Abstract
Objective
To determine the efficacy and safety of extended drotrecogin alfa (activated) (DAA) therapy.
Design
Multicentre, randomised, double-blind, placebo-controlled study.
Setting
Sixty-four intensive care units in nine countries.
Patients
Adults with severe sepsis and vasopressor-dependent hypotension after a 96-h infusion of standard DAA.
Interventions
A total of 193 patients received an intravenous infusion of extended DAA 24 µg/kg/h or sodium chloride placebo for a maximum of 72 h.
Measurements and results
At extended therapy initiation (baseline), DAA-group patients had lower protein C levels (P = 0.23) and higher vasopressor requirements, particularly for the primary vasopressor used, norepinephrine (P = 0.03), compared with placebo-group patients. DAA treatment did not result in a difference in the primary outcome of time to resolution of vasopressor-dependent hypotension versus placebo (P = 0.419). However, few patients reached resolution (DAA 34%, placebo 40%) as most continued to require vasopressor support after 72 additional hours of treatment. Treatment did not reduce 28-day all-cause mortality and in-hospital mortality or improve organ function compared with placebo, although there was a lower percentage change in D-dimers (P < 0.001) and increases in protein C levels were numerically greater on extended infusion. There was no difference in serious adverse events including bleeding events.
Conclusions
Extended DAA treatment did not result in more rapid resolution of vasopressor-dependent hypotension, despite demonstrating anticipated biological effects on D-dimer and protein C levels. A reduced planned sample size combined with baseline imbalances in protein C levels and vasopressor requirements may have limited the ability to demonstrate a clinical benefit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drotrecogin alfa (activated) (DAA) (recombinant human activated protein C) is indicated in the European Union for the treatment of adults with severe sepsis with multiple organ failure when added to best standard care. The use of DAA should be considered mainly in situations when therapy can be started within 24 h after the onset of organ failure [1]. DAA is the first specific pharmacological intervention to reduce mortality in severe sepsis, given as a 96-h intravenous infusion at a dose of 24 µg/kg/h [2, 3]. DAA has also been found to ameliorate cardiovascular and respiratory organ dysfunction [4], reduce markers of thrombin generation (D-dimer, prothrombin F1.2) and inflammation (interleukin-6), and result in a more rapid normalisation of protein C and plasminogen levels (important components of the antithrombotic and fibrinolytic systems), compared with placebo [1, 5]. The main safety concern associated with DAA treatment is bleeding [2, 3, 6, 7]. Studies have found that approximately 22% of patients remain vasopressor-dependent at the end of the standard 96-h infusion of DAA [4], suggesting that a longer treatment period could be beneficial for some patients. D-dimer levels decrease during DAA treatment but rapidly increase immediately after the end of the infusion [5], suggesting ongoing coagulopathy, although one study of 12 patients failed to show such an increase [8]. Therefore, this study investigated the effect of an extended infusion of DAA for up to 72 h on vasopressor requirements in adults with persistent septic shock on completion of a 96-h infusion of standard therapy [9].
Materials and methods
The study was approved by the site’s ethics committee and conducted in accordance with the Declaration of Helsinki. All patients or their legal representative gave written informed consent.
Design
This was a multicentre, randomised, double-blind, placebo-controlled study in which adults in intensive care units (ICUs) with persistent septic shock, after treatment with a 96-h infusion of standard DAA, were randomised to receive placebo or DAA for up to 72 additional hours. This resulted in a maximal length of infusion of up to 7 days compared to the standard treatment of approximately 4 days.
Patients were screened up to 12 h before the end of the 96-h standard DAA infusion. Within 12 h before the end of the standard infusion, eligible patients or their legal representative provided informed consent and were randomised to treatment (stratified by the investigator site). Patients were followed up for 24 days after initiation of the study drug (28 days after standard drug initiation). Patients still hospitalised at day 24 were followed up until they left hospital up until day 86.
Patients and study personnel remained blinded to treatment throughout the study, apart from a pharmacist or designee who obtained treatment assignments from an interactive voice response system and prepared the drug (covered to maintain the blind).
