Abstract
Background
Lithium dilution cardiac output by LiDCO™plus (LiDCO, Cambridge, UK) is a validated methodology for measuring cardiac output. It is used to calibrate a pulse pressure analysis algorithm (PulseCO) for the continuous measurement of subsequent changes in this variable. The variability of measurements, or precision, within patients of lithium dilution cardiac output has not previously been described.
Material and methods
Thirty-five hemodynamically stable patients in intensive care, with no significant variability in heart rate, mean arterial pressure or central venous pressure, were recruited. Fifty-three determinations of cardiac output were made, each using four lithium dilution measurement curves performed consecutively within a maximum period of 10 min. The coefficient of variation of the measurements was determined and used to derive the least significant change in cardiac output that this technique could reliably detect.
Results
For a single measurement, the coefficient of variation was 8%. This equates to the technique being able to detect a change (least significant change) between two measurements of 24%. Averaging two lithium dilution measurements improved the coefficient of variation to 6% with a least significant change of 17%. Using the average of three curves reduced the coefficient of variation to 5% with a least significant change of 14%.
Conclusions
To achieve a good precision with this technique, three lithium dilution measurements should be averaged. This will allow changes in cardiac output of more than 14% to be reliably detected. The understanding of the precision of this technique allows the user to know when a real change has happened to their patient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiac output measurement is one of the most widely used observations made in the intensive care unit (ICU). There is a commonly held belief that early manipulation of hemodynamic variables can improve the outcome of patients [1, 2]. One of the first devices available to measure cardiac output at the bedside was intermittent thermodilution (ITD) using the pulmonary artery catheter (PAC) [3]; however, more recently, a number of less invasive measurement systems have been introduced, which avoid some of the complications associated with pulmonary artery catheterization [4]. Less invasive devices using technologies such as pulse pressure or pulse contour analysis are now widely used [5]. A patented algorithm allows these devices to analyze the arterial pressure waveform and extrapolate this pressure trace to changes in stroke volume and therefore cardiac output. To accurately convert a pressure trace to a volume or flow-based variable, it is necessary to understand the arterial compliance. Unfortunately, the relationship between pressure and compliance is nonlinear, which has led to most companies marketing devices for this purpose to recommend an independent method of calibration. This calibration technique allows a pressure waveform to be accurately transformed into a volume-based measurement. It is vital, however, that this calibration is both accurate and precise, i.e. reliable. Accuracy describes how close to the actual value the measurements are. Precision refers to how close the repeated measurements of the same variable are to each other. A measurement can be accurate and precise (measurements are very close to each other and very close to the actual value), accurate and imprecise (i.e. measurements are close to the actual value but far from each other), inaccurate and precise (i.e. measurements are very close to each other but far from the actual value) and inaccurate and imprecise (i.e. measurements are basically randomly spread). This article investigates the precision of the lithium dilution technique.
LiDCO™plus (LiDCO, Cambridge, UK) uses an algorithm (PulseCO) based on the physics of conservation of mass and energy [6]. This is calibrated with a lithium dilution technique for independently measuring cardiac output. Although the lithium dilution cardiac output method of measurement is validated against arterial flow probes in animals and ITD from the PAC in humans [7, 8], there is little data that describe the precision of the technique or the number of measurements that need to be utilized to generate a level of precision that would be deemed to be acceptable to improve the reliability of the continuous algorithm and to be usefully used in routine clinical practice. Preliminary data was presented at a meeting in Bruxelles ISICEM conference in 2007 [9].
Materials and methods
This study was performed on the General Adult Intensive Care Unit of St George’s Hospital, London, following approval of the study protocol by the Local Research and Ethics Committee. Over a 30-day period, all patients receiving cardiac output monitoring with LiDCO™plus system for routine clinical indications were recruited. Inclusion criteria included the following: age greater than 18 years, weight more than 40 kg and the presence of both a central venous catheter and radial arterial line in situ. Exclusion criteria included the lack of consent from a patient, significant arrhythmias (bigemini or trigemini arrhythmias), new onset of atrial fibrillation or atrial fibrillation with uncontrolled ventricular response (i.e. HR > 100 bpm) and/or cardiovascular instability or the use of an interaortic counter-pulsation balloon pump. Four lithium dilution curves were performed according to the manufacturer’s recommendations in the shortest possible time (≤10 min) by skilled investigators with experience of in excess of two hundred lithium dilution calibrations (MC, DD and AR). This technique comprised a rapid injection of 0.3 mmol of lithium through a central venous catheter following the reaching of a steady state of serum lithium levels. The change in lithium levels were detected by blood being drawn out of the radial artery catheter over a specific lithium-selective sensor. Cardiac output was then measured from analysis of these lithium dilution curves utilizing a method of integrating the changes in lithium levels over time.
