Abstract
Purpose
Improvements in survival after septic shock will most likely rely on our capacity to manage individualized therapies based on the measurement of rapidly accessible biomarkers. As the early phase of septic shock is dominated by severe alterations of the cardiovascular system, the predictive value for mortality of pro-vasopressin (pro-AVP) and pro-adrenomedullin (pro-ADM), two vasoactive pro-hormones, was assessed.
Methods
In 99 consecutive patients, pro-hormone concentrations were measured (immunoluminometric assay) three times within the first week after the onset of septic shock.
Results
Pro-AVP and pro-ADM concentrations were significantly increased in non-survivors in comparison with survivors and were significantly associated with mortality after both univariate and multivariate analysis. Importantly, when assessed as a pair, pro-ADM and pro-AVP were even more informative.
Conclusions
Both Pro-ADM and pro-AVP appear to be good biomarkers for the prediction of 28-day mortality after septic shock. However, their association in a single variable tends to improve their predictive capacity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Septic syndromes remain the leading cause of death in intensive care units. In the United States alone, they develop in 750,000 people annually, of whom more than 210,000 die. Sepsis incidence has been continuously rising over the past 2 decades and is projected to increase significantly in the forthcoming years [1, 2]. Among these syndromes, septic shock possesses the highest severity, with a mortality ranging from 35 to 50% that has only slightly decreased over years [1, 3, 4].
One likely explanation for the overall only modest gain in survival is that the wide heterogeneity characterizing the septic patient population has not, so far, been appropriately taken into account. Indeed, septic syndromes include a heterogeneous mix of patients presenting with various co-morbidities (along with different long term treatment), different sites of infection (infected with different germs, more or less virulent), different organ failures, and different pro/anti-inflammatory responses [5, 6]. Thus, improvement in therapeutic interventions and the subsequent rise in survival will likely depend on our capacity to manage individualized therapies for each of these patients. Among tools desirable to achieve this objective, rapid access to biological markers tightly mirroring ongoing mechanisms and/or assessing risk and prognosis is now mandatory [5].
The early phase of septic shock is dominated by severe alterations of the cardiovascular system (ineffective tissue oxygen delivery, inappropriate peripheral vasodilatation, myocardial dysfunctions, altered blood flow distribution) that induce refractory hypotension to volume therapy [7]. In association with the major pro-inflammatory responses occurring within the first hours after septic shock, this likely participates in the development of multiple organ failure [8, 9]. Vasopressin (AVP), produced in the hypothalamus in response to hemodynamic and osmotic stimuli, is a vasopressive hormone that participates in osmoregulation [10]. It has been showed that AVP concentrations are elevated in the initial phase of septic shock, whereas relative AVP deficiency is reported in approximately one-third of late septic shock patients [11]. Adrenomedullin (ADM) is a vasodilator and exhibits natriuretic and diuretic properties [12]. This hormone has been shown to play a pivotal role in initiating the early hyperdynamic response in murine model of sepsis, and elevated levels of plasma ADM have been described early on in septic shock patients [13]. Recently, plasma concentrations of several fragments of precursors of these vasoactive hormones (pro-AVP and pro-ADM) have been reported to correlate with severity in septic patients [14, 15]. Briefly, on admission, concentrations of pro-AVP and pro-ADM were shown to increase gradually in correlation with severity ranging from systemic inflammatory response syndrome (SIRS) to severe forms of sepsis or with the pneumonia severity index (PSI) [14–20].
So far, no data are available for septic shock patients. The objective of this study was therefore to concomitantly assess the value of two precursors of vasoactive hormones (one vasodilator, pro-ADM, and one vasoconstrictor, pro-AVP) as predictors of 28-day mortality during the 1st week following the onset of septic shock.
