Abstract
Purpose
To determine whether the provision of early standard enteral nutrition (EN) confers treatment benefits to critically ill patients.
Methods
Medline and EMBASE were searched. Hand citation review of retrieved guidelines and systematic reviews were undertaken, and academic and industry experts were contacted.
Methodologically sound randomised controlled trials (RCTs) conducted in critically ill patient populations that compared the delivery of standard EN, provided within 24 h of intensive care unit (ICU) admission or injury, to standard care were included.
The primary analysis was conducted on clinically meaningful patient-oriented outcomes. Secondary analyses considered vomiting/regurgitation, pneumonia, bacteraemia, sepsis and multiple organ dysfunction syndrome. Meta-analyses were conducted using the odds ratio (OR) metric and a fixed effects model. The impact of heterogeneity was assessed using the I 2 metric.
Results
Six RCTs with 234 participants were analysed. The provision of early EN was associated with a significant reduction in mortality [OR = 0.34, 95% confidence interval (CI) 0.14–0.85] and pneumonia (OR = 0.31, 95% CI 0.12–0.78). There were no other significant differences in outcomes. A sensitivity analysis and a simulation exercise confirmed the presence of a mortality reduction.
Conclusion
Although the detection of a statistically significant reduction in mortality is promising, overall trial quality was low, trial size was small, and the findings may be restricted to the patient groups enrolled into included trials. The results of this meta-analysis should be confirmed by the conduct of a large multi-centre trial enrolling diverse critically ill patient groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the plethora of published guidelines recommending the provision of enteral nutrition (EN) within 24–48 h of intensive care unit (ICU) admission [1–4], observational studies reveal up to 40% of critically ill patients receive no nutritional support during their ICU stay [5]. Furthermore, 60% of patients who stay in the ICU at least 3 days remain unfed for 48 h or longer [4]. It is possible that current guideline recommendations for the provision of early EN in critical illness are not consistently translated into practice because they are not supported by sufficiently convincing evidence.
Previously published systematic reviews demonstrate the provision of early EN may have clinically important benefits in non-critically ill patient populations. In patients undergoing elective intestinal surgery, who were not critically ill, early EN resulted in a statistically significant reduction in mortality [relative risk (RR) 0.41, 95% confidence interval (CI) 0.18–0.93, P = 0.03, I 2 = 0.0%) [6]. Likewise, in non-critically ill patients hospitalised for an acute medical condition, early EN resulted in a statistically significant reduction in overall infectious complications (RR 0.45, 95% CI 0.3–0.66, P = 0.00006, heterogeneity P = 0.049) [7]. The only systematic review to focus on critically ill patients, published in 2003, failed to find any statistically significant benefits attributable to the provision of early EN [3].
The purpose of this project was to identify and synthesise the current evidence from methodologically sound randomised controlled trials (RCTs) conducted in critically ill patients and determine whether the provision of early standard EN confers a treatment benefit, on average, in the identified studies.
Materials and methods
Literature search
Medline (http://www.PubMed.org) and EMBASE (http://www.EMBASE.com) were searched using appropriately broad Medical Subject Heading and EMTREE terms for nutritional support and critical illness, crossed with phrases optimised to detect RCTs [8, 9].
Academic and industry experts were contacted, and reference lists of identified systematic reviews and evidence-based guidelines were hand searched. The search was not restricted by language. Complete details of the search process are available upon request. The search close out date was 1 October 2008.
Study selection
All controlled trials comparing primary feeding interventions published in any language were identified [10, 11]. Study selection was undertaken independently by at least three authors.
Early EN was defined as the provision of a standard EN formula via any feeding tube route within 24 h of initial injury or ICU admission [1, 2, 4]. A standard EN formula was considered to be any formula not supplemented with additional glutamine, arginine or other immune-enhancing ingredients. Appropriate comparison groups were accepted to include all forms of standard care, including standard EN provided later than 24 h after injury or ICU admission.
