Abstract
Introduction
A physiological review on renal replacement therapies (RRT) is a challenging task: there is nothing "physiologic" about RRT, since the most accurate, safe and perfectly delivered extracorporeal therapy would still be far from "physiologically" replacing the function of the native kidney.
Methods
This review will address the issues of physiology of fluid and solute removal, acid base control and impact on mortality during intermittent and continuous therapies: different RRT modalities and relative prescriptions will provide different "physiological clinical effects" to critically ill patients with acute kidney injury (AKI), with the aim of restoring lost "renal homeostasis". On the other side, however, the "pathophysiology" of RRT, consists with unwanted clinical effects caused by the same treatments, generally under-recognized by current literature but often encountered in clinical practice. Physiology and pathophysiology of different RRT modalities have been reviewed.
Conclusion
Physiology and pathophysiology of RRT often coexist during dialysis sessions. Improvement in renal recovery and survival from AKI will be achieved from optimization of therapy and increased awareness of potential benefits and dangers deriving from different RRT modalities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Physiology of RRT
Fluid removal
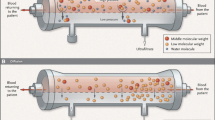
All critically ill patients need a high daily amount of volume infusions: blood and fresh frozen plasma, vasopressors and other continuous infusions, parenteral and enteral nutrition, which should be delivered without restriction or interruption. It is not uncommon for patients with acute kidney injury (AKI) and associated septic shock, to receive large amounts of fluid resuscitation, leading to fluid overload. The consequent positive fluid balance and tendency to interstitial edema causes the necessity for water removal and, possibly, the achievement of a negative daily fluid balance. The process of ultrafiltration occurs when plasma water is driven by a hydrostatic force through an extracorporeal circuit across a semi-permeable membrane and then removed from patient total body water. Extracorporeal renal replacement therapies (RRT) are typically utilized for ultrafiltration. Ultrafiltered water has a similar osmolarity to plasma water; for this reason the process of “isolated ultrafiltration” substantially corresponds to blood dehydration, with possible increase of hematocrit values and without modification of (small) solutes concentration.
Continuous renal replacement therapy (CRRT) slowly and continuously removes patient’s plasma water, mimicking urine output, whereas thrice weekly intermittent hemodialysis (IHD) must extract, in few hours, the equivalent of 2 days of administered fluids plus excess body water which may be present in the anuric patient. The intravascular volume depletion associated with excessive ultrafiltration rate is due to both the high rate of fluid removal required and the trans-cellular and interstitial fluid shifts caused by the rapid dialytic loss of solute [1]. The major consequence of rapid fluid removal is hemodynamic instability. In the case of a septic patient with AKI who is receiving a high amount of vasopressors because of hemodynamic instability and is needing appropriate fluid resuscitation, supplementation of nutrition and blood product administration. The renal replacement modality of choice seems to be the one that warrants slow fluid removal, prolonged it for many hours per day, in order to easily meet the higly variable required daily fluid balance. In particular, when volemic and uremic control are not a problem, an aggressive, protein-rich nutritional policy (1.5–2.5 g/day) can be implemented in the care of AKI patients receiving CRRT, resulting in a marked improvement in daily nitrogen balance with possible favorable effects on immune function and overall outcome [2–5]. Safe prescription of fluid loss during RRT requires intimate knowledge of the patient’s underlying condition, understanding of the process of ultrafiltration and close monitoring of the patient’s cardiovascular response to fluid removal. In order to preserve tissue perfusion in patients with AKI, it is important to optimize fluid balance by removing patient’s excess water without compromising the effective circulating fluid volume. It is still a matter of controversy which clinical parameter (actual patient weight/patient dry weight, mean arterial pressure, central venous pressure, wedge pressure, systemic saturation, mixed venous saturation, bioimpedance, etc.) or currently available monitorization (central venous catheter, swan ganz catheter, transesophageal echocardiography, etc.) should be utilized in order to uniformly define the concept of “volume overload”. In patients who are clinically fluid overloaded, however, it is extremely important to accurately evaluate the amount of fluid to remove [6]. In these kinds of patients, one of the main features of slow and constant ultrafiltration is the possibility for interstitial fluid to slowly and constantly refill the “dehydrated” bloodstream. This phenomenon is driven by hydrostatic and osmotic forces and allows for the elimination of high plasma water volumes per day with a reduced risk of hypovolemia and hypotension. In critically ill children the correction of water overload is considered a priority. It has been show that restoring adequate water content in small children is the main independent variable for outcome prediction [7, 8]. Similar results have been recently found in a large cohort of adult critically ill patients with AKI [9].