All lithium dilution curves were visually inspected to ensure that they conformed to an expected shape. When the device warned that a curve did not conform to a standard indicator dilution shape, it was rejected and wherever possible another lithium dilution measurement was performed. During the measurement process, all other interventions including changes to mechanical ventilation or cardiovascular therapy were withheld. A steady state was a necessary requirement before any measurement, i.e., all curves were performed within a 10-min period and the study was aborted if there was a change in either measured heart rate or blood pressure of more than 5% during the study period.
Statistical analysis
All data are described as means (SD), when were normally distributed, and medians (IQR) when were not normally distributed. The coefficient of variation (CV) for each independent set of cardiac output measurements was estimated according to the principles set out by Bland and Altman [8]. As the standard deviation (SD) of a single lithium dilution measurement is proportional to the magnitude of cardiac output, a log transformation was used prior to calculation of CV. Every lithium dilution measurement was defined as CO1, CO2, CO3 and CO4, and following log transformation, they were relabeled as LogCO1, LogCO2, LogCO3 and LogCO4. From this, the SD of the logarithmic transformation was calculated. The SD for LogCO1, LogCO2, LogCO3 and LogCO4 was then antilog-transformed to obtain the CV for each set of measurements. The mean of the CVs obtained from each study was then calculated.
While the SD is expected to be the same for every number of replicates (n), the standard error (SE) decreases with the number of replicates according to an exponential decay related to the number of measurements:
The CV for a series of replicates is given as follows: CV = SD/mean CO. The SE decreases together with the increase of the number. When SE is used instead of SD, it means that the variation can be classified as coefficient of error (CE), where CE = SE/mean CO. Using this relationship, the CE for n number of replicates was calculated. It is clear that there is a relationship between CE and CV as CE = CV/√n, with CE = CV when n = 1. Precision defined as 95% confidence is 2 SD. In terms of percentage, precision is 2 CV for single measurements and is 2 CE for averaged measurements.
To study the ability of a monitor to detect changes in cardiac output, it was necessary to understand how the CE of the technique affected the likelihood of detecting any given change with 95% probability. This variable is known as the least significant change (LSC), and it allows the user of the technology to understand for a measured change, if a real change has actually happened, or whether the measured change was just the result of a low precision in the measurement technique. This relationship was explored using the following equation [10]:
To assess the clinical utility of the device, we decided a priori that a LSC of 15% was the minimum that could be accepted for effective clinical practice. Factors relating to changes in the CE were explored by first identifying variables that were related to CV by either linear regression analysis for continuous variables or by nonparametric tests for discrete variables. Any factor that had a probability of less than 0.1 was then entered into a backward stepping multiple regression model. This was used to identify any factors that were significantly and independently related to the CV.
Results
Patient characteristics
Thirty-five patients were enrolled into the study, of whom 11 (31.5%) were male. Eleven (31.5%) of the 35 patients needed cardiac output monitoring due to an emergency deterioration in their clinical status, whereas 24 (68.5%) had cardiac output monitoring instituted as part of their routine postoperative clinical course. Seventeen (48.6%) of the 35 patients needed vasopressor support in terms of a norepinephrine infusion. Twenty-five patients were not ventilated. The median heart rate was 87 (75, 105) beats/min, mean arterial pressure 84 (72, 99) mmHg and central venous pressure 14 (8, 17) mmHg. The mean hemoglobin concentration was 10.3 (1.4 g/dl).
Bolus lithium dilution cardiac output
Cardiac output measurements: a total number of 53 studies (197 curves) were made from 35 patients (Fig. 1). In 15 studies, only three curves were acquired rather than four. All data points were then suitable for analysis. Twenty-three of the measurements (43%) were made while patients were receiving a norepinephrine infusion. The median value of 53 averages of cardiac output was 5.6 (4.6, 7.2) l/min. The median of the SDs of the four measurements in each subject was 0.5 l/min and the CV was 8.3%.