The primary efficacy outcome was time to resolution of vasopressor-dependent hypotension (dopamine ≥5 μg/kg/min; or epinephrine, phenylephrine, vasopressin, or norepinephrine at any dose) within 72 h. The dose of vasopressor was assessed approximately every 6 h, however no specific targets of hemodynamic therapy were provided. Secondary efficacy measures included 28-day all-cause mortality, 90-day in-hospital mortality, organ function (sequential organ failure assessment, SOFA [10]), and biomarker evaluations (protein C, D-dimers, prothrombin time). Safety measurements included monitoring of serious adverse events (SAEs) and adverse events (AEs); the following were collected: SAEs not considered clinical outcomes; bleeding events reported as non-serious AEs that occurred during the study infusion and that led to or contributed to the need for a transfusion of packed red blood cells; non-serious AEs that that were considered by the investigator to be study drug related; AEs, including bleeding events, that led to permanent discontinuation of study drug.
Patients
Patients were aged ≥18 years, had severe sepsis, and continued to require vasopressor support, having been treated with at least 84 h of a planned 96-h infusion of standard DAA.
Patients were excluded if they were expected to require extensive or multiple surgical procedures within the next 3 days, had a platelet count <30,000/mm3, were receiving therapeutic heparin [>15,000 IU/day of unfractionated heparin or larger doses of low molecular weight heparin than used for prophylaxis of deep venous thrombosis, or >15 IU/kg/h for renal replacement purposes], were not expected to survive 24 days given their pre-existing uncorrectable medical condition, had received treatment within the last 30 days with any drug that had not received regulatory approval, were pregnant or breastfeeding, were contraindicated for treatment with DAA, had not given written informed consent, or were no longer vasopressor dependent. Patients whose family or primary physician had not committed to aggressive management of the patient were also excluded.
Drug administration
DAA 24 μg/kg/h was administered as a maximum of 72-h extended infusion. There was to be no time interval between the standard and study infusions; however, a maximum of 2 h was allowed in case of unforeseen circumstances. Interruptions were acceptable as long as infusions were restarted within 24 h and within the 72-h treatment period. If a patient resolved their need for vasopressor support for 12 continuous hours before completion of treatment, the infusion was discontinued and not restarted. Patients randomised to placebo received sterile 0.9% sodium chloride.
Statistics
A sample of 270 patients was planned, based on calculations from PROWESS (recombinant human activated protein C worldwide evaluation in severe sepsis) [2]. From these calculations, 135 patients per group would have at least 81% power to detect a difference between treatment groups using the log rank statistic with a two-sided significance level of 0.1 if the true hazard ratio was 0.63. This corresponds to a difference of about 16.5% in time to resolution of vasopressor-dependent cardiovascular organ failure.
Analyses used the intent-to-treat (ITT) population (all randomised patients who received study drug for any length of time). Time to resolution of vasopressor-dependent hypotension was estimated for each group using the product-limit (Kaplan–Meier) method and a two-sided log-rank test was used for the primary comparison. For mortality, relative risk (RR) and odds ratio (OR) estimates with associated 95% confidence intervals (CIs) were calculated. For other efficacy variables and health outcomes, groups were compared with analysis of variance (ANOVA). Baseline characteristics at the initiation of extended therapy were compared using ANOVA or a chi-square test. Safety measurements were compared using Fishers exact test.
Results
Patients
The study was conducted at 64 centres in nine countries (Austria, Belgium, France, Germany, Italy, Poland, Spain, UK, US) in 2004–2007. Recruitment was slow because fewer patients than anticipated remained vasopressor dependent after a standard DAA infusion, and despite an increase in site numbers, the planned sample size was reduced to 200 patients to ensure that the study completed in an acceptable timeframe. This resulted in the minimum statistical difference that could be detected between the groups increasing to 19.2%.
A total of 201 patients entered the study and 199 were randomised. Six patients discontinued before receiving treatment; four DAA patients owing to entry criteria exclusion and two placebo patients owing to entry criteria exclusion and death. A total of 193 patients (94 DAA, 99 placebo) received study drug for any length of time and were included in the ITT and safety populations. Two ITT placebo patients discontinued from the study, one lost to follow-up and one patient decision. Fifty-three (27.5%) patients had protocol violations, most commonly study drug discontinuations.