All patients who are at risk of, or who already have, increased intracranial pressure (neurosurgical patients, patients with encephalitis or meningo-encephalitis, traumatic brain injury or acute liver failure) CRRT in preference to IHD is strongly indicated in case of AKI. In an evaluation of brain density by repeated computerized tomography (CT), La Greca and co authors found that after intermittent dialysis densitometric values fell significantly from normal values (27–40 Hounsfield units), especially in the basal ganglia; this might point to a build-up of fluid in the parenchyma. No significant variations was observed in repeated examinations of three normal individuals. Interestingly, the authors found no significant relation between biochemical data and the CT pattern, hypothesizing that decreased density of brain tissue and post-dialysis edema might be a consequence of the local production of “hydrogenic osmoles”, due to a reduction in the pH factor of CSF and cerebral tissue [10]. CRRT has been shown to prevent intracranial pressure increase associated with intermittent renal replacement therapies [11].
Solute removal
Physiology of solute removal is one of the main issues of dialysis and it is partially responsible for safety, tolerability and outcome of extracorporeal RRTs. Solute removal is a very broad concept that is generally described by the elimination of a marker solute. This marker solute should be reasonably representative of all solutes that are normally removed from blood by the kidney.
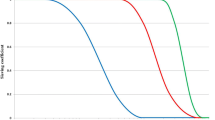
Unfortunately, a reference solute that represents all the solutes accumulating during AKI is not currently available, because kinetics and volume of distribution are different for each molecule. Then, “single-solute control” during RRT represents only a rough estimate of treatment efficiency. With these specifications, urea is generally utilized as “imperfect” marker molecule, because of its accumulation in all patients with AKI and the ease of serum level measurement. Furthermore, in spite of its moderate toxicity, urea is the final product of protein metabolism; its accumulation describes the need for dialysis and its removal describes treatment efficiency. It is a small molecule and its volume of distribution is similar to total body water. It is not bound to protein and it freely passes through tissues and cell membranes. Creatinine has similar characteristics and is another commonly used marker solute. One of the measures utilized to quantify urea/creatinine removal is dialysis dose. An in depth analysis of the concept of dialysis dose goes beyond the aim of this review and can be found elsewhere [12]. One of the main aspects of dose to be understood, however, is the concept of clearance (K); K is the volume of blood cleared from a given solute over a given time. K does not reflect the overall solute removal rate (mass transfer) but rather its value normalized by the serum concentration. Even when K remains stable over time, the removal rate will vary if the blood levels of the reference molecule change. K depends on solute molecular size, intercompartmental transmittance (Kc), transport modality (diffusion or convection), and circuit operational characteristics (blood flow rate, dialysate flow rate, ultrafiltration rate, hemodialyzer type and size). In its original conceivement, K is utilized to evaluate renal function among disparate individuals whose kidneys, however, are operating 24 h per day and urea/creatinine blood levels are at steady state. For this reason, the concept of K is easily applicable to continuous treatments and its utilization to describe intermittent therapies efficiency is a sort of “adaptation”. For this reason, since K represents only the instantaneous efficiency of the system, during treatments with different time schedules the information about the time span during which K is delivered is fundamental to compare the different RRT doses. For example, K is typically higher in IHD than in continuous renal replacement therapy (CRRT) and sustained low efficiency daily dialysis (SLEDD). However, mass removal may be greater during SLEDD or CRRT because the K is applied for 12/24 h (Table 1). In any case, from a physiological point of view, even if a continuous and an intermittent therapy were prescribed in order to provide exactly the same marker solute removal, still they could not be comparable: during continuous treatments, where a relatively low K is applied, a slow but prolonged removal of solutes approaches a pseudo-steady state slope (Fig. 1). In highly intermittent therapies, the intensive K, limited to 4–6 h per day, thrice a week, causes the saw tooth slope in solute removal and the eventual rebound during the time span without treatment. These peaks and valleys of solutes, bicarbonate, electrolytes, plasma osmolarity and volemia are not physiologic and might have a detrimental impact on patients’ hemodynamics, electrolyte, acid base and other “osmoles” balance. Furthermore, in the case of IHD, the Kc, i.e., the variable tendency of different tissues to “release” a solute into the bloodstream, is much more relevant than during slow efficiency treatments. As a matter of fact, finally, solute control is optimized during CRRT: it has been calculated that if the solute target was, for example, in a 70-kg patient, a mean blood urea nitrogen level of 60 mg/dL, this would be easily obtainable with a “standard” CVVH dose, but it might be very difficult to be reached by even intensive IHD regimens [13]. Some authors have recently suggested to express CRRT daily dose as K indexed to patient body weight. It is now recommended to administer a CRRT dose of at least 35 mL/(h kg) × 24 h [14–16]. Simplifying for low molecular weight solutes (see [15]), K equals replacement solution and/or dialysate flow, and CRRT “standard” dose may be expressed in our 70-kg patient as about 2,500 mL/h (35 mL/h × 70 kg) per 24 h or 60 L/day (2,500 mL/h × 24 h) of replacement solution during continuous venovenous hemofiltration (CVVH) or of dialysate during continuous venovenous hemodialysis (CVVHD) [12]. It is expected that this recommended dose to be modified in the next years, after the production of new evidence in this field (see below).