Patients and methods
Patients
This study was conducted in 99 consecutive patients with septic shock according to the diagnostic criteria of the ACCP/SCCM [21]. A majority (93 out of 99) of these patients had been examined in a previous study for their percentage of HLA-DR expressing monocytes [22]. They were admitted to the two participating ICUs (one medical, one surgical) of the Lyon-Sud University Hospital (Hospices Civils de Lyon, France). Septic shock was defined by an identifiable site of infection, evidence of a systemic inflammatory response manifested by at least two of the following criteria: (1) temperature >38 or <36°C; (2) heart rate >90 beats per minute; (3) respiratory rate >20 breaths per minute; (4) white blood cell count >12,000 or <4 000/mm3 and hypotension persisting despite fluid resuscitation and requiring vasopressor therapy. The onset of septic shock was defined by the beginning of vasopressive therapy. The only exclusion criteria were patients younger than 18 years old and the absence of circulating leukocytes. During the follow-up, clinical and biological parameters were collected. The data collection included demographic characteristics (age, gender); admission category (elective or emergency surgery, medicine); referral pattern (community, hospital or ICU-acquired septic shock); microbiological findings (characteristics of the infection such as the source and the identified microorganisms), the delay between the onset of septic shock and the admission in hospital and in ICU (septic shock was considered hospital-acquired when beginning at least 48 h after the admission to hospital); co-morbidities (chronic heart failure, chronic kidney failure, chronic hepatic failure, metastatic cancer, type I diabetes, malignant haemopathy, immunodepressive state or acquired immune deficiency syndrome) and the outcome after 28 days (death or survival). Three clinical scores were recorded: the severity of underlying medical condition according to the criteria of McCabe and Jackson (range 1–3) [23]; the severity at the onset of shock assessed by the Simplified Acute Physiology Score II (SAPS II, range 0–194) [24]; the sepsis-related organ failure assessment (SOFA, range 0–24) score at day 1–2 and 3–4 after the onset of shock [25].
Pro-hormone measurements were performed on residual blood after completing routine follow-up performed in the ICU, in accordance with the guidelines for clinical research of our institute (which waived the need for informed consent). Arterial blood samples were obtained at days 1–2, 3–4 and 5–7 after the onset of shock (one sample per period). As planned, blood samples were not collected on Saturdays and Sundays, during which time the laboratory did not operate, a fact that accounts for most of the missing values.
Measurements of pro-ADM and pro-AVP
Venous blood samples were collected in tubes containing ethylene-diamine-tetra-acetic acid (EDTA). After centrifugation, they were kept frozen at −80°C until assayed. Mid-regional pro-ADM and C-terminal pro-AVP were detected with newly developed chemiluminescence sandwich immunoassays, as extensively described elsewhere [26, 27]. Briefly, each immunoassay employed two polyclonal antibodies targeting specific fragments for each pro-hormone. Tubes were coated with one of these antibodies; the second one (labeled with an akridinium ester) was added to plasma samples (all kits from Brahms, Hennigsdorf, Germany). After incubation, tubes were washed several times with Lumitest wash solution (Brahms), and bound chemiluminescence was measured with a luminometer (Berthold, Germany). Analytical detection limits were 0.08 nmol/l for pro-ADM and 1.7 pmol/l for pro-AVP [26, 27]. In healthy individuals, median concentration for pro-AVP is 4.2 pmol/l [95% confidence interval (CI) 4.0–4.4 pmol/l]. Mean concentration for pro-ADM is 0.33 nmol/l (SD = 0.07) [26, 27].
Statistical analysis
Patients’ clinical and biological parameters were described using frequencies, percentages, medians and interquartile ranges (IQR). The two groups of patients (28-day survivors and non-survivors) were compared using Mann–Whitney U-test for continuous variables and Fisher’s exact test for categorical data. Predictive value for mortality was evaluated by analysis of receiver-operating characteristic (ROC) curves at days 1–2, 3–4 or 5–7. Survival estimate was based on the Kaplan–Meier method, and comparison of survival distribution was based on the log-rank test. Univariate and multivariate logistic regressions were used to identify the variables associated with the risk of death assessed by odds ratios (OR) and their 95% CI. The variables with a P value ≤0.15 in univariate analysis were entered in the multivariate model. The parameters included patient gender, age, number of co-morbidities, main diagnosis category, type of infection, site of infection, SAPS II, SOFA and pro-hormone measurements. The continuous variables, such as age, SAPS II and SOFA scores, were categorized using a cutoff value based on the population median. Pro-hormones were stratified using the best threshold indicated by the ROC curves analysis for mortality at day 1–2, 3–4 or 5–7 (i.e., maximized sensitivity and specificity). Pro-hormones were also assessed as a pair leading to four categories: pro-ADM and pro-AVP below their respective optimal thresholds, one above and one below optimal threshold (i.e., two categories) and finally both above thresholds. A P value <0.05 was considered as statistically significant. The analyses were performed using SPSS software (version 12.0, SPSS, Chicago, IL).
