Abstract
Purpose
During septic shock, muscle produces lactate and pyruvate by way of an exaggerated Na+, K+-ATPase-stimulated aerobic glycolysis associated with epinephrine stimulation. We hypothesized that patients with sepsis without shock and increased epinephrine levels or an increased muscle-to-serum lactate gradient are likely to evolve towards septic shock. Thus, in sepsis patients, we investigated (1) whether muscle produces lactate and pyruvate, and (2) whether muscle lactate production is linked to epinephrine levels and the severity of the patient's condition.
Methods
We studied 40 ventilated patients with sepsis without shock or hyperlactatemia and a control group of 10 ICU patients without infection. A microdialysis probe was inserted into the quadriceps muscle. Plasma lactate and pyruvate concentrations were measured in both the dialysate fluid and arterial blood samples every 6 h.
Results
There was no gradient between muscle and arterial levels for lactate and pyruvate in the control group. In the sepsis group, muscle lactate and pyruvate concentrations were consistently higher than the arterial levels (P < 0.01). Plasma epinephrine concentrations were also elevated (P < 0.05). A total of 15/40 patients further developed septic shock, and on admission these patients had significantly higher musculo-arterial gradients of lactate (2.9 ± 0.3 vs. 0.7 ± 0.2 mmol/l) (P < 0.05) and pyruvate (740 ± 60 vs. 200 ± 20 μmol/l) (P < 0.05), and higher levels of epinephrine concentrations (6.2 ± 0.7 vs. 2.5 ± 0.24 nmol/l) (P < 0.05). Both the lactate gradient and epinephrine concentrations measured on admission were good predictors of the evolution towards septic shock.
Conclusions
Muscle produces lactate and pyruvate during sepsis, and this production is highly correlated with plasma epinephrine secretion and severity of illness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperlactatemia in critically ill patients, and particularly those in shock, is usually interpreted as a marker of oxygen debt and global tissue hypoxia, and as a witness of anaerobic metabolism [1–3]. The notion that high lactate levels reflect tissue hypoxia has been questioned in light of data that suggest increased lactate production may occur as a result of aerobic glycolysis stimulated by beta-2-adrenergic receptor ligation [4, 5]. Indeed, several human and animal studies have demonstrated that epinephrine, via β2 stimulation, increases cyclic adenosine monophosphate production, thereby inducing stimulation of glycogenolysis and glycolysis leading to ATP production and activation of the Na+K+-ATPase pump [6, 7]. This activation consumes ATP, thereby leading to the generation of ADP. This ADP, via phosphofructokinase stimulation, reactivates glycolysis and hence generates more pyruvate and, consequently, more lactate. Muscle tissue, which represents approximately 40% of total body cell mass, is particularly involved in this process [8]. We recently demonstrated that, in human septic shock, muscle was a net producer of lactate and that this production could be totally inhibited by ouabain, thus confirming a Na+K+-ATPase-dependent mechanism that appears to be independent of tissue hypoxia [7, 9]. We also recently demonstrated in rat models of hemorrhagic and septic shock that muscle lactate production during shock states is related, at least partially, to increased Na+K+-ATPase activity under β2 stimulation [10].
Catecholamine secretion, especially epinephrine, is a component of the early systemic response to acute physiologic insult. Epinephrine levels increase after cardiac and non-cardiac surgery, brain injury, trauma, myocardial infarction and septic shock [11]. Indeed, the initial reaction to a severe insult, regardless of etiology, is catecholamine secretion. It therefore appears logical that patients with severe sepsis have increased epinephrine levels with a subsequent increase in muscle lactate and pyruvate levels. An increased plasma lactate level is part of the definition of severe sepsis but is not always present, depending on the rate of production compared to the rate of elimination.
The aim of this study was (1) to assess whether patients with sepsis without increased serum lactate levels have an increased muscle lactate and pyruvate production and its relationship with epinephrine levels, and (2) to demonstrate a link between this muscle lactate and pyruvate production, epinephrine concentration and the risk of developing septic shock within the first 48 h after ICU admission.