Trials reporting clinically meaningful patient-oriented outcomes [12] conducted in critically ill populations [13] were considered for inclusion. Only methodologically sound RCTs, which were free from major methodological flaws, were eligible (http://clinicalevidence.bmj.com/ceweb/about/appraisal.jsp, visited 6 March 2009). Major methodological flaws were defined a priori as pseudo-randomisation (clear failure to maintain allocation concealment) and excessive (>10%) loss to follow-up [14].
Publications based on subgroups of patients from larger published trials were not eligible for inclusion if the larger trial’s patient population was already deemed eligible.
Validity appraisal
All included trials were appraised on the reporting of three key methodological criteria: (1) the maintenance of allocation concealment, (2) the use of any form of blinding and (3) the completeness of patient follow-up [15]. Validity appraisal was undertaken independently by at least three authors.
Outcomes
All clinically meaningful patient-oriented outcomes (mortality, quality of life and physical function) [12] were considered in the primary analysis. In addition, vomiting/regurgitation, pneumonia, bacteraemia, sepsis and multiple organ dysfunction syndrome (MODS) were eligible for evaluation in the secondary analysis.
All phases of study selection, validity appraisal and data abstraction were undertaken by at least three reviewers. At each phase, majority decisions prevailed.
Statistical analysis
Analysis was conducted using a fixed effects model [16] with the odds ratio (OR) metric [17]. The underlying assumption behind the fixed effects model, that the true treatment effect of magnitude θ does not vary between studies, was assessed with a formal chi-square test of study × treatment effect homogeneity [16] and was quantified using the I 2 metric [18]. In the presence of important heterogeneity (heterogeneity P < 0.10), or if the I 2 metric exceeded 50% [19], the following a priori identified potential sources of heterogeneity were to be investigated via stratified analysis: (1) study quality, (2) disease groupings, (3) intervention timing and dose, (4) co-interventions and comparison intervention received and (5) outcome measurement and timing [20]. If the source of heterogeneity could not be identified, meta-analysis would not be undertaken, and results from contributing trials would be presented individually.
Analysis was conducted using RevMan Version 4.2 for Windows (The Cochrane Collaboration®, Oxford, England, 2003). A two-tailed P less than 0.05 was accepted to indicate statistical significance, while a two-tailed P greater than 0.05 but less than 0.10 was accepted to indicate a trend towards significance.
Sensitivity analysis
To assess the robustness of the underlying assumptions, a sensitivity analysis was conducted including all studies that were identified to be on-topic but were judged to be methodologically ‘unsound’.
Results
Literature search
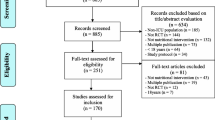
The primary literature search identified 4,800 unique abstracts. Review of abstracts (EAS, FS and GSD), reference lists of published guidelines and systematic reviews (PH, FS and GSD) and contact with academic and industry experts resulted in the retrieval of 675 papers for detailed eligibility review.
Study selection
The results of the detailed eligibility review of the 675 papers (EAS, PH, FS, AD and GSD) are presented in Fig. 1. Thirty clinical trials appeared to address questions regarding the timing of the delivery of EN. Twenty-four were excluded from further consideration for the following reasons: Seven trials did not commence early EN within 24 h of injury or ICU admission [21–27]; five trials were not conducted in critically ill patient populations [28–32]; four trials failed to report any clinically meaningful patient-oriented outcomes [33–36]; two trials evaluated the impact of early post-operative oral intake, not early EN [37, 38]; two trials commenced EN at the same time in both groups [39, 40]; one trial evaluated the impact of early immuno-enhanced EN [41]; one trial was based on a subgroup of patients published in a larger trial [42]; two trials were otherwise eligible but were excluded from the primary analysis due to excessive (>10%) loss to follow-up [43, 44].
Six methodologically sound RCTs qualified for inclusion in the primary analysis.