Results
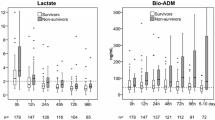
Ninety-nine patients with septic shock were included in the study (58 men and 41 women). Median age at admission was 67 years (IQR 54–76 years). At diagnosis of shock, median SAPS II was 51 (IQR 43–64), and median SOFA score was 10 (IQR 8–12). The overall 28 day-mortality was 35%. At the end of D3, 86 patients were still alive, and 81 were survivors at the end of D5. Demographic, clinical and biological data are presented in Table 1. As usually reported in similar cohorts of patients, SAPS II at admission, SOFA score at day 1–2 and 3–4 and the number of co-morbidities were significantly different between survivors and non-survivors. Parameters related to infection (main diagnosis category, germs, type and site of infection) or demographic characteristics (age and gender) were not different between these two groups. Overall measurements of the two pro-hormones are presented in Fig. 1. Pro-ADM and pro-AVP were significantly higher in non-survivors than in survivors during the whole follow-up.
Measurements of pro-ADM and pro-AVP in survivors and non-survivors at day 1–2, 3–4 and 5–7 after septic shock. Results are presented as box plots and individual values. White boxes represent survivors and grey boxes nonsurvivors. Numbers of samples are as follows: day 1–2 = 75 samples (50 S + 25 NS); day 3–4 = 71 samples (52 S + 19 NS); day 5–7 = 77 samples (59 S + 18NS). *P value <0.05, **P value ≤0.01 (Mann–Whitney U-test, corrected by the number of tests)
To evaluate the prognostic value, ROC curve analysis was performed for each pro-hormone at different time points (Table 2). At day 1–2, pro-AVP and pro-ADM presented with an area under the curve (AUC) of 0.76 and 0.71, respectively. At day 3–4, pro-AVP presented with a higher AUC (0.79) than pro-ADM (0.70). Finally, at day 5–7, pro-ADM exhibited the highest AUC (0.86 vs. 0.72 for pro-AVP). On the basis of optimal thresholds (determined according to ROC curve analysis, data not shown), prognostic parameters (PPV, NPV, LR+, LR−) were also calculated for the two pro-hormones (Table 3) indicating that pro-AVP and pro-ADM exhibit significant predictive value for mortality after septic shock.
The predictive capacity of the two pro-hormones was then tested after adjustment for other confounders in a multivariate logistic regression analysis. After univariate analysis, only SAPS II, co-morbidities, SOFA score and pro-hormones were included in the model (P ≤ 0.15, data not shown).
Pro-hormones were tested either independently (i.e., tested one by one with clinical variables) or concurrently (i.e., tested simultaneously as two distinct variables). When tested independently, in association with SAPS II score at day 1–2 and the number of comorbidities at day 3–4, only pro-AVP remained independently associated with mortality (ORD1–2 = 3.28; 95% CI 1.02–10.49; P = 0.046; ORD3–4 = 6.70; 95% CI 1.37–32.79; P = 0.019). At day 5–7, each pro-hormone entered in the model independently showed good prognostic value (pro-ADM: OR = 20.11; 95% CI 4.29–94.19; P < 0.001, pro-AVP OR = 5.18; 95% CI 1.49–17.98; P = 0.010).
When tested concurrently, at day 3–4 (Table 4), only pro-AVP appeared as an independent predictor of mortality (OR = 7.61; 95 %CI 1.30–44.44; P = 0.024). At day 5–7 (Table 4), only pro-ADM remained significantly associated with fatal outcome (OR = 15.35; 95% CI 3.16–74.57; P = 0.001).
Interestingly, when both pro-hormones were expressed as a pair (both above their respective cutoff ratio), this appeared as the best predictor for mortality at day 1–2 (OR = 5.50; 95% CI 1.23–24.68; P = 0.026) and day 5–7 (OR = 46.30; 95% CI 6.09–352.11; P < 0.001). This is also illustrated by survival curves after Kaplan–Meier analysis (Fig. 2).
