ICU) at Raymond Poincaré University Hospital. Adrenals were sampled in seven patients with septic shock (cases, Table 2) and seven patients with rapidly fatal acute illnesses (controls, Table 3).
In septic shock patients, adrenals weight was significantly greater than in controls (8.9 ± 5.0 versus 3.9 ± 0.7 g, p = 0.034). Lipid depletion scores were significantly higher in patients with septic shock than in controls (p = 0.011). Inflammation, necrosis and haemorrhagic scores within the medulla did not differ between patients with septic shock and controls (Table 4).
As compared with controls, septic shock patients had higher inflammation score for the whole cortex (p = 0.002) and in zona fasciculata (p = 0.007) and zona reticularis (p = 0.022) (Table 4). Similarly they had higher necrosis score for the whole cortex (p = 0.009) and in zona fasciculata (p = 0.023). Global haemorrhagic scores for cortex were significantly higher in patients with septic shock than in controls (p = 0.009). Haemorrhagic scores were higher in patients with septic shock than in controls for zona fasciculata (p = 0.023) and zona reticularis (p = 0.023).
Table 5 summarizes the correlations between zona fasciculata lesions and clinical and laboratory variables. Lipid depletion scores correlated only with mean blood pressure. Inflammation score did not correlate with any of the variables. Necrosis scores correlated with mean blood pressure, lactate levels, blood urea nitrogen, prothrombin ratio and platelets count. Haemorrhagic scores correlated with cumulative dose of catecholamines and shock duration. There was no correlation between adrenals morphologic lesions and basal or peak level of cortisol.
Discussion
This study showed that diffuse lipid depletion in zona fasciculata is a hallmark of human septic shock, experimental endotoxaemia and sepsis. Patients with septic shock showed signs of inflammation, necrosis and haemorrhage predominantly in the zona fasciculata, whereas the zona reticularis, the zona glomerulosa and the medulla remained almost unaffected.
Lipid depletion results from loss of cholesterol esters, which are normally abundant in adrenocortical cells. As a result, in the course of sepsis, the only sources of cholesterol esters for cortisol synthesis are circulating HDL and LDL via a SR-BI-dependent pathway [9]. The lack of correlation between adrenal lipid store depletion and basal cortisol level and cortisol response to ACTH may reflect that, as long as SR-BI is expressed in the adrenals, cortisol synthesis may not be compromised. In addition, all septic shock patients had renal insufficiency that may decrease plasma cortisol clearance [22]. In this case plasma cortisol levels depend more on cortisol accumulation than cortisol synthesis [2, 4]. Interestingly, lipid depletion in the zona fasciculata was independent of severity and duration of shock, presence of renal insufficiency, coagulation disorders or adrenal insufficiency. Moreover, both LPS and CLP mice showed intense lipid depletion in fasciculata with no evidence of inflammation, necrosis or haemorrhage. Our findings suggest that lipid depletion is a consequence of sepsis independently of organ dysfunction, or more particularly of adrenal insufficiency. The absence of morphologic and biological correlations between patients with or without adrenal insufficiency does not rule out the potential role of lipid depletion in adrenal failure. The fact that lipid depletion was more pronounced in lethal endotoxaemia than in mice with CLP-induced peritonitis may support the hypothesis of an intrinsic relationship between sepsis and lipid depletion.
Of note, patients with sepsis showed signs of inflammation, necrosis and haemorrhage almost specifically in the zona fasciculata, whereas the outer zona glomerulosa and the inner zona reticularis remained unaffected. These findings suggest that sepsis is associated with damage of cortisol-secreting cells and not of cells releasing aldosterone, dehydroepiandrosterone (DHEA) and its sulphated derivative (S-DHEA). Our results confirm previous observations of a lack of association between sepsis and DHEA and S-DHEA deficiency [23].
Previous observations of sepsis which showed dissociation between aldosterone levels and renin activity [24, 25] are not in contradiction with our findings of intact zona glomerulosa. In fact, these findings together suggest that aldosterone regulation is shifted from renin to ACTH and/or cytokine command. The morphologic integrity of the zona glomerulosa may question the need for mineralocorticoids supplementation. However, our findings may not rule out functional abnormalities of zona glomerulosa cells.
In conclusion, the current study suggests that lipid depletion, specific necrosis and haemorrhage of the zona fasciculata are hallmarks of sepsis. The lipid depletion suggests that SR-BI-mediated uptake is the only source of cholesterol ester supply for cortisol synthesis in sepsis. The integrity of the zona glomerulosa and the zona reticularis challenged the presence of mineralocorticoid insufficiency during sepsis and the need of a replacement treatment.