Included trial characteristics
The six included trials randomised a total of 234 patients, with a median of 37 patients and a range from 20 to 60 patients. The trials were conducted in: (1) ventilated medical and surgical ICU patients [45], (2) burn patients [46], (3) patients with severe pancreatitis and/or peritonitis [47] and (4) trauma patients [48–50]. Complete details of included trials are presented in Table 1.
Validity appraisal
Because none of the six included trials reported sufficient detail on the method of randomisation, it was unclear whether allocation concealment was maintained. None reported the use of any form of blinding. All six reported complete follow-up on clinically meaningful patient-oriented outcomes for all patients enrolled and randomised.
Clinically meaningful patient oriented outcomes
All included trials reported mortality; however, none reported quality of life, and none reported direct measures of physical function.
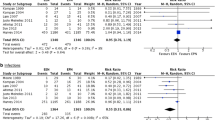
Hospital discharge mortality was reported in three trials [45, 47, 48]. One trial reported mortality over a 28-day follow-up period [46], and two trials reported ICU discharge mortality [49, 50].
As shown in Fig. 2, meta-analysis of RCTs revealed a statistically significant reduction in mortality in favour of early standard EN (OR = 0.34, P = 0.02) with no evidence of heterogeneity.
Complications and major ICU infections
Vomiting/aspiration
One trial reported the incidence of vomiting [46]; however, no trials reported the incidence of aspiration. There was no significant difference in vomiting rates between burn patients who received early standard EN compared to EN commenced at 48 h post injury (0/10 early EN patients vs. 1/10 delayed EN, Fisher’s exact P = 1.00).
Pneumonia
Two trials reported the incidence of pneumonia [45, 49]. Pooling of results (Fig. 3) demonstrated a statistically significant reduction in pneumonia attributable to the provision of early standard EN (OR = 0.31, P = 0.01), with no evidence of heterogeneity.
Bacteraemia
One trial reported the incidence of positive blood cultures [46]. There was no significant difference in positive blood culture rates between burn patients who received early EN compared to EN commenced at 48 h post injury (3/10 early EN patients vs. 7/10 delayed EN, Fisher’s exact P = 0.18).
Sepsis
No trials reported the incidence of sepsis as an outcome.
Multiple organ dysfunction syndrome
Two trials reported the incidence of MODS [47, 48], with one also reporting the severity of MODS (number of failed organs per patient) [48]. Pooling of results (Fig. 4) failed to demonstrate any differences between groups with regards to the incidence of MODS (OR = 0.94, P = 0.78, no evidence of heterogeneity). The single trial reporting severity of MODS demonstrated a trend towards fewer failed organ systems in patients receiving early EN (2.5 ± 0.7 vs. 3.1 ± 0.8 organ failures per patient, P = 0.057).
Sensitivity analysis
Two clinical trials met all eligibility criteria but were excluded from the primary analysis because of major methodological flaws. One trial failed to report outcomes on 16.0% (12/75) of enrolled patients [43], and the other failed to report outcomes on 15.6% (5/32) of enrolled patients [44]. Loss to follow-up was not reported by study arm in either trial.
Sensitivity analysis including these two additional trials provided evidence of a significant reduction in mortality attributable to early EN (OR = 0.40, P = 0.02, no evidence of heterogeneity, Fig. 5).
Discussion
We conducted an extensive literature search to detect RCTs evaluating the effectiveness of early standard EN in critically ill patients. We used an objective and repeatable definition of a critically ill patient population, and our primary conclusions were based on trials free from major methodological flaws.
Six clinical trials conducted in medical and surgical critically ill patients fulfilled our selection criteria. Meta-analysis of these trials revealed a statistically significant reduction in mortality and pneumonia attributable to the provision of standard EN within 24 h of injury or ICU admission. Although this meta-analysis is the first to demonstrate a statistically significant mortality benefit to critically ill patients, previous meta-analyses conducted in non-critically ill patient populations have documented statistically significant reductions in mortality [6] and infectious complications [7] attributable to the provision of early EN.