Typical solute removal patterns during different renal replacement therapies. The slow and steady clearance of continuous treatments allows lower average serum urea levels than during intermittent therapies, also avoiding potentially dangerous peaks of solute increase. Furthermore, since clearance does not reflect the overall solute removal rate (mass transfer) but rather its value normalized by the solute serum concentration, when solute concentration rapidly decreases (intermittent dialysis) the final result will be a lower mass transfer than when solute levels are steady (continuous treatments)
Acid–base control
Few studies have been done to detect which modality is better in terms of acid–base control [17]. Oligo anuric patients often have mild acidemia secondary to increased unmeasured anions (strong ion gap: SIG 12.3 mEq/L), hyperphosphatemia and hyperlactatemia. This acidosis is attenuated by the alkalizing effect of hypoalbuminemia. Uchino and co-workers [18] compared the effect on acid–base balance of IHD and CVVHDF. Before treatment, metabolic acidosis was common in both groups (63.2% for IHD and 54.3% for CVVHDF). Both IHD and CVVHDF corrected metabolic acidosis. However, the rate and degree of correction differed significantly. CVVHDF normalized metabolic acidosis more rapidly and more effectively during the first 24 h than did IHD (P < 0.01). IHD was also associated with a higher incidence of metabolic acidosis than was CVVHDF during the subsequent 2 weeks treatment period. Accordingly, CVVHDF can be considered physiologically superior to IHD in the correction of metabolic acidosis. In a comparison between CVVH and peritoneal dialysis, all patients randomized to CVVH achieved correction of acidosis by 50 h of treatment, compared with only 15% of those treated by peritoneal dialysis (P < 0.001) [19].
Rocktaschel showed that once CVVH is commenced, acidemia is corrected within 24 h. This change is associated with a decreased SIG, and decreased phosphate and chloride concentrations. After 3 days of CVVH, patients develop alkalemia secondary to metabolic alkalosis due to a further decrease in SIG and in serum phosphate concentration in the setting of persistent hypoalbuminemia [20].
Mortality
It has been hypothesized that safety features and “physiology” of different RRT modalities delivered for AKI in critically ill patients might partially give explanation for different clinical outcomes such as mortality, recovery of renal function and dialysis independence. Theoretically speaking, CRRT seems to represent the ideal therapy in order to “physiologically” restore renal homeostasis, steadily achieve adequate fluid balance, maintain hemodynamic stability and effectively control metabolic derangements of AKI and septic syndrome. However, four recently published randomized clinical trials and one multicenter observational study contested that outcomes with CRRT are superior to those of IHD [21–25]. None of these studies showed a superior outcome for CRRT compared with IHD. The results of these studies in some cases have been criticized for methodology and groups randomization [26]. Nevertheless, they do not support the belief that CRRT provides better outcomes than IHD. Three meta-analyses published in the last year agreed that CRRT does not differ from IHD with respect to in-hospital mortality, ICU mortality, number of surviving patients not requiring RRT and hemodynamic instability [27–29]. One of the common key points of these findings can be that IHD has become safer and more efficacious with contemporary dialytic techniques. Furthermore, a liberal and extended use of CRRT might have become less safe and/or efficacious than previously considered or expected. The concept that CRRT can provide more hemodynamic stability, more-effective volume homeostasis and better blood pressure support than IHD has been challenged by technical advances in the delivery of IHD, that have dramatically decreased the propensity of IHD to cause intradialytic hypotension. These advances include the introduction of volume-controlled dialysis machines, the routine use of biocompatible synthetic dialysis membranes, the use of bicarbonate based dialysate and the delivery of higher doses of dialysis, the utilization of “hybrid techniques” such as extended daily dialysis [30, 31]. In an important study, Schortgen et al. [32] demonstrated that there was a lower rate of hemodynamic instability and better outcomes after implementation of a clinical practice algorithm designed to improve hemodynamic tolerance to IHD. Recommendations included priming the dialysis circuit with isotonic saline, setting dialysate sodium concentration at above 145 mmol/L, discontinuing vasodilator therapy and setting dialysate temperature to below 37°C. Thus, the original rationale for the widely held assumption that CRRT is a superior therapy may have dissipated over time [33].