Methods
Study population
This study was approved by the institutional review board, and patients or their relatives provided written informed consent before enrollment.
To be included in the study, patients had to fulfill the following requirements: (1) a state of sepsis defined according to the ACCP/SCCM consensus criteria [12], (2) a mean arterial pressure (MAP) above or equal to 65 mmHg without vasopressor therapy and (3) a normal plasma lactate concentration (<2.2 mmol/l). All patients were monitored with an arterial catheter (radial or femoral site). Patients previously treated with beta blockers were not included.
A control group of ten hemodynamically stable ICU patients without infection was also studied.
Intervention
The treatment of sepsis was conducted according to international recommendations [13, 14]. Adequate volume resuscitation was assumed to have been achieved when respiratory variations in pulse pressure (in supine patients without spontaneous respiratory efforts) were less than 13% or when additional fluid administration did not yield further increases in the cardiac index (CI). After optimal volume resuscitation and in case of persistent hypotension, we used the lowest effective dose of norepinephrine to maintain MAP between 65 and 70 mmHg. Cardiac index was estimated as per the decision of the physician in charge by echocardiography or PiCCO (PiCCO, Pulsion Medical Systems, Munich, Germany) monitoring combined with intermittent or continuous central venous oxygen saturation (ScvO2) measurement through the venous central catheter placed with the tip in the superior vena cava. Patients were sedated with the lowest effective dose of remifentanyl [50–100 µg/h combined when necessary with a low dose of midazolam (1–5 mg/h].
Microdialysis technique
A microdialysis catheter (CMA 60, CMA/Microdialysis, Stockholm, Sweden) was inserted on admission into the quadriceps femoris muscle. The probe was connected to a microinjection pump that was continuously infused with lactate-free Ringer’s solution. The length of the microdialysis membrane (30 mm) together with a very slow perfusion flow rate of 0.3 μl/min guarantees 100 percent recovery (i.e., uptake of molecules from the interstitial space) for molecules up to 20 kDa, thus providing true tissue concentrations. A 1-h equilibration period was allowed following insertion of the probe, after which measurements were performed every 6 h for 24 h.
Measurements
Lactate, pyruvate and glucose concentrations were measured every 6 h in the dialysate fluid using a CMA 600 analyser (Microdialysis AB, Solna, Sweden) as well as in arterial blood.
For lactate, arterial blood samples were collected in fluoride-oxalate-containing tubes. Lactate was measured by an enzymatic colorimetric method adapted to the Wako automatic analyzer (Biochem Systems, Paris, France). Normal blood values are <2.2 mmol/l.
For pyruvate, arterial blood samples were immediately deproteinized by addition of iced perchloric acid (1 mol/l) and analyzed. Pyruvate was measured by the enzymatic UV method. The normal range of values in blood is 40–68 μmol/l.
Plasma epinephrine concentration was measured using a HPLC electrochemical detection technique and UV detection (Primesep 200, SIELC Technologies, Prospect Heights, IL). Normal blood values are <0.55 nmol/l.
Statistical analysis
Normally distributed (Kolmogorov-Smirnov test) quantitative variables were analyzed by the Student’s t test and results expressed as mean ± SD. Non-normally distributed variables were studied with the non-parametric Mann-Whitney U test and results expressed as median (IQR). The change in measurements over time was calculated for the microdialysis catheter as well as for blood and analyzed using the repeated measures ANOVA test. To determine the sensitivity and specificity of the lactate gradient and epinephrine concentration in predicting septic shock, a ROC curve was constructed. Analysis was completed with the Prism 4.0c software (Graphpad Software, Inc.), and a two-tailed P value of less than 0.05 was considered to indicate statistical significance.
Results
Forty consecutive patients were included with a mean age of 67 ± 12 years. The characteristics of the study groups are shown in Table 1. An infectious microorganism was documented in 36/40 patients.