EN, bacterial translocation, multiple organ dysfunction syndrome and mortality
The progressive failure of multiple organ systems is a leading cause of morbidity and mortality in critical illness [51, 52]. It has been proposed that the gut may be the ‘motor’ that drives the progression of multiple organ dysfunction syndrome (MODS) in critical illness [53].
The gut is an intricate ecosystem that is composed of at least three main components: the epithelium, the mucosal immune system and the commensal flora [54]. In the early stages of critical illness, all three components undergo change. Gut immune function is compromised through mucosal atrophy, increased intestinal permeability, and a reduction in gut associated lymphoid tissue and IgA secretion [55, 56]. The gut flora also changes in critically ill patients, with a decrease in anaerobic bacteria, an increase in pathogenic bacteria with antibiotic selection pressures towards resistant strains [54]. Complex interactions arising from these changes lead to the translocation of pathogenic bacteria from the gut, stimulating systemic cytokine release, and resulting in an increase in infectious complications. It is hypothesised that the resultant cytokine storm drives the critically ill patient towards uncontrollable MODS, thus increasing the risk of mortality [53, 54]. Ample evidence highlights the role EN may play in ameliorating the changes [55, 56]. Recent research sheds light on a novel mechanistic pathway.
Intestinal alkaline phosphatase (iAP) is a brush–border protein expressed exclusively in villus-associated enterocytes and is known to actively detoxify bacterial lipopolysaccharide (LPS) and reduce bacterial translocation [57, 58]. The expression and function of iAP is lost in critical illness in the presence of short-term fasting, but is maintained with the provision of EN 58. The authors of this seminal work conclude “it is likely that the iAP silencing that occurs during starvation is a key component of the gut mucosal barrier dysfunction seen in critically ill patients” [58].
The provision of early standard EN, resulting in preservation of the gut-associated lymphoid tissue, gut barrier function and ability to detoxify LPS [55–58] could explain our key finding of a reduction in pneumonia and mortality. Although only one RCT in our systematic review explicitly reported a composite measure of the severity of MODS, a strong trend towards a reduction in the number of organ system failures was documented in this RCT in patients who received early EN [48].
EN within 24 h of injury or ICU admission
The only other published meta-analysis addressing the effects of early EN in critical illness reported evidence of a trend towards a reduction in mortality [RR 0.52, P = 0.08, heterogeneity P = 0.67] [3], which is consistent with our findings. It is likely that our results were found to be statistically significant because we focused exclusively on trials that began early EN within 24 h of injury or ICU admission. This definition of early nutritional support has been promoted by internationally recognised evidence-based guidelines [1, 2, 4]. Extending the definition of early to include trials that provided EN within 60 h [21] or 72 h [26] of injury may dilute the mortality benefit attributable to the provision of EN within a shorter 24 h window.
As a simulation exercise, we repeated Heyland et al.’s (2003) meta-analysis but excluded the three trials they identified as commencing EN later than 24 h [21–23]. We used their analytic technique (random effects model with the RR metric) and re-analysed the five trials their systematic review identified as providing EN within 24 h [43, 46–48, 50]. This simulation exercise revealed a statistically significant reduction in mortality attributable to the provision of EN within 24 h of injury or ICU admission (RR = 0.26, 95% CI 0.08–0.83, P = 0.02, I 2 = 0%). Concurrence of the results of this simulation exercise with the findings of our current meta-analysis, which uses slightly different selection criteria by placing an emphasis on methodologically sound trials, reinforces the potential importance of defining early as within 24 h of injury or ICU admission. Furthermore, since this simulation exercise was based on trials included in Heyland et al.’s (2003) meta-analysis, it demonstrates that evidence of a mortality reduction has been present in our literature for some time.