There is also urgent need for prospective high-quality and suitably powered trials to adequately address the impact of “dialysis dose” on mortality. New high level of evidence is however coming from two very recent trials. A (small) randomized controlled trial on 200 critically ill patients with AKI concluded that patient survival or renal recovery was not different between patients receiving high-dosage (35 mL/(kg h) or standard-dosage (20 mL/(kg h) CVVHDF [34]. In the second study, under the sponsorship of The Veterans Affairs/National Institutes of Health (VA/NIH) Acute Renal Failure Trial Network, 1,124 critically ill patients with AKI and failure of at least one nonrenal organ or sepsis were randomly assigned to receive intensive or less-intensive RRT [35]. The study was a multicenter, prospective, randomized, parallel-group trial conducted between November 2003 and July 2007 at 27 VA and university-affiliated medical centers. In both groups only hemodynamically stable patients underwent IHD, whereas hemodynamically unstable patients underwent CVVHDF or SLED. Patients receiving the intensive treatment strategy underwent IHD and SLED six times per week and CVVHDF at 35 mL/h per kilogram of body weight; for patients receiving the less-intensive treatment strategy, the corresponding treatments were provided thrice weekly and at 20 mL/h per kilogram. The rate of death from any cause by day 60 was 53.6% with intensive therapy and 51.5% with less-intensive therapy. There was no significant difference between the two groups in the duration of RRT or in the rate of recovery of kidney function or nonrenal organ failure. This is the first multicenter clinical trial with adequate statistical power on different RRT strategies, so far. The findings of this study contrast with other single center trials [18, 19] and are similar to smaller studies by Tolwani and Bouman [34, 36]. However, these results add high level of evidence to the debate on dialysis dose and are going to be discussed for a long time. Nonetheless, many concerns about external validity of the study have risen. First of all, patients were allowed to be transitioned from one dialysis method to another. In this condition, as discussed above, the dialysis dose is impossible to compare and unlikely to be equivalent. Finally it is not known if the study findings can be generalized to different health care systems from United States and to different RRT approaches (for example the sole use of continuous venovenous hemofiltration of many European and Australian centers). In any case, it is possible that other strategies than only increasing RRT dose might help AKI patients. As the accompanying editorial correctly points out, current approaches to dialysis are probably adequate to replace critical functions such as regulation of volume and electrolyte and acid–base homeostasis. Still lacking are methods that efficiently down-regulate the inflammatory response, which might play a major role in the pathophysiology of AKI [37].
Pathophysiology of RRT
Although considerable attention has focused on the perceived benefits of CRRT, there has been less emphasis on the possibility that CRRT might confer increased risk [38]. As a continuous extracorporeal therapy, CRRT requires continuous contact of patient’s blood with foreign surfaces. This event activates the coagulation and complement cascade, leukocytes and platelets [39]. Activated leukocytes release inflammatory mediators and induce oxidative stress, transforming lipids and proteins and contributing to endothelial injury. Activated platelets aggregate and stimulate thrombin generation. Thus, bioincompatibility of RRT materials potentially enhances coagulation and inflammation pathways that are already triggered in the critically ill patients and that RRT is called to treat.
Continuous anticoagulation does increase bleeding risk. Conversely, clotting of the extracorporeal circuit also occurs frequently with CRRT, which might contribute to blood loss and could exacerbate anemia in critically ill patients. Interestingly, it has been shown that citrate anticoagulation abolishes polymorphonuclear and platelet degranulation in the filter in chronic hemodialysis. Furthermore, lower levels of oxidized low-density lipoprotein were found, indicating less lipid peroxidation [40]; citrate may thus have beneficial effects beyond the reduction of bleeding.