Precision of lithium dilution cardiac output
With a single measurement, a mean CV of 8% (±4%) was observed. If the mean of three lithium dilution curves was used, the CE was reduced to 5%. If it was desired to measure cardiac output with a precision of ±10%, then at least three averaged curves are necessary (Fig. 2).
In clinical practice, it is often more useful to detect a change in cardiac output from a baseline. For this, it is necessary to understand how the precision of a monitor influences its ability to detect change. This can be expressed as the LSC. The LSC is the minimum change that needs to be detected by a device to recognize a real change. In practice, the more precise the monitor is, the smaller the LSC is. Our a priori criteria were achieving a LSC change of less than 15%. Figure 3 demonstrates the number of measurements that must be obtained with the lithium dilution system to detect with 95% certainty that a change in cardiac output from a baseline has occurred. It can be seen that, to have a LSC lower than 15% (14%) change, three averaged lithium dilution curves would need to be undertaken. Two curves would only allow a change in cardiac output of greater than 17% to be trusted. Averaging four would allow changes in cardiac output as small as 11.8% to be trusted.
Variables influencing the precision of lithium dilution cardiac output
Univariate factors significantly associated with the CV of the lithium dilution system included weight (r 2 = 0.12, P = 0.03) and the hemoglobin concentration (r 2 = 0.20, P = 0.008; Tables 1, 2). There were no significant relationships found for the diagnostic category, height, age, gender, heart rate, mean arterial pressure, central venous pressure or the plasma sodium concentration. A multiple regression model was constructed to determine multivariate independent factors that influenced the CV. The only significant independent factor found that influenced the CV was the hemoglobin concentration with an increase in the CV of 1.6 times for every increase in hemoglobin of 1 g/dl (see Tables 3–5 in additional ESM material).
Discussion
This study demonstrates that at least three lithium dilution measurements should be performed for the technique to have sufficient precision to be used to calibrate an independent form of cardiac output measurement or to detect significant changes in cardiac output during clinical practice. This is the first report of the intrapatient variability of a transpulmonary cardiac output measurement technique. In theory, transpulmonary techniques [11, 12] should be more precise than pulmonary thermodilution, because they are less affected by the respiratory cycle. Investigators have previously explored methods of minimizing the error of the ITD technique [11–19]. However, it is unclear as to whether the same principles hold true with a transpulmonary methodology.
The lithium dilution technique [20] has previously been validated in a variety of different clinical scenarios against ITD with a good level of agreement between the two techniques [7, 21–23]. Validation studies on agreement between two techniques, however, do not provide useful information about the precision of either individual method. When using ITD determination of cardiac output, it is known that the average of three to four measurement curves represents the best compromise between precision and practicality, allowing the clinician to confidently accept a change detected by the monitor as real [18]. If a monitoring device or technique is highly precise, it will be able to detect small changes in a variable but if it is imprecise it will only detect much larger changes. Nilsson demonstrated that the ITD is a very precise tool and that an average of four ITD curves allows the clinician to detect changes in cardiac output of more than 7%. When using less invasive devices, we may be willing to accept lower levels of precision but this must be understood by the clinician to avoid arriving at inappropriate conclusions. This concept is especially important when one technique is used to calibrate another. In pulse power algorithms, the calibration is vital to have an accurate starting point to convert changes in arterial pressure to changes in arterial volume. Recalibration is usually recommended when major hemodynamic changes occur; therefore, knowing how precise this process is means that it is possible to better understand the changes between two calibrations and how precise the calibration technique must be before the accuracy of the whole system is impaired.
This study is similar to work by Nilsson et al. [18], in which they found that averaging four intermittent pulmonary thermodilution curves performed with iced saline enabled the clinician to achieve an error of about 5% and to detect changes in cardiac output of 7%. In clinical practice, it is usual to use room temperature saline boluses to perform ITD. This will inevitably lead to increased error, secondary to a reduced signal-to-noise ratio, and may increase the precision of the technique to as much as 20% (10% error) [24, 25]. If a less invasive technique for monitoring cardiac output is to replace the ‘gold’ standard ITD, it must have a level of precision at least similar to the traditional methodology [26, 27]. Our results suggest that the precision of a single lithium dilution curve for the measurement of cardiac output is worse than the normally accepted values for ITD. When two, three or four measurements are averaged, the precision improves to be at least as good as ITD and improves to a level whereby the technique is precise enough to improve the confidence and precision of an independent measurement technique. At this level of precision, the technique is able to detect small changes (less than 12%) in cardiac output, which enables the device to be used for routine clinical practice—for instance, in the detection of change following a fluid challenge.