Kaplan–Meier survival curves stratified according to pro-ADM and pro-AVP measurements considered as a pair at day 1–2, 3–4 and 5–7 after the onset of septic shock. For each time point, log rank test revealed a P value <0.001. CO: respective cutoff as provided in Table 3
Finally, the influence of renal parameters and catecholamine treatment on the predictive value of both pro-hormones was evaluated, especially since maximal catecholamine dosages and creatininemia (measured within day 1–2 and day 3–4) presented with a P value <0.15 after univariate analysis. As creatininemia and maximal catecholamine dosages are already included in the calculation of the SOFA score, they were not added to the initial multivariate model. However, in a separate multivariate analysis including the SAPS II score, creatininemia, catecholamine dosages, number of co-morbidities and both pro-hormones (expressed concurrently or as categories), both pro-hormones kept an independent prognostic value (Table S1). Thus, the increase in pro-AVP and pro-ADM observed after septic shock is most likely not solely due to an altered renal function or high catecholamine dosages in these patients.
Discussion
It has been suggested that early and adequate prognosis and risk assessment after septic shock may facilitate improved care of the patients through tailored therapy [5, 6]. In this context, since the first hours after the onset of shock are dominated by alterations of the cardiovascular system (especially major peripheral vasodilation [7–9]), the measurement of biomarkers (vasodilatator/vasoconstrictor) playing a role in the homeostasis of this system (or reflecting its alterations) may be of major interest.
Because vasoactive hormones, in contrast with their precursors, are rapidly cleared from the circulation (half-life of some hours for pro-ADM and days for pro-AVP vs. a few minutes for active hormones) [10, 12, 26, 27], their plasmatic measurement is technically difficult. As generation of both hormone and precursor is a stoichiometric process, the measurement of pro-hormone fragments offers a seducing alternative for assessing the release of vasoactive hormones.
To date, few data are available regarding the potential of vasoactive pro-hormones as biomarkers in septic shock patients. Studies measuring these pro-hormones in patients with pneumonia have only included a small number of patients and have mainly considered one pro-hormone at a time [14–17]. For example, in the work of Seligman et al. [16], as observed in our study, pro-AVP remained significantly associated with mortality after multivariate analysis. Concerning pro-ADM, two studies have reported its good prognostic value in critically ill patients [15] and in patients with community-acquired pneumonia [17]. AUCs were calculated at 0.81 and 0.76, respectively, with cutoff values of 3.9 and 1.8 nmol/l, in keeping with the present results (AUC 0.71, 0.70, 0.86; thresholds 5, 5, 4 nmol/l for the three time points).
Beyond this first step of analysis, we further investigated the potential of pro-AVP and pro-ADM to remain independently predictive for mortality in a multivariate analysis. At day 1–2, we observed that along with SAPS II, pro-AVP remained independently associated with mortality as well as pro-AVP and pro-ADM when assessed as a pair and above their respective cut-off ratio. At day 3–4, after adjustment for clinical confounders and pro-ADM, pro-AVP was the sole parameter predictive for fatal outcome. Later on, at day 5–7, along with the number of co-morbidities, pro-ADM was independently associated with mortality as well as its combination with pro-AVP. Of note, the interest of combining pro-hormone measurements in a single parameter has been reported in another study in which pro-endothelin-1 (pro-ET-1, precursor of the vasopressive hormone endothelin-1) and pro-ADM were assessed as a ratio in 30 septic shock patients. In this study, AUC was improved from 0.49 for pro-ET-1 alone and 0.76 for pro-ADM alone to 0.81 for the ratio [28]. The optimized information provided by the use of combined pro-hormone measurements is also clearly illustrated by survival curves in our study (Fig. 2). The risk of death was higher when both markers were found to be above their respective cutoff ratio. Importantly, at day 1–2, this tended to indicate an early risk of death, as most patients in this group died within the first days after the onset of shock. This could be of major interest if we are to perform an aggressive management of this particular subgroup of patients. Noteworthily, in accordance with our study, it has been shown that in post-acute myocardial infarction the association of pro-hormones as a pair provided additional prognostic information regarding the occurrence of death or delayed heart failure [29–31].
Another interesting result is that pro-hormone measurements provide different information depending on their sampling time after the onset of shock. Pro-AVP showed a better predictive capacity when measured at day 1–2 and 3–4, whereas at day 5–7, pro-ADM was clearly a better predictor. In the early phase of septic shock, pro-AVP may be more informative as it reflects the counter-regulatory vasoactive response activated in response to the early loss of vascular tone and decreased arterial pressure. As pathophysiology evolves over time, choice and interest of biomarkers may evolve too. Therefore, this supports the importance of the measurement of a panel of biomarkers over time rather than a single one.
