Abstract
Objective
Assessment of electrocardiographic (ECG) effects of dexmedetomidine.
Design
Prospective observational study including children 0–17 years of age with congenital heart disease (CHD) and children following cardiothoracic surgery. Patients who did not receive dexmedetomidine were used as a control group. All patients had two ECGs: one baseline, pre-dexmedetomidine (T1) and one during dexmedetomidine infusion (T2).
Measurements and results
Fifty-one patients, median age of 0.5 years (IQR = 3.4), and 25 patients, age 0.25 (IQR = 2.9), were included in the dexmedetomidine and control groups, respectively. Forty received a dexmedetomidine-loading dose of 1 µg/kg (IQR = 0.5). At T2, the dexmedetomidine infusion was 1 µg/kg/h (IQR = 0.5). In the dexmedetomidine group, heart rate (HR) decreased from 140 ± 22 to 115 ± 23 (P < 0.001); PR, PRc and PR index changed from 115 ± 28 to 122 ± 29 ms (P = 0.01), 174 ± 38 to 167 ± 35 ms (P = 0.07) and 15,882 ± 3,565 to 13,792 ± 3,311 (P < 0.001), respectively. QRS decreased from 84 ± 21 to 80 ± 21 ms (P = 0.02), and QTc had no change (433 ± 47 to 435 ± 36 ms). When compared to the control group, none of the ECG intervals had any difference other than a trend towards lower HR (P = 0.08). Neonates and infants had a bigger drop in the HR compared to older children (P < 0.001), while other parameters were similar. At T2 none of the dexmedetomidine group patients had atrioventricular block or other arrhythmia. Four patients in the control group had accelerated junctional rhythm.
Conclusions
Use of dexmedetomidine in patients with CHD and patients following cardiothoracic surgery is not associated with any significant ECG interval abnormalities other than a trend towards lower HR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dexmedetomidine is a highly selective alpha 2-agonist with sedative and analgesic properties that has been shown to reduce the postoperative requirements of additional intravenous sedative and analgesic agents. Additionally, dexmedetomidine appears to maintain the patient’s underlying hemodynamic stability and has minimal respiratory side effects [1, 2]. Though the US Food and Drug Administration has approved its use only in adult patients, there have been an increasing number of reports on its use in pediatric patients. Dexmedetomidine has been used to provide sedation and analgesia in the pediatric intensive care unit, for procedural sedation and treatment of withdrawal symptoms from opioids, and recently it has be used as a treatment option for perioperative supraventricular arrhythmias [3–10].
Dexmedetomidine mediates its effects through a complex mechanism that involves both presynaptic and postsynaptic receptor activation. Activation of the presynaptic alpha-2 adrenoreceptors on sympathetic nerves and the central nervous system induces sympatholysis, which is responsible for the hypotensive and bradycardic side effects. Though dexmedetomidine appears to have a wide safety margin, there have been concerns related to the development of sudden sinus node pauses, symptomatic bradycardia and its potential to cause atrioventricular nodal block and prolongation of the QTc interval [11–13]. The aim of this study was to characterize the electrocardiographic (ECG) effects of dexmedetomidine in children with congenital heart disease (CHD) and children undergoing cardiothoracic surgery.
Materials and methods
This prospective, observational study was approved by the Institutional Review Board, and informed consent was obtained from all patients. Patients 0–17 years old who were admitted to the cardiac intensive care unit (CICU) from June 2008 to January 2009 and who were scheduled to receive dexmedetomidine were enrolled in the study.
To evaluate the effect of dexmedetomidine, two 12-lead ECGs per patient were obtained: a baseline ECG before the start of dexmedetomidine (T1) and a second ECG while on dexmedetomidine infusion (T2). Both ECGs were part of the routine CICU care. One ECG is routinely obtained upon admission of a patient, and another one is obtained on the morning of each CICU day. Dexmedetomidine dose and timing were determined by the clinical team. The usual loading dose of dexmedetomidine in our institution is 0.5–1.0 µg/kg followed by a continuous infusion of 0.2–1.5 µg/kg/h. Data collected and analyzed included standard demographic information, diagnosis and surgical procedure performed, dexmedetomidine dose and other medications that patients received at the time of the study. Furthermore, the following laboratory values were analyzed: potassium (K+), magnesium (Mg2+) and ionized calcium (iCa2+) levels at T1 and T2. All ECGs were analyzed manually for heart rate (HR) and PR, QRS and QT intervals. The PR and QT intervals were corrected for HR, PRc and QTc, respectively, using Bazett’s formula (corrected value = observed value/√[R − R′]) [14]. To further assess the influence of HR on the PR interval, the PR index was calculated by multiplying the PR interval with the HR [15]. All ECGs were analyzed separately by two investigators, both blinded to the timing of dexmedetomidine.