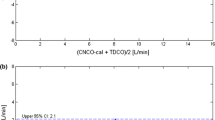
The relevance of precision of this technique can be seen in the following example. A cardiac output measurement with a CV of 8% implies that, with 95% confidence, our results will be in a ±17% range, i.e., a cardiac output of 5 l/min could represent any value from 4.2 to 5.8 l/min. This may lead to overtreatment of some patients and undertreatment of others. An average of two lithium dilution curves improves the CE by 29%. It is possible to construct a “5 l/min isocardiac output” graph as seen in Fig. 4, where it is clear that increasing the number of measurements that are averaged leads to narrow confidence intervals.
This study identifies the hemoglobin concentration as being the only independent factor that influences the precision of lithium dilution cardiac output measurement. Weight was also found to be a significant variable on univariate analysis but not on the multiple regression (Tables 3–5 in additional ESM material). This may be because weight is not an independent factor that determines precision, but may also relate to the power of the study being insufficient to statistically detect this as an issue. Lithium dilution measurement is based on the Stewart–Hamilton equation. To utilize this approach, it is assumed that lithium is only distributed in the plasma, which is estimated with reference to the hemoglobin concentration and by inference from the haematocrit. Despite the demonstrated increase in variability with increasing hemoglobin concentration being small, it is statistically significant and should be taken into consideration if very precise measurements are required.
There are a number of limitations to this study. This study was performed on a group of patients on the general intensive care unit. It therefore deliberately did not include children, patients following cardiac surgery or neurosurgery or patients undergoing general anesthesia. The results of this study, therefore, cannot be extrapolated to these patient groups. The aims of the study were to identify the CV for lithium dilution cardiac output measurements. The fundamental hypothesis for this has to be that any changes detected are from the technique itself rather than from changes in the measured variable—for instance, changing hemodynamic status of the patient. To minimize this risk, we performed the four measurements in as short a time as possible (less than 10 min). We also only studied patients who were relatively hemodynamically stable as defined by having changes in heart rate and mean arterial pressure of less than 5% for the study period. It is possible that, in less cardiovascularly stable patients, the variability that we detected may be significantly higher, making the technique less robust unless more measurements are used to get the averaged value.
Conclusions
The precision of the lithium dilution technique to measure cardiac output improves when averaging increasing numbers of measurements. A good compromise between acceptable levels of precision and clinical utility is the use of three lithium dilution curves. This will enable a high level of precision to be obtained, which will allow small but significant changes in cardiac output to be detected.
References
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M (2001) Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345:1368–1377
Pearse R, Dawson D, Fawcett J, Rhodes A, Grounds RM, Bennett ED (2005) Early goal-directed therapy after major surgery reduces complications and duration of hospital stay. A randomised, controlled trial [ISRCTN38797445]. Crit Care 9:R687–R693
Swan HJ, Ganz W, Forrester J, Marcus H, Diamond G, Chonette D (1970) Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. N Engl J Med 283:447–451
Connors AF Jr, Speroff T, Dawson NV, Thomas C, Harrell FE Jr, Wagner D, Desbiens N, Goldman L, Wu AW, Califf RM, Fulkerson WJ Jr, Vidaillet H, Broste S, Bellamy P, Lynn J, Knaus WA (1996) The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators. JAMA 276:889–897
Cecconi M, Wilson J, Rhodes A (2006) Pulse pressure analysis. In: Vincent JL (ed) Yearbook of intensive care and emergency medicine. Springer, Berlin, pp 176–184
Rhodes A, Sunderland R (2004) Arterial pulse pressure analysis: the LiDCOplus system. Update in intensive care and emergency medicine. In: Pinsky MR, Payen D (eds) Functional hemodynamic monitoring. Springer, Heidelberg, pp 183–192