Demographic and baseline characteristics are presented in Table 1. Protein C levels were lower in the DAA group. More DAA patients were admitted from acute care hospitals (20.2 vs. 15.2%, P = 0.422) and had a history of cancer (26.1 vs. 16.0%, P = 0.089), and significantly more placebo patients had a past history of myocardial infarction (18.3 vs. 6.5%, P = 0.015). According to SOFA scores, the greatest level of dysfunction was evident in the cardiovascular system with 84.2% and 71.4% of patients rated as grade 4 before the 96-h infusion of standard treatment and at baseline, respectively. There were no other significant differences between the groups except for markers of cardiovascular dysfunction, where more DAA patients had the highest cardiovascular SOFA score (P = 0.026). There was also a trend (P = 0.062) for more DAA patients to have the highest score before the 96-h infusion of standard treatment. The greater level of cardiovascular dysfunction in the DAA group was supported by higher vasopressor doses (CVI, P = 0.006), particularly in the primary vasopressor used, norepinephrine (P = 0.034, Table 1).
96-h standard drug treatment
The mean number of hours of standard treatment was 99.0 (median 96, range 89.5–124.0). There was a mean of 0.5 (median 0, range −0.3 to 17.0) hours between the end of standard treatment and the start of study treatment.
Efficacy
Primary outcome
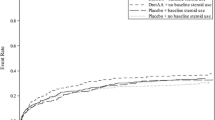
Vasopressor-dependent hypotension resolved in 32 DAA patients (34.0%) and 40 placebo patients (40.4%). The difference between the groups was not statistically significant (P = 0.419; Fig. 1). Sixty-two (66.0%) of the 94 extended DAA-group patients and 59 (59.6%) of the 99 placebo patients were counted as no resolution. Patients were considered as having no resolution if they continued to require vasopressor support after 72 h of treatment or stopped the infusion for the last time during the 72 h for reasons other than because they resolved their need for vasopressor support for >12 continuous hours [i.e. death (n = 17), procedure (n = 2)], or resolved their need for vasopressor support for <12 h (n = 2).
Secondary outcomes
By 28 days after the start of the 96-h infusion of standard drug therapy, 37 DAA patients (39.8%) and 31 placebo patients (32.3%) had died (OR 1.39, 95% CI 0.76–2.51; RR 1.23, 95% CI 0.84–1.81; P = 0.283). Ninety-day in-hospital mortality was similar between groups: 51 DAA patients (54.3%) and 43 placebo patients (43.9%; OR 1.53, 95% CI 0.86 to 2.68; RR 1.24, 95% CI 0.92 to 1.65; P = 0.15). The main causes of death were sepsis-induced multi-organ failure and refractory septic shock.
Repeated measures analyses indicated that cardiovascular and renal SOFA scores improved over time (P values, <0.001 and 0.011, respectively), whereas respiration and liver scores worsened (P values, 0.024 and 0.050, respectively). However, there were no significant differences between the two treatment groups in any mean organ system SOFA score (Table 2). There were also no treatment-group differences in: hospital-, ICU-, catecholamine-, renal replacement therapy-, and mechanical ventilation-free days; time to discharge from hospital and ICU; time to discontinuation of catecholamines, renal replacement therapy, and mechanical ventilation (data not shown).
Mean thrombin generation (D-dimers) did not fall significantly from baseline to end point (5.2–5.1) in DAA patients, but increased in placebo patients (4.6–6.3; difference in least squares means −1.78, 95% CI −3.34 to −0.23; P < 0.001). There were no statistically significant differences for change in prothrombin time or protein C; however, DAA patients had lower protein C levels at baseline compared with placebo patients. At study end point, levels were roughly equivalent and therefore protein C had increased by a greater extent in the drug group compared with placebo, although this change did not reach statistical significance (P = 0.216) (Table 3).