Our study has some limitations. This preliminary study was not specifically designed to correlate cardiovascular parameters to the present biomarkers. Our results therefore need to be confirmed in future prospective studies collecting renal and hemodynamic parameters in addition with pro-hormone measurements. Similarly, vasoactive therapies such low dose of corticoids administrated during septic shock were not taken into account, although we may hypothesize that these drugs may have influenced pro-hormone release by modulating blood pressure [32]. Besides, 14 values are missing for non-survivors, and 10 values are missing for survivors at D1–2. This was expected as blood samples were not collected on Saturdays and Sundays when the laboratory did not operate (also explaining the 48-h interval regarding the blood sampling). However, since as many non-surviving as surviving patients values are missing, we believe this cannot bias the results. Finally, though a population of 99 septic shock patients constitutes a rather large cohort regarding this specific condition, we can suspect that our multivariate statistical analysis may have been underpowered, leading to large 95% CIs, especially when pro-AVP and pro-ADM were concomitantly assessed in the model (and even more if we excluded the patients with missing values).
Conclusion
The present work illustrates the potential of combining measurements of different biomarkers in a single parameter and suggests that choice of biomarkers for monitoring patients may also evolve over time. The promising potential of pro-ADM and pro-AVP to guide clinical decisions during initial management of septic shock patients now needs to be investigated in an appropriately designed multicentered study.
References
Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 9:1303–1310
Dombrovskiy VY, Martin AA, Sunderram J, Paz HL (2007) Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med 35:1244–1250
Annane D, Bellissant E, Cavaillon JM (2005) Septic shock. Lancet 365:63–78
Hotchkiss RS, Karl IE (2003) The pathophysiology and treatment of sepsis. N Engl J M 348:138–150
Carlet J, Cohen J, Calandra T, Opal SM, Masur H (2008) Sepsis: time to reconsider the concept. Crit Care Med 36:964–966
Marshall JC (2008) Sepsis: rethinking the approach to clinical research. J Leukoc Biol 83:471–482
Hollenberg SM, Ahrens TS, Annane D, Astiz ME, Chalfin DB, Dasta JF, Heard SO, Martin C, Napolitano LM, Susla GM, Totaro R, Vincent JL, Zanotti-Cavazzoni S (2004) Practice parameters for hemodynamic support of sepsis in adult patients: 2004 update. Crit Care Med 32:1928–1948
Flierl MA, Rittirsch D, Huber-Lang MS, Sarma JV, Ward PA (2008) Molecular events in the cardiomyopathy of sepsis. Mol Med 14:327–336
Matsuda N, Hattori Y (2007) Vascular biology in sepsis: pathophysiological and therapeutic significance of vascular dysfunction. J Smooth Muscle Res 43:117–137
Treschan TA, Peters J (2006) The vasopressin system: physiology and clinical strategies. Anesthesiology 105:599–612
Sharshar T, Blanchard A, Paillard M, Raphael JC, Gajdos P, Annane D (2003) Circulating vasopressin levels in septic shock. Crit Care Med 31:1752–1758
Hinson JP, Kapas S, Smith DM (2000) Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev 21:138–167
Zudaire E, Portal-Núñez S, Cuttitta F (2006) The central role of adrenomedullin in host defense. J Leukoc Biol 80:237–244
Morgenthaler NG, Müller B, Struck J, Bergmann A, Redl H, Christ-Crain M (2007) Copeptin, a stable peptide of the arginine vasopressin precursor, is elevated in hemorrhagic and septic shock. Shock 28:219–226
Christ-Crain M, Morgenthaler NG, Struck J, Harbarth S, Bergmann A, Müller B (2005) Mid-regional pro-adrenomedullin as a prognostic marker in sepsis: an observational study. Crit Care 9:R816–R824
Seligman R, Papassotiriou J, Morgenthaler NG, Meisner M, Teixeira PJ (2008) Copeptin, a novel prognostic biomarker in ventilator-associated pneumonia. Crit Care 12:R11
Christ-Crain M, Morgenthaler NG, Stolz D, Müller C, Bingisser R, Harbarth S, Tamm M, Struck J, Bergmann A, Müller B (2006) Pro-adrenomedullin to predict severity and outcome in community-acquired pneumonia. Crit Care 10:R96