Strengths and limitations
We conducted an extensive and exhaustive literature search that was not restricted to the English language. Although it is unlikely that published studies were missed, we did not explicitly search the grey literature to identify conference abstracts of unpublished studies. Contact with recognised experts and industry representatives did not yield any unpublished studies, and inspection of the funnel plot does not reveal obvious evidence of a negative study publication bias. It is likely our literature search identified all eligible trials.
We undertook a formal sensitivity analysis and conducted a simulation exercise to investigate the robustness of our assumptions. The formal sensitivity analysis included RCTs with major methodological flaws identified during our current search, whilst the simulation exercise was conducted using the selection criteria and analytic techniques employed in a previous publication on this topic [3]. The results of the sensitivity analysis and the simulation exercise both support our primary findings: both demonstrated a statistically significant reduction in mortality attributed to the provision of standard EN within 24 h of injury or ICU admission.
Overall, the RCTs included in our meta-analysis were small and of poor quality; however, none of the RCTs included in our primary analysis had major methodological flaws. Methodological flaws and reporting deficiencies have been documented in trials of nutritional support in the past [13]. There is a pressing need for improvements in the conduct and reporting of future trials in this field [59].
The patient groups enrolled into the included trials appear to be clinically heterogeneous. The strength of standard EN formula used, nutritional goals set, use of supplemental parenteral nutrition and comparator groups also differ between trials. Because there is no evidence of statistical heterogeneity and the magnitude of the observed treatment effect is reasonably similar across all included trials, we can conclude that it is valid to obtain an overall summary estimate despite these apparent differences [18–20]. Within the constraints of the patient groups and interventions evaluated in the included trials, the presence of a reasonably consistent treatment effect in the face of differences in study design suggests that the benefits of early EN may be independent of patient population, strength of EN formula used, nutritional goals set, use of supplemental parenteral nutrition and comparator groups. This hypothesis should be confirmed in a subsequent multi-centre clinical trial.
Conclusions
Authoritative guidelines from the European Society of Clinical Nutrition and Metabolism [1], evidence-based guidelines from Australia and New Zealand [4] and Canadian guidelines [2] all recommend that EN should be commenced within 24 h of ICU admission in patients expected to remain in the ICU for at least 2 days. Unfortunately, 40 to 60% of patients who are eligible for early EN still fail to receive EN within 48 h of ICU admission [4].
Meta-analysis conducted on the methodologically sound clinical trials identified by our systematic review of the literature revealed a statistically significant reduction in mortality and pneumonia attributable to the provision of standard EN within 24 h of injury or ICU admission. These findings are robust and were confirmed by sensitivity analysis and a simulation study. Because the included clinical trials may not represent all patient groups, we recommend the use of judicious clinical judgement in applying these findings to clinical practice. The primary findings of this meta-analysis need to be confirmed by the conduct of a large scale multi-centre clinical trial.