Control group
The ICU control group included ten hemodynamically stable patients without sepsis admitted for intracerebral haemorrhage. The mean age was 63 ± 5 years. The lactate level was 0.90 ± 0.2 mmol/l, the blood pyruvate level was 0.100 ± 0.04 mmol/l, and the lactate/pyruvate ratio was 8.4 ± 1.5. There was no gradient between muscle and arterial levels for lactate and pyruvate in the control group. The epinephrine level was slightly elevated when compared to our reference values (1.3 ± 0.1 nmol/l vs. 0.55 nmol/l; P = 0.02).
Sepsis group
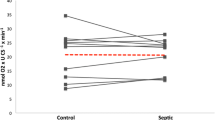
The lactate level was 1.5 ± 0.3 mmol/l, the blood pyruvate level was 0.200 ± 0.05 mmol/l, and the lactate/pyruvate ratio was 7.5 ± 1.4. Muscle lactate concentrations were always higher than arterial lactate levels during the study period with a mean positive gradient of 1.58 ± 0.2 mmol/l (P = 0.001) (Fig. 1 and Figure 1 online supplement). Similarly, the muscle pyruvate concentration was always higher than the arterial pyruvate levels during the study period with a mean positive gradient of 370 ± 30 μmol/l (P = 0.03) (Figure 2, online supplement). At T0, there was a strong correlation between the lactate gradient and epinephrine concentration (r 2 = 0.7084, P < 0.0001) (Fig. 2).
Evolution of lactate gradient (muscle lactate-arterial lactate) during the first 24 h. Open squares sepsis group (septic patients who did not develop septic shock; filled squares septic shock group (septic patients who developed septic shock). Numerical values indicate the number of patients in septic shock at the time of measurement. Values are expressed as mean ± SD
Plasma glucose was elevated in all septic patients (8.2 ± 0.80 mmol/l). Epinephrine concentrations were also elevated when compared to reference values but also compared to the control population (3.4 ± 0.4 nmol/l vs. 0.55 nmol/l vs. 1.3 ± 0.1 nmol/l, respectively) (P = 0.01) (Fig. 3).
Fifteen out of 40 patients further developed septic shock within 48 h after severe sepsis diagnosis. The only difference between these patients and patients who did not develop septic shock was significantly higher levels of the musculo-arterial gradient for both lactate (2.9 ± 0.3 vs. 0.7 ± 0.2) (Fig. 1 and Figure 1 online supplement) and pyruvate (740 ± 60 vs. 200 ± 20 μmol/l) (Figure 2 online supplement) and higher levels of epinephrine concentrations (6.2 ± 0.7 nmol/l vs. 2.5 ± 0.24 nmol/l) (Fig. 4). Moreover, these patients exhibited slightly increased lactate and pyruvate musculo-arterial gradients during the first 24 h (Fig. 1 and Figure 2 online supplement). Conversely, patients who did not develop septic shock normalized their gradients after 24 h (P = 0.004) (Fig. 1 and Figure 2 online supplement). Glucose concentrations did not differ between the two groups. The correlation between epinephrine concentration and lactate gradient was performed by means of a linear regression test.
To determine the optimal cutoff value of the musculo-arterial lactate gradient and epinephrine concentration on admission in predicting the evolution to septic shock, a ROC curve was constructed (Figure 3 online supplement and Figs. 4, 5). The area under the curve was, respectively, 0.96 ± 0.03 (95% CI, 0.90–1.02, P < 0.0001) and 0.94 ± 0.05 (95% CI, 0.83–1.052, P < 0.0001). At a value of 1.5 mmol/l, the musculo-arterial lactate gradient predicted the evolution to septic shock with a sensitivity of 100% (95% CI, 78–100%) and a specificity of 92% (95% CI, 74–99%). The positive likelihood ratio was 12.5. Conversely, at a value equal or superior to 5.2 nmol/l, the epinephrine concentration predicted the evolution to septic shock with a sensitivity of 93% (95% CI, 68–99%) and a specificity of 100% (95% CI, 86–100%). The positive likelihood ratio was 25.
Discussion
This study demonstrates that patients with sepsis but without shock have increased epinephrine concentrations associated with muscle production of lactate and pyruvate despite normal plasma values. Moreover, patients who subsequently developed septic shock exhibited higher levels of both epinephrine and muscle lactate and pyruvate concentrations on admission.