Masiá M, Papassotiriou J, Morgenthaler NG, Hernández I, Shum C, Gutiérrez F (2007) Midregional pro-A-type natriuretic peptide and carboxy-terminal provasopressin may predict prognosis in community-acquired pneumonia. Clin Chem 53:2193–2201
Müller B, Morgenthaler N, Stolz D, Schuetz P, Müller C, Bingisser R, Bergmann A, Tamm M, Christ-Crain M (2007) Circulating levels of copeptin, a novel biomarker, in lower respiratory tract infections. Eur J Clin Invest 37:145–152
Krüger S, Papassotiriou J, Marre R, Richter K, Schumann C, von Baum H, Morgenthaler NG, Suttorp N, Welte T, CAPNETZ Study Group (2007) CAPNETZ Study Group: pro-atrial natriuretic peptide and pro-vasopressin to predict severity and prognosis in community-acquired pneumonia: results from the German competence network CAPNETZ. Intensive Care Med 33:2069–2078
Bone RC (1992) Toward an epidemiology and natural history of SIRS (systemic inflammatory response syndrome). JAMA 268:3452–3455
Monneret G, Lepape A, Voirin N, Bohé J, Venet F, Debard AL, Thizy H, Bienvenu J, Gueyffier F, Vanhems P (2006) Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med 32:1175–1183
McCabe WR, Jackson GC (1962) Gram-negative bacteremia. Arch Intern Med 110:847–864
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S (1998) Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Crit Care Med 26:1793–1800
Morgenthaler NG, Struck J, Alonso C, Bergmann A (2005) Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem 51:1823–1829
Morgenthaler NG, Struck J, Alonso C, Bergmann A (2006) Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 52:112–119
Schuetz P, Christ-Crain M, Morgenthaler NG, Struck J, Bergmann A, Müller B (2007) Circulating precursor levels of endothelin-1 and adrenomedullin, two endothelium-derived, counteracting substances, in sepsis. Endothelium 14:345–351
Khan SQ, Dhillon O, Struck J, Quinn P, Morgenthaler NG, Squire IB, Davies JE, Bergmann A, Ng LL (2007) C-terminal pro-endothelin-1 offers additional prognostic information in patients after acute myocardial infarction: Leicester acute myocardial infarction peptide (LAMP) study. Am Heart J 154:736–742
Khan SQ, O’Brien RJ, Struck J, Struck J, Quinn PA, Morgenthaler NG, Squire IB, Davies JE, Bergmann A, Ng LL (2007) Prognostic value of midregional pro-adrenomedullin in patients with acute myocardial infarction: the LAMP (Leicester acute myocardial infarction peptide) study. J Am Coll Cardiol 49:1525–1532
Khan SQ, Dhillon OS, O’Brien RJ, Struck J, Quinn PA, Morgenthaler NG, Squire IB, Davies JE, Bergmann A, Ng LL (2007) C-terminal provasopressin (copeptin) as a novel and prognostic marker in acute myocardial infarction: Leicester acute myocardial infarction peptide (LAMP) study. Circulation 115:2103–2110
de Kruif MD, Lemaire LC, Giebelen IA, Struck J, Morgenthaler NG, Papassotiriou J, Elliott PJ, van der Poll T (2008) The influence of corticosteroids on the release of novel biomarkers in human endotoxemia. Intensive Care Med 34:518–522
Acknowledgments
This work was supported by the Hospices Civils de Lyon and was conducted thanks to the support of Brahms—we especially thank Dr. N.G. Morgenthaler and Dr. A. Bergmann. All measurements were performed in the Research Department of Brahms AG in a totally blinded fashion without knowledge of clinical parameters. This work was also conducted thanks to the logistical support (H. Thizy, F. Gueyffier) from the Centre d’Investigation Clinique (CIC 201) de Lyon (Clinical Research Centre) of INSERM and Hospices Civils de Lyon.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is discussed in the editorial available at: doi:10.1007/s00134-009-1613-2.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guignant, C., Voirin, N., Venet, F. et al. Assessment of pro-vasopressin and pro-adrenomedullin as predictors of 28-day mortality in septic shock patients. Intensive Care Med 35, 1859–1867 (2009). https://doi.org/10.1007/s00134-009-1610-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-009-1610-5