References
Kreymann KG, Berger MM, Deutz NE, Hiesmayr M, Jolliet P, Kazandjiev G, Nitenberg G, van den Berghe G, Wernerman J, DGEM (German Society for Nutritional Medicine), Ebner C, Hartl W, Heymann C, Spies C, ESPEN (European Society for Parenteral, Enteral Nutrition) (2006) ESPEN guidelines on enteral nutrition: intensive care. Clin Nutr 25:210–223
Martin CM, Doig GS, Heyland DK, Morrison T, Sibbald WJ (2004) Cluster randomized clinical trial of algorithms for critical care enteral and parenteral therapy (ACCEPT). CMAJ 170:197–204
Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P (2003) Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. J Parenter Enteral Nutr 27:355–373
Doig GS, Simpson F, Finfer S, Delaney A, Davies AR, Mitchell I, Dobb G, Nutrition Guidelines Investigators of the ANZICS Clinical Trials Group (2008) Effect of evidence-based feeding guidelines on mortality of critically ill adults: a cluster randomized controlled trial. JAMA 300:2731–2741
Heyland DK, Schroter-Noppe D, Drover JW, Jain M, Keefe L, Dhaliwal R, Day A (2003) Nutrition support in the critical care setting: current practice in Canadian ICUs–opportunities for improvement? J Parenter Enteral Nutr 27:74–83
Lewis SJ, Andersen HK, Thomas S (2009) Early enteral nutrition within 24 h of intestinal surgery versus later commencement of feeding: a systematic review and meta-analysis. J Gastrointest Surg 13:569–575
Marik PE, Zaloga GP (2001) Early enteral nutrition in acutely ill patients: a systematic review. Crit Care Med 29:2264–2270
Haynes RB, McKibbon KA, Wilczynski NL, Walter SD, Werre SR (2005) Optimal search strategies for retrieving scientifically strong studies of treatment from Medline: analytical survey. BMJ 330:1179
Wong SS, Wilczynski NL, Haynes RB (2006) Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. J Med Libr Assoc 94:41–47
Egger M, Juni P, Bartlett C, Holenstein F, Sterne J (2003) How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technol Assess 7:1–76
Juni P, Holenstein F, Sterne J, Bartlett C, Egger M (2002) Direction and impact of language bias in meta-analyses of controlled trials: empirical study. Int J Epidemiol 31:115–123
Prentice RL (1989) Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med 8:431–440
Doig GS, Simpson F, Delaney A (2005) A review of the true methodological quality of nutritional support trials conducted in the critically ill: time for improvement. Anesth Analg 100:527–533
Graf J, Doig GS, Cook DJ, Vincent JL, Sibbald WJ (2002) Randomized, controlled clinical trials in sepsis: has methodological quality improved over time? Crit Care Med 30:461–472
Juni P, Altman DG, Egger M (2001) Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 323:42–46
Villar J, Mackey ME, Carroli G, Donner A (2001) Meta-analyses in systematic reviews of randomized controlled trials in perinatal medicine: comparison of fixed and random effects models. Stat Med 20:3635–3647
Deeks JJ (2002) Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Stat Med 21:1575–1600
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Hatala R, Keitz S, Wyer P, Guyatt G (2005) Tips for learners of evidence-based medicine: 4 assessing heterogeneity of primary studies in systematic reviews and whether to combine their results. CMAJ 172:661–665
Glasziou PP, Sanders SL (2002) Investigating causes of heterogeneity in systematic reviews. Stat Med 21:1503–1511
Minard G, Kudsk KA, Melton S, Patton JH, Tolley EA (2000) Early versus delayed feeding with an immune-enhancing diet in patients with severe head injuries. J Parenter Enteral Nutr 24:145–149
Eyer SD, Micon LT, Konstantinides FN, Edlund DA, Rooney KA, Luxenberg MG, Cerra FB (1993) Early enteral feeding does not attenuate metabolic response after blunt trauma. J Trauma 34:639–643
Singh G, Ram RP, Khanna SK (1998) Early postoperative enteral feeding in patients with nontraumatic intestinal perforation and peritonitis. J Am Coll Surg 187:142–146