References
Polito A, Aboab J, Annane D (2007) The hypothalamic pituitary adrenal axis in sepsis. Novartis Found Symp 280:182–199
Marik PE, Pastores SM, Annane D, Meduri GU, Sprung CL, Arlt W, Keh D, Briegel J, Beishuizen A, Dimopoulou I, Tsagarakis S, Singer M, Chrousos GP, Zaloga G, Bokhari F, Vogeser M, American College of Critical Care Medicine (2008) Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med 36:1937–1949
Annane D, Maxime V, Ibrahim F, Alvarez JC, Abe E, Boudou P (2003) Diagnosis of adrenal insufficiency in severe sepsis and septic shock. Am J Respir Crit Care Med 174:1319–1326
Prigent H, Maxime V, Annane D (2004) Science review: mechanisms of impaired adrenal function in sepsis and molecular actions of glucocorticoids. Crit Care 8:243–252
Mason JI, Rainey WE (1987) Steroidogenesis in the human fetal adrenal: a role for cholesterol synthesized de novo. J Clin Endocrinol Metab 64:140–147
Borkowski A, Delcroix C, Levin S (1972) Metabolism of adrenal cholesterol in man. I. In vivo studies. J Clin Invest 51:1664–1678
Borkowski A, Delcroix C, Levin S (1972) Metabolism of adrenal cholesterol in man. II. In vitro studies including a comparison of adrenal cholesterol synthesis with the synthesis of the clucocorticosteroid hormones. J Clin Invest 51:1679–1687
van Leeuwen HJ, Heezius EC, Dallinga GM, van Strijp JA, Verhoef J, van Kessel KP (2003) Lipoprotein metabolism in patients with severe sepsis. Crit Care Med 31:1359–1366
Temel RE, Trigatti B, DeMattos RB, Azhar S, Krieger M, Williams DL (1997) Scavenger receptor class B, type I (SR-BI) is the major route for the delivery of high density lipoprotein cholesterol to the steroidogenic pathway in cultured mouse adrenocortical cells. Proc Natl Acad Sci USA 94:13600–13605
Cai L, Ji A, de Beer FC, Tannock LR, van der Westhuyzen DR (2008) SR-BI protects against endotoxemia in mice through its roles in glucocorticoid production and hepatic clearance. J Clin Invest 118:364–375
Hoekstra M, Meurs I, Koenders M, Out R, Hildebrand RB, Kruijt JK, Van Eck M, Van Berkel TJ (2008) Absence of HDL cholesteryl ester uptake in mice via SR-BI impairs an adequate adrenal glucocorticoid-mediated stress response to fasting. J Lipid Res 49:738–745
American College of Chest Physicians/Society of Critical Care Medicine (1992) Consensus Conference: definitions for sepsis and multiple organ failure, and guidelines for the use of innovative therapies in sepsis. Crit Care Med 20:864–874
Wichterman KA, Baue AE, Chaudry IH (1980) Sepsis and septic shock—a review of laboratory models and a proposal. J Surg Res 29:189–201
Le Gall JR, Lemeshow S, Saulnier F (1993) A new simplified acute physiology score (SAPSII) based on a European/North American multicenter study. JAMA 270:2957–2963
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
Movat HZ (1955) Demonstration of all connective tissue elements in a single section. Arch Pathol 60:289–295
Gurr G (1953) A practical manual of medical and biological stain technics. Interscience, New York, p 65
Mowry RW (1956) Alcian blue technics for the histochemical study of acidic carbohydrates. J Histochem Cytochem 4:407
Chiffelle TL, Putt FA (1951) Propylene and ethylene glycol as solvents for Sudan IV and Sudan Black B. Stain Technol 26:51–56
Weber AFM, Philipps MG, Bell JT (1956) An improved method fort he Schultz cholesterol test. J Histochem Cytochem 4:308–309
Sharshar T, Gray F, Lorin de la Grandmaison G, Hopkinson NS, Ross E, Dorandeu A, Orlikowski D, Raphael JC, Gajdos P, Annane D (2003) Apoptosis of neurons in cardiovascular autonomic centres triggered by inducible nitric oxide synthase after death from septic shock. Lancet 362:1799–1805
Melby JC, Spink WW (1958) Comparative studies on adrenal cortical function and cortisol metabolism in healthy adults and in patients with shock due to infection. J Clin Invest 37:1791–1798
Arlt W, Hammer F, Sanning P, Butcher SK, Lord JM, Allolio B, Annane D, Stewart PM (2006) Dissociation of serum dehydroepiandrosterone and dehydroepiandrosterone sulfate in septic shock. J Clin Endocrinol Metab 91:2548–2554
Lichtarowicz-Krynska EJ, Cole TJ, Camacho-Hubner C, Britto J, Levin M, Klein N, Aynsley-Green A (2004) Circulating aldosterone levels are unexpectedly low in children with acute meningococcal disease. J Clin Endocrinol Metab 89:1410–1414
du Cheyron D, Lesage A, Daubin C, Ramakers M, Charbonneau P (2003) Hyperreninemic hypoaldosteronism: a possible etiological factor of septic shock-induced acute renal failure. Intensive Care Med 29:1703–1709
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Polito, A., Lorin de la Grandmaison, G., Mansart, A. et al. Human and experimental septic shock are characterized by depletion of lipid droplets in the adrenals. Intensive Care Med 36, 1852–1858 (2010). https://doi.org/10.1007/s00134-010-1987-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-1987-1