RRT has important metabolic consequences because it is associated with large nonselective solute shifts. In the normal kidney, tubular modification of the glomerular filtrate includes re-absorption of beneficial substances such as amino acids, water-soluble vitamins and trace elements. During RRT these substances are lost, thereby reducing antioxidant defense. Amino acids are lost through the filter and it has been estimated that they represent at most approximately 10% of overall amino acid supplementation [41]. A negative balance of water-soluble vitamins, glutamine, carnitine, selenium and copper has been shown [42]. Zinc is also lost, but total balance appeared to be positive, because the replacement solution contained zinc. These micronutrients are crucial for antioxidant defense [43]. Consequently, patients on RRT need a sufficient intake of protein and micronutrients to compensate for increased losses. Moreover, CRRT corrects metabolic acidosis by removing metabolic acids and replacing buffer. In addition to citrate, lactate and bicarbonate are the most frequently used buffers. If liver function and tissue perfusion are not severely disturbed, and CRRT dose is sufficient, lactate buffering is generally safe and adequate. However, the generation of buffer from lactate requires three molecules of oxygen for each molecule of bicarbonate. Furthermore, with the exclusive use of lactate-based fluids patients develop hyperlactatemia [44]. Even if it is generally postulated that hyperlactatemia is not harmful, it indicates that the infused amount of lactate (about 150–300 g a day depending on CRRT dose) overcomes metabolic capacity [45]. If exogenous lactate supply exceeds the capacity of the citric acid cycle, a higher proportion of lactate is used for gluconeogenesis, which is not energy-effective. In patients with multiple organ failure, lactate buffering (compared with bicarbonate buffering) increased glucose intolerance [45]. Furthermore, RRT may alter actual serum lactate level and impair the utilization of this molecule as a marker of metabolic acidosis or systemic hypoperfusion.
The majority of critically ill patients receive some form of antimicrobial treatment during their stay in the intensive care unit (ICU). Clinical data on the removal of antimicrobial drugs by CVVH are scarce and in most cases give general informations, whereas antibiotic removal rate is highly variable [46, 47]. Clearance of antibiotics is influenced by prescribed RRT dose and modality, protein binding, drug–membrane interaction, size and charge of molecules, characteristics and lifespan of the hemofilter. However, although interpatient variability and the potential need of monitoring serum levels of each molecule, it has been shown that for clinical practice dose adjustment according to predicted CVVH removal provides a reliable estimate compared to effective CVVH removal for many antibiotics (amoxicillin, ceftazidime, ciprofloxacin, flucloxacillin, fluconazole and metronidazole) [48]. Nevertheless, plasma levels should be strictly monitored when CVVH is performed for those antibiotics that are eliminated predominantly by the kidney, and that have a low therapeutic threshold (e.g. vancomycin). In this case, especially when high volume RRT is used, the risk of therapy underdosing exists [49].
During CRRT heat is lost as a result of blood leaving the body, cooling by the dialysate and/or the infusion of large amounts of replacement fluids. Although short-term adverse effects of cooling on gastric mucosal acidosis (pHi) were not seen [50], long-term cooling has huge metabolic consequences. Among others, loss of heat implies loss of energy, shivering, which increases oxygen demands, vasoconstriction, inhibition of leukocyte function and coagulation. In an experimental septic-shock hemofiltration model, the nonheating of replacement fluids caused early death [51] even if there are no current clinical trials in humans. The heater of several modern devices for CRRT is inadequate, especially if higher volumes are exchanged. Monitoring of body temperature, prevention of hypothermia and timely provision of external heat are recommended.