Grahm TW, Zadrozny DB, Harrington T (1989) The benefits of early jejunal hyperalimentation in the head-injured patient. Neurosurgery 25:729–735
Cabre E, Rodriguez-Iglesias P, Caballeria J, Quer JC, Sanchez-Lombrana JL, Pares A, Papo M, Planas R, Gassull MA (2000) Short- and long-term outcome of severe alcohol-induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology 32:36–42
Dvorak MF, Noonan VK, Belanger L, Bruun B, Wing PC, Boyd MC, Fisher C (2004) Early versus late enteral feeding in patients with acute cervical spinal cord injury: a pilot study. Spine 29:E175–E180
Malhotra A, Mathur AK, Gupta S (2004) Early enteral nutrition after surgical treatment of gut perforations: a prospective randomised study. J Postgrad Med 50:102–106
Page RD, Oo AY, Russell GN, Pennefather SH (2002) Intravenous hydration versus naso-jejunal enteral feeding after esophagectomy: a randomised study. Eur J Cardiothorac Surg 22:666–672
De Ledinghen V, Beau P, Mannant P-R, Borderie C, Ripault M-P, Silvain C, Beauchant M (1997) Early feeding or enteral nutrition in patients with cirrhosis after bleeding from esophageal varices? A randomized controlled study. Dig Dis Sci 42:536–541
Seri S, Aquilio E (1984) Effects of early nutritional support in patients with abdominal trauma. Ital J Surg Sci 14:223–227
Carr CS, Ling KDE, Boulos P, Singer M (1996) Randomised trial of safety and efficacy of immediate postoperative enteral feeding in patients undergoing gastrointestinal resection. BMJ 312:869–871
Kaur N, Gupta MK, Minocha VR (2005) Early enteral feeding by nasoenteric tubes in patients with perforation peritonitis. World J Surg 29:1023–1027
Schroeder D, Gillanders L, Mahr K, Hill GL (1991) Effects of immediate postoperative enteral nutrition on body composition, muscle function, and wound healing. J Parenter Enteral Nutr 15:376–383
Hasse JM, Blue LS, Liepa GU, Goldstein RM, Jennings LW, Mor E, Hyusberg BS, Levy MF, Gonwa TA, Klintmalm GB (1995) Early enteral nutrition support in patients undergoing liver transplantation. J Parenter Enteral Nutr 19:437–443
Watters JM, Kirkpatrick SM, Norris SB, Shamji FM, Wells GA (1997) Immediate postoperative enteral feeding results in impaired respiratory mechanics and decreased mobility. Ann Surg 226:369–380
Sagar S, Harland P, Shields R (1979) Early postoperative feeding with elemental diet. Br Med J 1:293–295
Beier-Holgersen R, Brandstrup B (1999) Influence of early postoperative enteral nutrition versus placebo on cell-mediated immunity, as measured with the Multitest CMI. Scand J Gastroenterol 34:98–102
Schilder JM, Hurteau JA, Look KY, Moore DH, Raff G, Stehman FB, Sutton GP (1997) A prospective controlled trial of early postoperative oral intake following major abdominal gynecologic surgery. Gynecol Oncol 67:235–240
Ibrahim EH, Mehringer L, Prentice D, Sherman G, Schaiff R, Fraser V, Kolleff MH (2002) Early versus late enteral feeding of mechanically ventilated patients: results of a clinical trial. J Parenter Enteral Nutr 26:174–181
Taylor SJ, Fettes SB, Jewkes C, Nelson RJ (1999) Prospective, randomized, controlled trial to determine the effect of early enhanced enteral nutrition on clinical outcome in mechanically ventilated patients suffering head injury. Crit Care Med 27:2525–2531
Heslin MJ, Latkany L, Leung D, Brooks AD, Hochwald SN, Pisters PW, Shike M, Brennan MF (1997) A prospective, randomized trial of early enteral feeding after resection of upper gastrointestinal malignancy. Ann Surg 226:567–577
Pupelis G, Austrums E, Jansone A, Sprucs R, Wehbi H (2000) Randomised trial of safety and efficacy of postoperative enteral feeding in patients with severe pancreatitis: preliminary report. Eur J Surg 166:383–387
Moore EE, Jones TN (1986) Benefits of immediate jejunostomy feeding after major abdominal trauma—a prospective, randomized study. J Trauma 26:874–881