Exclusion criteria included patients who had received any antiarrhythmic medications within 7 days of the study, patients with ongoing arrhythmias at the time of the baseline ECG and patients receiving medications with potential ECG effects, specifically calcium channel and beta blockers, ketamine, amphotericin B and fluconazole. Patients receiving digoxin for anti-arrhythmic purposes were also excluded; however, if the indication for digoxin was for inotropic support, they were included in the study. Patients who were on dexmedetomidine at T1 but not at T2 were also excluded.
For a control group, a retrospective chart review was performed to identify patients who met the following conditions: postoperative cardiac surgery, did not receive dexmedetomidine, had two ECGs performed (T1: immediately after surgery and T2: first postoperative day) and satisfied the same exclusion criteria. Patients in this group received either fentanyl or morphine as needed for analgesia. For study analysis purposes, the effect of these drugs on the ECG was considered minimal if patients did not receive a dose within 5 h of T2. These patients were therefore considered as they had not received any fentanyl or morphine.
Statistical analysis was performed using SPPS version 16.0. The sample results were tested for normality of distribution using the Shapiro–Wilk test. Descriptive data, including demographic information, dexmedetomidine dose and duration and the various ECG intervals, are presented as mean ± standard deviation or as median (interquartile range, IQR) where appropriate. Differences in ECG data and electrolyte values were compared between the two time points using a paired sample t test. Further subgroup analysis among neonates, infants and older children was performed with analysis of variance (ANOVA) by Kruskal–Wallis test or with one-way ANOVA where appropriate. For post hoc examination the Tukey’s test was applied. Comparison between low-dose and high-dose dexmedetomidine groups was performed with an unpaired t test or Mann–Whitney test where appropriate. Correlation between K+, Mg2+ and iCa2+ levels and ECG intervals was assessed using the Pearson’s correlation coefficient. Statistical tests were two-sided, and P < 0.05 was considered significant. Comparison of ECG parameters between the dexmedetomidine and control groups was performed using an unpaired sample t test. Interobserver reliability was calculated for every ECG interval using the Cronbach’s alpha reliability coefficient. A value of ≥0.8 was considered good reliability.
Results
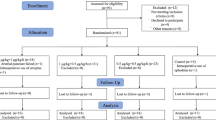
A total of 55 patients were screened for eligibility, and 51 were finally enrolled. Four patients were excluded because of postoperative arrhythmias, specifically junctional accelerated rhythm at T1. The baseline characteristics, diagnosis and procedures performed in both the dexmedetomidine and control groups are presented in Tables 1 and 2. The control group included 25 post cardiothoracic surgery patients, none of whom had received dexmedetomidine prior to T2.
The dexmedetomidine infusion dose was adjusted by the clinical team according to the patient’s clinical response as well as per the intensive care unit sedation scales. Due to the fact that dexmedetomidine has a terminal t 1/2 of approximately 2 h and to ensure that the ECG at T2 was obtained during a steady-state plasma concentration, we further analyzed the dexmedetomidine dose requirements for the 2 h prior to T2. In 44 (86%) patients, the dexmedetomidine infusion dose remained unchanged for the last 2 h prior to T2. The complete description of dexmedetomidine duration and dose requirement is shown in Table 1. None of the patients had an interruption of the dexmedetomidine infusion between T1 and T2. All ECGs were analyzed by two investigators, with an interobserver reliability that ranged from 0.88 for QTc (lowest) to 0.97 for HR (highest).
Heart rate decreased by approximately 17 ± 15% (P < 0.001), and QRS decreased by 4 ± 16% (P = 0.02). QTc interval remained unchanged. The uncorrected PR interval was statistically increased; however, when corrected for HR, both the PRc and PR indexes were actually shorter (P = 0.07 and P < 0.001, respectively) (Table 3). Using Pearson’s correlation coefficient, it was also shown that there was a statistically significant negative correlation between the HR and PR interval (−0.4, P = 0.009). Despite the observed changes after the administration of dexmedetomidine, when compared with the control group, we did not find any significant differences other than a tendency towards a lower HR in the dexmedetomidine group (Table 3). None of the ECGs showed any evidence of any type of atrioventricular block. One patient in the dexmedetomidine group had sinus bradycardia (10 years old; HR, 64 bpm), and four patients in the control group had accelerated junctional rhythm (HR 118 ± 27 bpm). Eight patients (16%) in the dexmedetomidine group and 17 patients (68%) in the control group were receiving an opioid infusion (fentanyl or morphine) at the time of T2 (Table 1).