Also of concern are recent reports that technical problems with the delivery of CRRT, including machine malfunction, medication errors and compounding errors, might contribute to increased patient morbidity and mortality. Detection of safety problems and/or adverse events is particularly difficult when there are high rates of expected morbidity and mortality in the population undergoing a procedure, as is the case with CRRT in critically ill patients with AKI. Currently, few available studies provide substantive information on the safety or adverse effects of CRRT or IHD. The possibility of making fluid balance errors during CRRT was easier with the early technology and the advent of automated machines has partially overcome this problem. Furthermore, a new generation of dedicated CRRT machines has been recently released with strict safety features [52, 53]. Nevertheless, there are conditions and operation modes in which the potential for fluid balance errors is still present. For example, the simultaneous use of other extracorporeal therapies continuously tests CRRT machines safety and accuracy features. Dr. Shaheen and colleagues [54] recently presented their experience with two different subgroups of children: one group that required hemofiltration alone and the second group that required hemofiltration and extracorporeal membrane oxygenation (ECMO). Not surprisingly, the authors identified a higher mortality rate in those patients requiring CVVH and ECMO compared with those patients requiring hemofiltration alone. The authors promoted the concept that certain therapies should be reserved to experienced teams. Accuracy and safety features of modern CRRT machines allow effective and harmless net ultrafiltration delivery in high-risk pediatric patients, when coupled with constant clinical monitorization and deep knowledge of the extracorporeal circuits. We recently showed that net ultrafiltration error ranges between −1% and about 2% of prescribed rate [55]. We currently have no information on how “acceptable” is a machine-provoked error and net ultrafiltration unbalance cannot be reduced to zero, because “external” factors must be considered, such as uncorrect opening of dialysis solution bags, delay in bag substitution, troubleshooting. Finally, the CRRT delivery in parallel with ECMO circuit might have an important role in net ultrafiltration derangements [55].
Conclusions
Physiology and physiopathology of RRT are very important and often coexistent features during dialysis sessions. An in depth knowledge of both may help operators to approach the ideal treatment and help to tailor RRT to critically ill patients. It is possible that a further improvement in renal recovery and survival from AKI will depend from increased awareness of potential benefits and dangers that different RRT modalities can carry to different patients.
References
Lauer A, Alvis R, Avram M (1988) Hemodynamic consequences of continuous arteriovenous hemofìltration. Am J Kidney Dis 12:110–115
Bouffard Y, Viale JP, Annat G, Delafosse B, Guillaume C, Motin J (1987) Energy expenditure in the acute renal failure patient mechanically ventilated. Intensive Care Med 13:401–404
Frankenfield DC, Reynolds HN (1995) Nutritional effect of continuous hemodiafiltration. Nutrition 11:388–393
Kierdorf HP (1995) The nutritional management of acute renal failure in the intensive care unit. New Horiz 3:699–707
Bellomo R, Tan K, Bhonagiri S, Gopal I, Seacombe J, Daskalakis M, Boyce N (2002) High protein intake during continuous hemodialfiltration: impact on aminoacids and nitrogen balance. Int J Artif Organs 25:261–268
Gibney N, Cerda J, Davenport A, Ramirez J, Singbartl K, Leblanc M, Ronco C (2008) Volume management by renal replacement therapy in acute kidney injury. Int J Artif Organs 31:145–155
Goldstein SL, Currier H, Graf C, Cosio CC, Brewer ED, Sachdeva R (2001) Outcome in children receiving continuous veno-venous hemofiltration. Pediatrics 107:1309–1312
Foland JA, Fortenberry JD, Warshaw BL, Pettignano R, Merritt RK, Heard ML, Rogers K, Reid C, Tanner AJ, Easley KA (2004) Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med 32:1771–1776
Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL, The Sepsis Occurrence in Acutely Ill Patients (SOAP) Investigators (2008) A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care 12:R74
La Greca G, Dettori P, Biasioli S, Fabris A, Feriani M, Pinna V, Pisani E, Ronco C (1980) Brain density studies during dialysis. Lancet 13:580