Linton RA, Band DM, Haire KM (1993) A new method of measuring cardiac output in man using lithium dilution. Br J Anaesth 71:262–266
Bland JM, Altman DG (1999) Measuring agreement in method comparison studies. Stat Methods Med Res 8:135–160
Cecconi M, Al-Subaie N, Canete M, Dawson D, Puntis M, Poloniecki J, Grounds R, Rhodes A (2007) Lithium dilution cardiac output measurement in the critically ill patient: determination of precision of the technique. Crit Care 11:P294
Lodder MC, Lems WF, Ader HJ, Marthinsen AE, van Coeverden SC, Lips P, Netelenbos JC, Dijkmans BA, Roos JC (2004) Reproducibility of bone mineral density measurement in daily practice. Ann Rheum Dis 63:285–289
Jansen JR, Schreuder JJ, Settels JJ, Kornet L, Penn OC, Mulder PG, Versprille A, Wesseling KH (1996) Single injection thermodilution. A flow-corrected method. Anesthesiology 85:481–490
Jansen JR, Schreuder JJ, Punt KD, van den Berg PC, Alfieri O (2001) Mean cardiac output by thermodilution with a single controlled injection. Crit Care Med 29:1868–1873
Jansen JR, Schreuder JJ, Bogaard JM, van Rooyen W, Versprille A (1981) Thermodilution technique for measurement of cardiac output during artificial ventilation. J Appl Physiol 51:584–591
Jansen JR, Versprille A (1986) Improvement of cardiac output estimation by the thermodilution method during mechanical ventilation. Intensive Care Med 12:71–79
Jansen JR, Schreuder JJ, Settels JJ, Kloek JJ, Versprille A (1990) An adequate strategy for the thermodilution technique in patients during mechanical ventilation. Intensive Care Med 16:422–425
Jansen JR (1995) The thermodilution method for the clinical assessment of cardiac output. Intensive Care Med 21:691–697
Berthelsen PG, Eldrup N, Nilsson LB, Rasmussen JP (2002) Thermodilution cardiac output. Cold vs room temperature injectate and the importance of measuring the injectate temperature in the right atrium. Acta Anaesthesiol Scand 46:1103–1110
Nilsson LB, Nilsson JC, Skovgaard LT, Berthelsen PG (2004) Thermodilution cardiac output—are three injections enough? Acta Anaesthesiol Scand 48:1322–1327
Ostergaard M, Nilsson LB, Nilsson JC, Rasmussen JP, Berthelsen PG (2005) Precision of bolus thermodilution cardiac output measurements in patients with atrial fibrillation. Acta Anaesthesiol Scand 49:366–372
Pearse RM, Ikram K, Barry J (2004) Equipment review: an appraisal of the LiDCO plus method of measuring cardiac output. Crit Care 8:190–195
Costa MG, Della Rocca G, Chiarandini P, Mattelig S, Pompei L, Barriga MS, Reynolds T, Cecconi M, Pietropaoli P (2008) Continuous and intermittent cardiac output measurement in hyperdynamic conditions: pulmonary artery catheter vs. lithium dilution technique. Intensive Care Med 34:257–263
Jonas MM, Tanser SJ (2002) Lithium dilution measurement of cardiac output and arterial pulse waveform analysis: an indicator dilution calibrated beat-by-beat system for continuous estimation of cardiac output. Curr Opin Crit Care 8:257–261
Jonas MM, Kelly FE, Linton RA, Band DM, O’Brien TK, Linton NW (1999) A comparison of lithium dilution cardiac output measurements made using central and antecubital venous injection of lithium chloride. J Clin Monit Comput 15:525–528
McGee WT, Horswell JL, Calderon J, Janvier G, Van Severen T, Van den Berghe G, Kozikowski L (2007) Validation of a continuous, arterial pressure-based cardiac output measurement: a multicenter, prospective clinical trial. Crit Care 11:R105
Squara P, Denjean D, Estagnasie P, Brusset A, Dib JC, Dubois C (2007) Noninvasive cardiac output monitoring (NICOM): a clinical validation. Intensive Care Med 33:1191–1194
Critchley LA, Critchley JA (1999) A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput 15:85–91
Cecconi M, Grounds M, Rhodes A (2007) Methodologies for assessing agreement between two methods of clinical measurement: are we as good as we think we are? Curr Opin Crit Care 13:294–296
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cecconi, M., Dawson, D., Grounds, R.M. et al. Lithium dilution cardiac output measurement in the critically ill patient: determination of precision of the technique. Intensive Care Med 35, 498–504 (2009). https://doi.org/10.1007/s00134-008-1292-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-008-1292-4