Peck MD, Kessler M, Cairns BA, Chang YH, Ivanova A, Schooler W (2004) Early enteral nutrition does not decrease hypermetabolism associated with burn injury. J Trauma 57:1143–1148
Nguyen NQ, Fraser RJ, Bryant LK, Burgstad C, Chapman MJ, Bellon M, Wishart J, Holloway RH, Horowitz M (2008) The impact of delaying enteral feeding on gastric emptying, plasma cholecystokinin, and peptide YY concentrations in critically ill patients. Crit Care Med 36:1469–1474
Chiarelli A, Enzi G, Casadei A, Baggio B, Valerio A, Mazzoleni F (1990) Very early nutrition supplementation in burned patients. Am J Clin Nutr 51:1035–1039
Pupelis G, Selga G, Austrums E, Kaminski A (2001) Jejunal feeding, even when instituted late, improves outcomes in patients with severe pancreatitis and peritonitis. Nutrition 17:91–94
Kompan L, Kremzar B, Gadzijev E, Prosek M (1999) Effects of early enteral nutrition on intestinal permeability and the development of multiple organ failure after multiple injury. Intensive Care Med 25:157–161
Kompan L, Vidmar G, Spindler-Vesel A, Pecar J (2004) Is early enteral nutrition a risk factor for gastric intolerance and pneumonia? Clin Nutr 23:527–532
Chuntrasakul C, Siltharm S, Chinswangwatanakul V, Pongprasobchai T, Chockvivatanavanit S, Bunnak A (1996) Early nutritional support in severe traumatic patients. J Med Assoc Thai 79:21–26
Marshall JC (2001) Inflammation, coagulopathy, and the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med 29:S99–S106
Vincent JL, Zambon M (2006) Why do patients who have acute lung injury/acute respiratory distress syndrome die from multiple organ dysfunction syndrome? Implications for management. Clin Chest Med 27:725–731
Carrico CJ, Meakins JL, Marshall JC, Fry D, Maier RV (1986) Multiple-organ-failure syndrome. Arch Surg 121:196–208
Clark JA, Coopersmith CM (2007) Intestinal crosstalk: a new paradigm for understanding the gut as the “motor” of critical illness. Shock 28:384–393
Kudsk KA (1998) Early enteral nutrition in surgical patients. Nutrition 14:541–544
Magnotti LJ, Deitch EA (2005) Burns, bacterial translocation, gut barrier function, and failure. J Burn Care Rehabil 26:383–391
Tuin A, Poelstra K, Jager-Krikken A, Bok L, Raaben W, Velders MP, Dijkstra G (2009) Role of alkaline phosphatase in colitis in man and rats. Gut 58:379–387
Goldberg RF, Austen WG Jr, Zhang X, Munene G, Mostafa G, Biswas S, McCormack M, Eberlin KR, Nguyen JT, Tatlidede HS, Warren HS, Narisawa S, Millán JL, Hodin RA (2008) Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc Natl Acad Sci USA 105:3551–3556
Doig GS, Simpson F, Sweetman EA (2009) Evidence-based nutrition support in the intensive care unit: an update on reported trial quality. Curr Opin Clin Nutr Metab Care 12:201–206
Acknowledgments
The conduct of this study was not funded. Ethics approval was not required to conduct this integrative meta-epidemiological study.
Conflict of interest statement
GSD has received academic research grants from Fresenius Kabi Deutschland GmbH and Baxter Healthcare Pty Ltd., and speaker's honoraria from Baxter Healthcare Pty Ltd. FS has received academic research grants from Fresenius Kabi Deutschland GmbH and Baxter Healthcare Pty Ltd., and speakers honoraria from Pharmatel-Fesenius Kabi Pty Ltd. EAS has received an academic research grant from Baxter Healthcare Pty Ltd. ARD has received an academic research grant from Cook Medical. PTH declares no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Doig, G.S., Heighes, P.T., Simpson, F. et al. Early enteral nutrition, provided within 24 h of injury or intensive care unit admission, significantly reduces mortality in critically ill patients: a meta-analysis of randomised controlled trials. Intensive Care Med 35, 2018–2027 (2009). https://doi.org/10.1007/s00134-009-1664-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-009-1664-4