Exploratory analyses suggested that baseline protein C levels predicted outcome; patients with levels >40% had better estimated survival at 28 days compared with those with levels ≤40% (P = 0.03). A patient was a non-responder if their protein C decreased or remained stable from baseline to end point; a patient was a responder if levels increased from baseline. There were more protein C responders after treatment with DAA compared with placebo (58.1 vs. 41.9%, P = 0.07). Responders had higher 28-day survival (Fig. 2), although the difference was not significant (P = 0.10). There was no difference between responders and non-responders in time to resolution of vasopressor-dependent hypotension (P = 0.25).
Safety
Overall numbers of AEs, SAEs, and bleeding events are summarised by study period in Table 4. Nine patients in each of the treatment groups reported at least one SAE, most commonly cardiac disorders. Two patients experienced SAEs that were considered related to study drug: one DAA patient with two events (colitis ischemic, retroperitoneal haemorrhage) and one placebo patient with one event (haemoptysis). Three DAA patients and four placebo patients reported non-serious AEs, mostly cardiac and vascular disorders.
Discussion
Continued administration of DAA for up to 72 additional hours after a 96-h infusion of the standard drug did not result in a more rapid resolution of vasopressor-dependent hypotension versus placebo. Few patients reached resolution as most had either died or continued to require vasopressor support at the end of follow-up. These results should be interpreted cautiously as there was a reduction in the planned sample size from 270 to 200 patients, which resulted in estimates having wide CIs (for estimates of resolution of vasopressor support, the 95% CIs were 24.4–43.6% for the extended DAA group and 30.7–50.1% for the placebo group). No standard process for withdrawal of vasopressor was implemented, which may have contributed to this variability. There were also potentially relevant baseline group differences in vasopressor requirements and protein C levels, which are important because low protein C levels have been linked to a worse outcome in severe sepsis [11]. In addition, recruitment was prolonged, which could have contributed to the heterogeneity of the sample.
Although there was no difference in the primary objective, the biological activity of extended DAA was demonstrated; thrombin generation was not significantly reduced in DAA patients, but increased in placebo patients after the withdrawal of standard DAA. In addition, there were potentially clinically relevant increases in protein C after treatment with DAA. Compared with placebo, protein C increased by a greater extent in DAA patients, but owing to lower baseline levels, final protein C levels were similar, which would predict similar clinical outcomes. The failure of extended DAA in weaning patients with prolonged septic shock from vasoactive support could also be related to causes other than persistent sepsis-induced cardiovascular dysfunction. It is well known that septic shock causes alterations in myocardial and vascular adrenergic receptor-mediated responses in animals [12] and humans [13]. However, two well-established lines of evidence in chronic heart failure [14] and prolonged exercise [15] suggest that a down-regulation of adrenoreceptors contributes to cardiac dysfunction due to a pronounced activation of the sympathetic system. In the same line, the reduced sensitivity to catecholamines and consequent failure of weaning from vasoactive support may, in part, be due to a desensitization of adrenergic receptor stimulation induced by the stimulatory effect of endogenous and exogenous catecholamines [16].
Exploratory analyses suggested baseline protein C predicts outcome, which agrees with other studies [17–21]. Additionally, patients were more likely to be protein C responders (levels increased from baseline) with DAA treatment compared with placebo, and responders had a more favourable outcome (Fig. 2); although non-responders in the DAA group had the overall worst outcome. Further research is needed, but improvements in protein C could be a benefit of extended treatment. However, failure of improvement in protein C despite treatment with DAA may be indicative of a poor prognosis, or that other aspects of care should be reviewed. The potential role of protein C as a biomarker to guide therapy with DAA is currently being investigated in the Research Evaluating Serial PC levels in severe sepsis patients On DrotAA (RESPOND) study [11].