Davenport A, Will EJ, Davison AM (1993) Effect of renal replacement therapy on patients with combined acute renal failure and fulminant hepatic failure. Kidney Int 43:S245–S251
Ricci Z, Ronco C (2008) Dose and efficiency of renal replacement therapy: continuous renal replacement therapy versus intermittent hemodialysis versus slow extended daily dialysis. Crit Care Med 36:S229–S237
Clark WR, Mueller BA, Alaka KJ, Macias VL (1994) A comparison of metabolic control by continuous and intermittent therapies in acute renal lailure. J Am Soc Nephrol 4:1113–1120
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL (2004) Surviving sepsis campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 32:858–873
Ronco C, Bellomo R, Homel P, Brendolan A, Dan M, Piccinni P, La Greca G (2000) Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet 356:26–30
Saudan P, Niederberger M, De Seigneux S, Romand J, Pugin J, Perneger T, Martin PY (2006) Adding a dialysis dose to continuous hemofiltration increases survival in patients with acute renal failure. Kidney Int 70:1312–1317
Naka T, Bellomo R (2004) Bench-to-bedside review: treating acid–base abnormalities in the intensive care unit—the role of renal replacement therapy. Crit Care 8:108–114
Uchino S, Bellomo R, Ronco C (2001) Intermittent versus continous renal replacement therapy in the ICU: impact on electrolyte and acid–base balance. Intensive Care Med 27:1037–1043
Phu NH, Hien TT, Mai NT, Chau TT, Chuong LV, Loc PP, Winearls C, Farrar J, White N, Day N (2002) Hemofitlration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med 347:895–902
Rocktaschel J, Morimatsu H, Uchino S, Ronco C, Bellomo R (2003) Impact of continuous veno-venous hemofiltration on acidbase balance. Int J Artif Organs 26:19–25
Mehta RL, McDonald B, Gabbai FB, Pahl M, Pascual MT, Farkas A, Kaplan RM, Collaborative Group for Treatment of ARF in the ICU (2001) A randomized clinical trial of continuous versus intermittent dialysis for acute renal failure. Kidney Int 60:1154–1163
Uehlinger DE, Jakob SM, Ferrari P, Eichelberger M, Huynh-Do U, Marti HP, Mohaupt MG, Vogt B, Rothen HU, Regli B, Takala J, Frey FJ (2005) Comparison of continuous and intermittent renal replacement therapy for acute renal failure. Nephrol Dial Transplant 20:1630–1637
Augustine JJ, Sandy D, Seifert TH, Paganini EP (2004) A randomized controlled trial comparing intermittent with continuous dialysis in patients with ARF. Am J Kidney Dis 44:1000–1007
Vinsonneau C, Camus C, Combes A, Costa de Beauregard MA, Klouche K, Boulain T, Pallot JL, Chiche JD, Taupin P, Landais P, Dhainaut JF, For the Hemodiafe Study Group (2006) Continuous venovenous haemodiafi ltration versus intermittent haemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: a multicentre randomised trial. Lancet 368:379–385
Cho KC, Himmelfarb J, Paganini E, Ikizler TA, Soroko SH, Mehta RL, Chertow GM (2006) Survival by dialysis modality in critically ill patients with acute kidney injury. J Am Soc Nephrol 17:3132–3138
Kellum J, Palevsky P (2006) Renal support in acute kidney injury. Lancet 368:344–345
Pannu N, Klarenbach S, Wiebe N, Manns B, Tonelli M, Alberta Kidney Disease Network (2008) Renal replacement therapy in patients with acute renal failure: a systematic review. JAMA 299:793–805
Bagshaw SM, Berthiaume LR, Delaney A, Bellomo R (2008) Continuous versus intermittent renal replacement therapy for critically ill patients with acute kidney injury: a meta-analysis. Crit Care Med 36:610–617
Rabindranath K, Adams J, Macleod AM, Muirhead N (2007) Intermittent versus continuous renal replacement therapy for acute renal failure in adults. Cochrane Database Syst Rev 18:CD003773
Kielstein JT, Kretschmer U, Ernst T, Hafer C, Bahr MJ, Haller H, Fliser D (2004) Efficacy and cardiovascular tolerability of extended dialysis in critically ill patients: a randomized controlled study. Am J Kidney Dis 43:342–349
Baldwin I, Naka T, Koch B, Fealy N, Bellomo R (2007) A pilot randomised controlled comparison of continuous veno-venous haemofiltration and extended daily dialysis with filtration: effect on small solutes and acid–base balance. Intensive Care Med 33:830–835
Schortgen F, Soubrier N, Delclaux C, Thuong M, Girou E, Brun-Buisson C, Lemaire F, Brochard L (2000) Hemodynamic tolerance of intermittent hemodialysis in critically ill patients: usefulness of practice guidelines. Am J Respir Crit Care Med 162:197–202
Himmelfarb J (2007) Continuous dialysis is not superior to intermittent dialysis in acute kidney injury of the critically ill patient. Nat Clin Pract Nephrol 3:120–121