The K+, Mg2+ and iCa2+ levels were all statistically different at T2. The K+ increased from 3.5 ± 0.6 to 3.9 ± 0.6 mEq/l (P = 0.005), the Mg2+ decreased from 2.2 ± 0.4 to 1.9 ± 0.3 mg/dl (P = 0.003) and iCa2+ decreased from 1.3 ± 0.2 to 1.2 ± 0.1 mg/dl (0.001). None of these electrolyte changes, however, correlated with any of the ECG interval changes.
To evaluate if younger patients responded differently to dexmedetomidine, we performed a subgroup analysis among neonates, infants and older children (Table 4). Neonates and infants had a statistically bigger drop in HR compared to the older patients; however, there were no differences in the remaining ECG parameters with the exception of the PR interval. As expected, the PR interval was longer in the older patients at baseline and remained longer during the dexmedetomidine infusion; however, the percent change from baseline did not differ among the age groups.
To evaluate if any of the ECG changes were dose dependent, we compared patients with dexmedetomidine infusions running at less than 0.8 µg/kg/h (lower dose group) versus patients with infusions running at more than 1 µg/kg/h (higher dose group) at T2 (Table 5). No difference was found in any of the ECG parameters.
Five patients were receiving digoxin (approximate mean dose of 8 µg/kg/day) for inotropic support at the time of dexmedetomidine administration. The mean age was 4 ± 5 months; all patients received a dexmedetomidine loading dose of 0.9 ± 0.7 µg/kg, and the dexmedetomidine infusion dose at T2 was 0.9 ± 0.3 µg/kg. The characteristic results of these patients appeared to be similar to the rest of the patients. HR changed from 140 ± 8 to 115 ± 20 bpm (P = 0.03); there was no change in the PR interval [108 ± 19 to 112 ± 19 ms (P = 0.59)], QRS [84 ± 13 to 82 ± 15 ms (P = 0.6)] and QTc [409 ± 42 to 393 ± 34 ms (P = 0.6)]. None had any significant bradycardia or any atrioventricular block at T2.
Discussion
Dexmedetomidine’s unique safety profile of causing minimal respiratory depression while providing excellent analgesia, anxiolysis, decreased delirium, a distinctive type of “arousable sedation” and its potential anti-arrhythmogenic effects have made it one of the main sedative agents in the armamentarium of cardiac anesthesiologists and intensivists [9, 16, 17]. Despite dexmedetomidine’s increased usage in the pediatric population, its effects on the ECG and cardiac conduction tissue are not well described in the literature.
In this study, dexmedetomidine was used to provide sedation and analgesia in patients with CHD during the perioperative period and after cardiac catheterization. The baseline, pre-dexmedetomidine ECG data were compared to the ECG data obtained at a median of 13 h after dexmedetomidine initiation. In addition, we compared ECG data between patients who received dexmedetomidine and a control group who received mostly an opioid infusion.
Though the HR decreased as expected by approximately 17%, when compared to the control group, there was no substantial difference (P = 0.08). This HR effect appeared to be more pronounced in neonates and infants, and we believe that this difference is most likely related to the parasympathetic system domination and immaturity of the sympathetic nervous system that exists in this younger age. A comparison between a lower and a higher dose of dexmedetomidine (median 0 5. vs. 1 µg/kg/h) showed no significant differences in the heart rate or other ECG parameters. This was not surprising since most of dexmedetomidine’s sympatholytic effect occurs already at the lower doses. In a study by Snapir et al. [18], epinephrine and norepinephrine plasma levels decreased on the average by approximately 70% during a low-dose dexmedetomidine infusion level (0.5 ng/ml), and only slight further decreases were noted during high-dose dexmedetomidine infusion levels (5 ng/ml).
The PR interval, which represents the intra-atrial and atrioventricular nodal conduction system, was mostly unaffected by dexmedetomidine. The PR interval, similar to the QT interval, is affected by HR, and it’s important to take that inter-relationship into account. This effect has been well demonstrated in previous studies that have shown that there is a significant negative linear association between the two [19–21]. In our study, though the uncorrected PR interval was increased, when this was adjusted for HR, there was no significant difference. On the contrary, there was a tendency towards a shorter PRc interval and a significantly decreased PR index. No difference was observed when compared to the control group.
An unexpected finding was the statistically shorter QRS duration. The QRS duration usually decreases with increased sympathetic activity, i.e., exercise, etc., and therefore a sympatholytic agent like dexmedetomidine would be expected to have no effect or if anything increase the QRS duration. This finding could not be explained with our study design; however, the fact that there was a similar shortening in the control group makes us believe that this may represent the natural postoperative course after cardiothoracic surgery. The QTc, a measure of ventricular repolarization, remained unchanged.