Extended administration of DAA did not reduce 28-day and in-hospital mortality compared with placebo. Previous work has shown that DAA is successful in reducing mortality when infused for up to 96 h [2, 3]. Mortality in this study was higher than has been previously found, although a relatively high mortality is to be expected with a population of such critically ill patients. In PROWESS, 28-day mortality following a 96-h infusion was lower at 24.7% in the DAA group (n = 850) and 30.8% in the placebo group (n = 840) [2], compared with 39.8 and 32.3%, respectively, in this study. However, it is difficult to compare mortality between the two treatment groups in this study owing to the small sample size and baseline imbalances in underlying diseases, vasopressor use, and protein C. Despite these limitations, one might speculate that extended DAA therapy may have been associated with a higher mortality. As the only known adverse effect of DAA treatment is bleeding, it could be hypothesised that if DAA treatment was associated with harm, more bleeding events should have been expected; in the present study, this was not the case, and there were no haemorrhagic or study drug related deaths. However, these study results do not support the use of infusions of longer than 4 days in current clinical practice.
Extended DAA treatment had an acceptable safety profile, with similar numbers of SAEs between DAA- and placebo-treated patients. In addition, the incidence of bleeding-related events was low with only one patient in each group experiencing serious bleeding during the infusion period. Given the study design, SAEs were not retrospectively collected from the standard DAA infusion period, and it is unlikely that patients experiencing serious bleeding events during this period would have continued standard treatment to be eligible for inclusion in this study. Thus, these results underestimate the full risks at the start of a planned course of potentially extended therapy; however, they do suggest that in a patient who has completed a standard 96-h infusion of DAA, the risks of continuing therapy at that stage may be relatively small; although it is difficult to make firm conclusions given the sample size and overall low event rate.
In conclusion, extended DAA treatment did not result in a difference in the primary outcome of time to resolution of vasopressor-dependent hypotension versus placebo. In addition, extended treatment did not reduce mortality or improve organ function compared with placebo, although some anticipated biological effects on D-dimer and protein C levels were found. The results of the study should be interpreted cautiously as a small sample size and baseline imbalances in protein C levels and vasopressor requirements disfavoured DAA and may have limited the ability to demonstrate a clinical benefit.
References
Xigris summary of product characteristics. EMEA, Xigris: product information. http://www.emea.europa.eu/humandocs/Humans/EPAR/xigris/Xigris.htm. Accessed July 2007
Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ Jr, Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group (2001) Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 344:699–709
Vincent JL, Bernard GR, Beale R, Doig C, Putensen C, Dhainaut JF, Artigas A, Fumagalli R, Macias W, Wright T, Wong K, Sundin DP, Turlo MA, Janes J (2005) Drotrecogin alfa (activated) treatment in severe sepsis from the global open-label trial ENHANCE: further evidence for survival and safety and implications for early treatment. Crit Care Med 33:1–12
Vincent JL, Angus DC, Artigas A, Kalil A, Basson BR, Jamal HH, Johnson G 3rd, Bernard GR; Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) Study Group (2003) Effects of drotrecogin alfa (activated) on organ dysfunction in the PROWESS trial. Crit Care Med 31:834–840
Dhainaut JF, Yan SB, Margolis BD, Lorente JA, Russell JA, Freebairn RC, Spapen HD, Riess H, Basson B, Johnson G 3rd, Kinasewitz GT, PROWESS Sepsis Study Group (2003) Drotrecogin alfa (activated) (recombinant human activated protein C) reduces host coagulopathy response in patients with severe sepsis. Thromb Haemost 90:642–653
Abraham E, Laterre PF, Garg R, Levy H, Talwar D, Trzaskoma BL, François B, Guy JS, Brückmann M, Rea-Neto A, Rossaint R, Perrotin D, Sablotzki A, Arkins N, Utterback BG, Macias WL, Administration of Drotrecogin Alfa (Activated) in Early Stage Severe Sepsis (ADDRESS) Study Group (2005) Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N Engl J Med 353:1332–1340