Tolwani AJ, Campbell RC, Stofan BS, Lai KR, Oster RA, Wille KM (2008) Standard versus high-dose CVVHDF for ICU-related acute renal failure. J Am Soc Nephrol 19:1233–1238
The VA/NIH Acute Renal Failure Trial Network (2008) Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 359:7–20
Bouman CS, Oudemans-Van Straaten HM, Tijssen JG, Zandstra DF, Kesecioglu J (2002) Effects of early high-volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: a prospective, randomized trial. Crit Care Med 30:2205–2211
Bonventre JV (2008) Dialysis in acute kidney injury—more is not better. N Engl J Med 359:82–84
Oudemans van Straaten HM (2007) Primum non nocere, safety of continuous renal replacement therapy. Curr Opin Crit Care 13:635–637
Gorbet MB, Sefton MV (2004) Biomaterial-associated thrombosis: roles of coagulation factors, complement, platelets and leukocytes. Biomaterials 25:5681–5703
Gritters M, Grooteman MP, Schoorl M, Schoorl M, Bartels PC, Scheffer PG, Teerlink T, Schalkwijk CG, Spreeuwenberg M, Nubé MJ (2006) Citrate anticoagulation abolishes degranulation of polymorphonuclear cells and platelets and reduces oxidative stress during haemodialysis. Nephrol Dial Transplant 21:153–159
Wooley JA, Btaiche IF, Good KL (2005) Metabolic and nutritional aspects of acute renal failure in critically ill Patients Requiring Continuous Renal Replacement Therapy. Nutr in Clin Pract 20:176–191
Story DA, Ronco C, Bellomo R (1999) Trace element and vitamin concentrations and losses in critically ill patients treated with continuous venovenous hemofiltration. Crit Care Med 27:220–223
Heyland DK, Dhaliwal R, Suchner U, Berger MM (2005) Antioxidant nutrients: a systematic review of trace elements and vitamins in the critically ill patient. Intensive Care Med 31:327–337
Cole L, Bellomo R, Baldwin I, Hayhoe M, Ronco C (2003) The impact of lactate buffered high volume hemofiltraiton on acid base balance. Intensive Care Med 29:1113–1120
Bollmann MD, Revelly JP, Tappy L, Berger MM, Schaller MD, Cayeux MC, Martinez A, Chioléro RL (2004) Effect of bicarbonate and lactate buffer on glucose and lactate metabolism during hemodiafiltration in patients with multiple organ failure. Intensive Care Med 30:1103–1110
Vidal L, Shavit M, Fraser A, Paul M, Leibovici L (2005) Systematic comparison of four sources of drug information regarding adjustment of dose for renal function. BMJ 331:263
Trotman RL, Williamson JC, Shoemaker DM, Salzer WL (2005) Antibiotic dosing in critically ill adult patients receiving continuous renal replacement therapy. Clin Infect Dis 41:1159–1166
Bouman CS, van Kan HJ, Koopmans RP, Korevaar JC, Schultz MJ, Vroom MB (2006) Discrepancies between observed and predicted continuous veno venous hemofiltration removal of antimicrobial agents in critically ill patients and the effect of dosing. Intensive Care Med 32:2013–2019
Uchino S, Cole L, Morimatsu H, Goldsmith D, Bellomo R (2002) Clearance of vancomycin during high volume hemofiltration: impact of pre-dilution. Intensive Care Med 28:1664–1667
John S, Griesbach D, Baumgartel M, Weihprecht H, Schmieder RE, Geiger H (2001) Effects of continuous haemofiltration vs intermittent haemodialysis on systemic haemodynamics and splanchnic regional perfusion in septic shock patients: a prospective, randomized clinical trial. Nephrol Dial Transplant 16:320–327
Rogiers P, Sun Q, Dimopoulos G, Tu Z, Pauwels D, Manhaeghe C, Su F, Vincent JL (2006) Bloodwarming during hemofiltration can improve hemodynamics and outcome in ovine septic shock. Anesthesiology 104:1216–1222
Ricci Z, Bonello M, Salvatori G, Ratanarat R, Brendolan A, Dan M, Ronco C (2004) Continuous renal replacement technology: from adaptive technology and early dedicated machines towards flexible multipurpose machine platforms. Blood Purif 22:269–276
Ronco C, Ricci Z, Bellomo R, Baldwin I, Kellum J (2005) Management of fluid balance in CRRT: a technical approach. Int J Artif Organs 28:765–776
Shaheen IS, Harvey B, Watson AR, Pandya HC, Mayer A, Thomas D (2007) Continuous venovenous hemofiltration with or without extracorporeal membrane oxygenation in children. Pediatr Crit Care Med 8:362–365
Ricci Z, Morelli S, Vitale V, Di Chiara L, Cruz D, Picardo S (2007) Management of fluid balance in continuous renal replacement therapy: technical evaluation in the pediatric setting. Int J Artif Organs 30:896–901
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ronco, C., Ricci, Z. Renal replacement therapies: physiological review. Intensive Care Med 34, 2139–2146 (2008). https://doi.org/10.1007/s00134-008-1258-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-008-1258-6