Our findings do differ slightly from a previous study by Hammer et al. [13]. This study involved 12 children, 5–17 years of age, who underwent electrophysiology study and ablation of supraventricular accessory pathways, and had electrophysiologic variables measured before and during administration of dexmedetomidine (1 µg/kg over 10 min followed by a 10-min continuous infusion of 0.7 µg/kg/h). The study concluded that the HR was decreased, QRS was unchanged, QTc was prolonged, and atrioventricular nodal function was depressed, as evidenced by Wenckeback cycle length prolongation and prolongation of the PR interval. This study had fewer patients, and the measurements were performed under the effect of other drugs, i.e., ketamine and propofol. Though the electrophysiologic effects of these latter drugs are not well described, they could have potentially influenced the dexmedetomidine effects in a synergistic or additive manner. Furthermore, this study did not take into consideration the HR-PR interval relationship, and thus no adjustments were made.
Although our patient population included a diverse group of patients including single ventricle and other complex CHDs, none of the ECGs showed any evidence of atrioventricular block or significant bradycardia. The five patients who were on digoxin at the time of the study did not appear to have any significant differences in the ECG parameters compared with other patients, and none had any significant bradycardia. This is contrary to a case report by Berkenbosch et al. [22] in which an infant who was concurrently receiving dexmedetomidine and digoxin developed significant bradycardia requiring discontinuation of the dexmedetomidine infusion. Nonetheless, despite our encouraging experiences, it is very important to note that patients who receive dexmedetomidine along with other sympatholytic or parasympathomimetic medications should be monitored closely for the development of significant bradycardia and/or hypotension [23, 24].
Because electrolyte abnormalities can have a significant effect on the ECG, we compared the baseline K+, Mg2+ and iCa2+ levels with the levels obtained at T2. At the time of the second ECG, patients had a statistically increased K+ and decreased Mg2+ and iCa2+ level. Though a decreased Mg2+ and iCa2+ level can cause prolongation of the QTc interval and an increased K+ level could cause prolongation of the PR and QRS intervals, none of these changes was observed in this study [25, 26]. On the contrary, there was a tendency towards a shorter PRc and QRS intervals.
Because this study included only children with CHD and patients following cardiopulmonary bypass, these results may not be applicable to other patient populations. The metabolic alterations following CPB and the potential influences that these might have on the ECG intervals are not addressed by this study. In addition, it is important to note that we only assessed ECG changes from 12-lead ECGs and not from a continuous telemetry. Therefore, changes that may have occurred in the interim, i.e., arrhythmias, are not addressed.
Overall, dexmedetomidine, a relatively new alpha 2-adrenergic agonist, appears to have several unique properties that make it an attractive agent for intensivists caring for patients after cardiothoracic surgery. Some of these properties include an opioid sparing effect and minimal respiratory depression, which allows for early extubation without the interruption of continuous sedation and analgesia. Though the current study adds to the body of literature and demonstrates dexmedetomidine’s wide electrophysiologic safety margin, dexmedetomidine’s inherent sympatholytic properties warrant close monitoring to avoid significant bradycardia and/or hypotension.
Conclusion
The use of dexmedetomidine in patients with CHD and patients following cardiothoracic surgery is not associated with any significant or any atypical ECG interval abnormalities other than an expected trend towards a decrease in heart rate.