Muscle produces lactate during sepsis
The microdialysis technique involves the insertion in a given tissue (brain, muscle, adipose tissue) of a semi-permeable membrane continuously infused with dialysis solution. At the tissue level, the solutes present in the interstitium freely diffuse into the catheter according to their concentration gradient. At a very low perfusion flow rate (0.3 μl/min) such as that used in this study, the gradient between muscle interstitium and arterial blood concentration indicates whether muscle produces or utilizes a specific substrate [15].
In the present study, lactate and pyruvate levels were always higher in muscle than in blood. This contrasts sharply to the findings observed in volunteers where the gradient between muscle and arterial blood was very low. This argues strongly in favor of muscle lactate production during severe sepsis [16]. As skeletal muscles account for approximately 40 percent of the total body-cell mass, this tissue may represent a major source of lactate during sepsis. On the other hand, despite a marked increase in lactate production, our patients did not have hyperlactatemia. The blood level of lactate reflects a balance between the production and metabolism of lactate by various tissues. In general, this concentration is less than 2 mmol/l, although daily production of lactate is actually 1,500 mmol/l [8]. Generated lactate can be transformed into oxaloacetate or alanine via the pyruvate pathway or can be utilized directly by periportal hepatocytes (60%) to produce glycogen and glucose (neoglycogenesis and neoglucogenesis; Cori cycle). The kidney also participates in the metabolism of lactate (30%), with the cortex classically acting as a metabolizer by neoglucogenesis and the medulla as a producer of lactate [8]. Since the blood lactate level reflects a balance between production and clearance, for any given mechanism that leads to increased lactate production, the blood level of lactate can be either elevated or normal, depending on the rates of lactate clearance. In this study, in the absence of tissue hypoxia and with normal liver and kidney function, it is likely that the increased rate of lactate production was compensated by an increase in lactate clearance.
Muscle lactate production is correlated with epinephrine concentration
According to previous experimental and clinical studies, it is likely that excess muscle lactate production during severe sepsis is linked to the higher levels of circulating epinephrine that we observed. Indeed, in the present study, we observed a strong correlation (r 2 = 0.7084) between epinephrine concentration and muscle-arterial blood lactate gradient. Moreover, we previously demonstrated the link between increased epinephrine levels and lactate muscle production in a rat study [10]. Epinephrine, which is one of the main stress mediators, acts on alpha, beta 1 as well as beta 2 adrenoreceptors [17]. The latter are considered to be responsible for the metabolic actions of epinephrine including hyperglycemia, hyperlactatemia and hypokalemia [18]. Epinephrine is associated with an increase in lactate concentration not only in patients with sepsis, but also in healthy volunteers at rest and during exercise. In the present study, our patients did not have any signs of hypoxia since they were normotensive, normolactatemic and had a normal lactate/pyruvate ratio, hence arguing for a non-hypoxic mechanism to explain muscle lactate formation.
Accelerated aerobic glycolysis during sepsis without shock
The basis for aerobic lactate muscle production during sepsis without shock is not straightforward. Accelerated aerobic glycolysis, a definite state when the rate of glucose metabolism exceeds the oxidative capacity of the mitochondria, is present during states associated with an increase in endogenous or exogenous catecholamines [19]. During sepsis, epinephrine-induced muscle lactate production may serve as the main metabolic precursor for the liver via the Cori cycle to produce glucose that may be used by glucose-dependent organs [20].
Muscle lactate production and plasma epinephrine concentrations are predictive of septic shock
Of further interest is the fact that the persistence and even the accentuation of the initial stress responses characterized by a persistent increase in epinephrine concentration is an early marker of severity despite the absence of systemic hemodynamic disorders. Muscle microdialysis is an invasive, expensive and difficult technique, and its use as a monitoring tool during sepsis is difficult for consideration in a routine manner. Nevertheless, the concept of a muscle lactate gradient as a predictor of septic shock is new. More useful for clinical practice is the fact that plasma epinephrine is also linked to patient prognosis. We are currently investigating the value of combined indirect signs of elevated epinephrine concentration, such as hyperglycemia, hypokaliemia and tachycardia, in predicting prognosis in sepsis.