Nadel S, Goldstein B, Williams MD, Dalton H, Peters M, Macias WL, Abd-Allah SA, Levy H, Angle R, Wang D, Sundin DP, Giroir B, REsearching severe Sepsis, Organ dysfunction in children: a gLobal perspective (RESOLVE) study group (2007) Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase II randomised controlled trial. Lancet 369:836–843
de Pont AC, Bakhtiari K, Hutten BA, de Jonge E, Vroom MB, Meijers JC, Büller HR, Levi M (2005) Recombinant human activated protein C resets thrombin generation in patients with severe sepsis—a case control study. Crit Care 9:R490–R497
Dhainaut J, Antonelli M, Wright P, Belger M, Cobas-Meyer M, Mignini M, Janes J (2008) Extended drotrecogin alfa (activated) therapy in patients with persistent requirement for vasopressors support after 96-hour infusion with commercial drotrecogin alfa (activated). Crit Care 12(Suppl 2):P205
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
Shorr AF, Nelson DR, Wyncoll DL, Reinhart K, Brunkhorst F, Vail GM, Janes J (2008) Protein C: a potential biomarker in severe sepsis and a possible tool for monitoring treatment with drotrecogin alfa (activated). Crit Care 12:R45
Boillot A, Massol J, Maupoil V, Grelier R, Capellier G, Berthelot A, Barale F (1996) Alterations of myocardial and vascular adrenergic receptor-mediated responses in Escherichia coli-induced septic shock in the rat. Crit Care Med 24:1373–1380
Bernardin G, Strosberg AD, Bernard A, Mattei M, Marullo S (1998) Beta-adrenergic receptor-dependent and -independent stimulation of adenylate cyclase is impaired during severe sepsis in humans. Intensive Care Med 24:1315–1322
Lohse MJ, Engelhardt S, Eschenhagen T (2003) What is the role of beta-adrenergic signaling in heart failure? Circ Res 93:898–906
Hart E, Dawson E, Rasmussen P, George K, Secher NH, Whyte G, Shave R (2006) Beta-adrenergic receptor desensitization in man: insight into post-exercise attenuation of cardiac function. J Physiol 577:717–725
Reithmann C, Hallström S, Pilz G, Kapsner T, Schlag G, Werdan K (1993) Desensitization of rat cardiomyocyte adenylyl cyclase stimulation by plasma of noradrenaline-treated patients with septic shock. Circ Shock 41:48–59
Fourrier F, Chopin C, Goudemand J, Hendrycx S, Caron C, Rime A, Marey A, Lestavel P (1992) Septic shock, multiple organ failure, and disseminated intravascular coagulation: Compared patterns of antithrombin III, protein C, and protein S deficiencies. Chest 101:816–834
Mesters RM, Helterbrand J, Utterback BG, Yan B, Chao YB, Fernandez JA, Griffin JH, Hartman DL (2000) Prognostic value of protein C concentrations in neutropenic patients at high risk of severe septic complications. Crit Care Med 28:2209–2216
Yan SB, Helterbrand JD, Hartman DL, Wright TJ, Bernard GR (2001) Low levels of protein C are associated with poor outcome in severe sepsis. Chest 120:915–922
Fisher CJ Jr, Yan SB (2000) Protein C levels as a prognostic indicator of outcome in sepsis and related diseases. Crit Care Med 28:S49–56
Lorente JA, Garcia-Frade LJ, Landin L, de Pablo R, Torrado C, Renes E, Garcia-Avello A (1993) Time course of hemostatic abnormalities in sepsis and its relation to outcome. Chest 103:1536–1542
Acknowledgments
The authors acknowledge the efforts of all the investigators, study coordinators, and pharmacists involved in this clinical trial. This study was sponsored by Eli Lilly and Company. The authors would like to thank Nancy Milligan of Dianthus Medical Limited for preparing the first draft of the manuscript on behalf of Eli Lilly and Co Ltd in accordance with the European Medical Writers Association guidelines.
Conflict of interest statement
J.-F. D, and J. C. have served as consultants for Eli Lilly and Company; M. A., P. W., A. D., J. R. and S. L. report no conflict of interests at this time; M. B. is an employee of Eli Lilly and Company; M. C.-M., C. M, M. A. M. and J. J. are employees and stockholders of Eli Lilly and Company.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00134-009-1502-8
Rights and permissions
About this article
Cite this article
Dhainaut, JF., Antonelli, M., Wright, P. et al. Extended drotrecogin alfa (activated) treatment in patients with prolonged septic shock. Intensive Care Med 35, 1187–1195 (2009). https://doi.org/10.1007/s00134-009-1436-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-009-1436-1