References
Triltsch AE, Welte M, von Homeyer P, Grosse J, Genähr A, Moshirzadeh M, Sidiropoulos A, Konertz W, Kox WJ, Spies CD (2002) Bispectral index-guided sedation with dexmedetomidine in intensive care: a prospective, randomized, double blind, placebo-controlled phase II study. Crit Care Med 30:1007–1014
Venn RM, Bradshaw CJ, Spencer R, Brealey D, Caudwell E, Naughton C, Vedio A, Singer M, Feneck R, Treacher D, Willatts SM, Grounds RM (1999) Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia 54:1136–1142
Mukhtar AM, Obayah EM, Hassona AM (2006) The use of dexmedetomidine in pediatric cardiac surgery. Anesth Analg 103:52–56
Chrysostomou C, Di Filippo S, Manrique AM, Schmitt CG, Orr RA, Casta A, Suchoza E, Janosky J, Davis PJ, Munoz R (2006) Use of dexmedetomidine in children after cardiac and thoracic surgery. Pediatr Crit Care Med 7:126–131
Chrysostomou C, Sanchez De Toledo J, Avolio T, Motoa MV, Berry D, Morell VO, Orr R, Munoz R (2009) Dexmedetomidine use in a pediatric cardiac intensive care unit: Can we use it in infants after cardiac surgery? Pediatr Crit Care Med [Epub ahead of print]
Tobias JD, Berkenbosch JW (2004) Sedation during mechanical ventilation in infants and children: dexmedetomidine versus midazolam. South Med J 97:451–455
Munro HM, Tirotta CF, Felix DE, Lagueruela RG, Madril DR, Zahn EM, Nykanen DG (2007) Initial experience with dexmedetomidine for diagnostic and interventional cardiac catheterization in children. Paediatr Anaesth 17:109–112
Barton KP, Munoz R, Morell VO, Chrysostomou C (2008) Dexmedetomidine as the primary sedative during invasive procedures in infants and toddlers with congenital heart disease. Pediatr Crit Care Med 9:612–615
Chrysostomou C, Beerman L, Shiderly D, Berry D, Morell VO, Munoz R (2008) Dexmedetomidine: a novel drug for the treatment of atrial, junctional tachyarrhythmias during the perioperative period for congenital cardiac surgery: a preliminary study. Anesth Analg 107:1514–1522
Tobias JD (2006) Dexmedetomidine to treat opioid withdrawal in infants following prolonged sedation in the pediatric ICU. J Opioid Manag 2:201–205
Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD (2000) The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology 93:382–394
Bloor BC, Ward DS, Belleville JP, Maze M (1992) Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology 77:1134–1142
Hammer GB, Drover DR, Cao H, Jackson E, Williams GD, Ramamoorthy C, Van Hare GF, Niksch A, Dubin AM (2008) The effects of dexmedetomidine on cardiac electrophysiology in children. Anesth Analg 106:79–83
Mirvis DM, Goldberger AL (2005) Electrocardiography. In: Zipes DP, Libby P, Bonow RO, Braunwald E (eds) Braunwald’s heart disease: a textbook of cardiovascular medicine. Elsevier Saunders, Philadelphia, p 118
Cagli K, Ozbakir C, Ergun K, Bakuy V, Circi R, Circi P (2006) Electrocardiographic changes after coronary artery surgery. Asian Cardiovasc Thorac Ann 14:294–299
Chrysostomou C, Schmitt CG (2008) Dexmedetomidine: sedation, analgesia and beyond. Expert Opin Drug Metab Toxicol 4:619–627
Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, Whitten P, Margolis BD, Byrne DW, Ely EW, Rocha MG (2009) Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 301:489–499
Snapir A, Posti J, Kentala E, Koskenvuo J, Sundell J, Tuunanen H, Hakala K, Scheinin H, Knuuti J, Scheinin M (2006) Effects of low and high plasma concentrations of dexmedetomidine on myocardial perfusion and cardiac function in healthy male subjects. Anesthesiology 105:902–910
Atterhög JH, Loogna E (1977) P–R interval in relation to heart rate during exercise and the influence of posture and autonomic tone. J Electrocardiol 10:331–336
Alimurung MM, Massell BF (1956) The normal P–R interval in infants and children. Circulation 13:257–262
Danter WR, Carruthers SG (1990) The heart rate-PR interval relationship: a model for evaluating drug actions on SA and AV nodal function. Br J Clin Pharmacol 30:490–492
Berkenbosch JW, Tobias JD (2003) Development of bradycardia during sedation with dexmedetomidine in an infant concurrently receiving digoxin. Pediatr Crit Care Med 4:203–205
Shah AN, Koneru J, Nicoara A, Goldfeder LB, Thomas K, Ehlert FA (2007) Dexmedetomidine related cardiac arrest in a patient with permanent pacemaker; a cautionary tale. Pacing Clin Electrophysiol 30:1158–1160
Sichrovsky TC, Mittal S, Steinberg JS (2008) Dexmedetomidine sedation leading to refractory cardiogenic shock. Anesth Analg 106:1784–1786
Herndon RF, Meroney WH, Pearson CM (1955) The electrocardiographic effects of alterations in concentration of plasma chemicals. Am Heart J 50:188–202
Weiner M, Epstein FH (1970) Signs and symptoms of electrolyte disorders. Yale J Biol Med 43:76–109
Acknowledgments
Support for this work was provided by the Department of Pediatric Cardiology at Children’s Hospital of Pittsburgh and by Hospira Inc., Lake Forest, Illinois.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chrysostomou, C., Komarlu, R., Lichtenstein, S. et al. Electrocardiographic effects of dexmedetomidine in patients with congenital heart disease. Intensive Care Med 36, 836–842 (2010). https://doi.org/10.1007/s00134-010-1782-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-1782-z