Conclusions
Muscle lactate production (1) is present in severe sepsis despite the absence of systemic hyperlactatemia, (2) is correlated with the intensity of stress-induced epinephrine secretion and (3) is an early marker of septic shock.
References
Cohen RD, Woods HF (1983) Lactic acidosis revisited. Diabetes 32:181–191
Bakker J, Coffernils M, Leon M, Gris P, Vincent JL (1991) Blood lactate levels are superior to oxygen-derived variables in predicting outcome in human septic shock. Chest 99:956–962
Kompanje EJ, Jansen TC, van der Hoven B, Bakker J (2007) The first demonstration of lactic acid in human blood in shock by Johann Joseph Scherer (1814–1869) in January 1843. Intensive Care Med 33:1967–1971
James JH, Fang CH, Schrantz SJ, Hasselgren PO, Paul RJ, Fischer JE (1996) Linkage of aerobic glycolysis to sodium-potassium transport in rat skeletal muscle. Implications for increased muscle lactate production in sepsis. J Clin Invest 98:2388–2397
James JH, Luchette FA, McCarter FD, Fischer JE (1999) Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet 354:505–508
James JH, Wagner KR, King JK, Leffler RE, Upputuri RK, Balasubramaniam A, Friend LA, Shelly DA, Paul RJ, Fischer JE (1999) Stimulation of both aerobic glycolysis and Na+-K+-ATPase activity in skeletal muscle by epinephrine or amylin. Am J Physiol 277:E176–E186
Levy B, Gibot S, Franck P, Cravoisy A, Bollaert PE (2005) Relation between muscle Na+K+ ATPase activity and raised lactate concentrations in septic shock: a prospective study. Lancet 365:871–875
Levy B (2006) Lactate and shock state: the metabolic view. Curr Opin Crit Care 12:315–321
Leverve XM (1999) Energy metabolism in critically ill patients: lactate is a major oxidizable substrate. Curr Opin Clin Nutr Metab Care 2:165–169
Levy B, Desebbe O, Montemont C, Gibot S (2008) Increased aerobic glycolysis through beta2 stimulation is a common mechanism involved in lactate formation during shock states. Shock 30:417–421
Barth E, Albuszies G, Baumgart K, Matejovic M, Wachter U, Vogt J, Radermacher P, Calzia E (2007) Glucose metabolism and catecholamines. Crit Care Med 35:S508–S518
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS International sepsis definitions conference. Crit Care Med 31:1250–1256
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M (2001) Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345:1368–1377
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL (2008) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med 34:17–60
Rosdahl H, Ungerstedt U, Henriksson J (1997) Microdialysis in human skeletal muscle and adipose tissue at low flow rates is possible if dextran-70 is added to prevent loss of perfusion fluid. Acta Physiol Scand 159:261–262
Daniel AM, Shizgal HM, MacLean LD (1978) The anatomic and metabolic source of lactate in shock. Surg Gynecol Obstet 147:697–700
Clausen T, Flatman JA (1980) Beta 2-adrenoceptors mediate the stimulating effect of adrenaline on active electrogenic Na-K-transport in rat soleus muscle. Br J Pharmacol 68:749–755
Bearn AG, Billing B, Sherlock S (1951) The effect of adrenaline and noradrenaline on hepatic blood flow and splanchnic carbohydrate metabolism in man. J Physiol 115:430–441
Gladden LB (2004) Lactate metabolism—a new paradigm for the third millennium. J Physiol 558:5–30
Bakker J, Jansen TC (2007) Don’t take vitals, take a lactate. Intensive Care Med 33:1863–1865
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Levy, B., Perez, P., Gibot, S. et al. Increased muscle-to-serum lactate gradient predicts progression towards septic shock in septic patients. Intensive Care Med 36, 1703–1709 (2010). https://doi.org/10.1007/s00134-010-1938-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-1938-x