Abstract
Objective
Recent experimental evidence suggests that matrix metalloproteinases (MMPs) are implicated in the pathophysiology of traumatic brain injury (TBI) by increasing blood-brain barrier permeability and exacerbating posttraumatic edema. We examined the acute profile of MMP-2 and MMP-9 in the plasma of patients with moderate or severe TBI and in the brain extracellular fluid (ECF).
Design
Prospective observational study.
Setting
Neurotraumatology intensive care unit of a tertiary university hospital.
Patients
Twenty patients with moderate or severe TBI were included and three groups were used as controls: 20 patients with a mild head injury and normal CT scan, 15 moderate polytrauma patients without TBI, and 20 healthy volunteers.
Interventions
Plasma samples were collected within the first 12 h and at 24 h post-injury. Simultaneous brain microdialysate and plasma samples were obtained in four moderate-severe TBI patients at additional timepoints: 48, 72, and 96 h post-TBI.
Measurements and main results
Gelatinases (MMP-2 and MMP-9) were measured by gelatin zymography. A significant increase in plasma gelatinases was observed at baseline when compared with healthy volunteers in the study group. This early increase was followed by a significant decrease at 24 h post-injury. Brain microdialysis samples presented a similar time profile as plasma samples for both gelatinases.
Conclusions
High levels of gelatinases were found in plasma and brain ECF in the early phase of TBI, indicating that both local and systemic trauma-induced upregulation of gelatinases in the acute phase might play an important role in the pathophysiology of TBI and could be a future therapeutic target.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) is the leading cause of death and disability worldwide among the population under the age of 45 years. Severe TBI is an extremely heterogeneous and dynamic process where mechanical injury triggers vascular, metabolic, cellular, and molecular processes known as secondary injuries [1]. Two of the less understood processes activated by TBI are the neuroinflammatory response and the increase in vascular permeability of the blood-brain barrier (BBB). Alteration in BBB permeability, and consequently brain edema, are an integral part of the secondary injury process, although the timing of this dysfunction and the exact mechanisms involved are still highly speculative.
Matrix metalloproteinases (MMPs), also called matrixins, are a family of more than 20 neutral proteases that are able to degrade or modify almost all extracellular matrix (ECM) components, including collagen, laminin, and proteoglycans [2]. The MMPs and their potential deleterious effects are tightly regulated at transcriptional and post-transcriptional levels through proform activation and by MMP tissue inhibitors (TIMPs) [3]. Two members of this family have a very specific and marked activity against gelatin and are termed gelatinases. One enzyme of 72 kDa has been called MMP-2 (gelatinase A), and the other, a 92-kDa protein, is MMP-9 (gelatinase B). Both gelatinases specifically degrade type-IV collagen, laminin, and fibronectin, which are the major components of the basal membrane of the cerebral blood vessels [4]. The MMPs are secreted as a proenzyme (proMMPs) or latent inactive form by endothelial cells, astrocytes, and neurons. These proforms could be activated by a variety of molecules and its activation plays an important role in several physiological processes, such as embryonic development, tissue resorption, and remodeling [2, 5].
Recent evidence shows that MMPs are overexpressed in several pathological states affecting the brain [6] and also are involved in the pathophysiology of severe TBI [7–12]. In vitro studies have demonstrated that the secretion of gelatinases is significantly increased in cortical cultures in which mechanical injury has been simulated [12]. There is also evidence showing that several inflammatory cytokines, such as interleukin-1β and tumor necrosis factor-α (TNF-α), induce MMP overexpression following brain injury, both in astrocytes and microglial cells [13]. Animal models have shown that levels of MMP-9 messenger RNA are increased following TBI, and that in the MMP-9 knockout mice there is a significant reduction of brain damage after induced TBI when compared with wild animals [14, 15]. It is currently accepted that the balance between MMP and TIMP activity determines the net degrading potential of the ECM. Preliminary clinical studies have shown increased MMP-9 in the blood of TBI patients [16], and Horstmann et al. have defined its temporal profile in severe stroke [6]; however, in human TBI, data about the presence of gelatinases in the brain extracellular fluid (ECF) and their temporal profile, both in plasma and ECF, are still lacking.
This work focuses primarily on studying the plasma levels of gelatinases (MMP-2 and MMP-9) in the acute phase of moderate or severe TBI, and on determining their temporal pattern in the early phase after injury. A secondary aim was to study the levels of both enzymes in the ECF of the injured brain in a selected group of patients monitored with microdialysis and a 100-kDa cut-off membrane. To clarify whether the systemic levels found are a consequence of either the release of gelatinases from the injured brain or a systemic brain-induced inflammatory response, or the result of both mechanisms combined, we used three different control groups. For establishing the baseline levels, healthy volunteers, mild TBI patients without significant polytrauma, and a normal CT scan and moderate polytrauma patients without TBI were used. To compare the temporal profile, a cohort of moderate polytrauma patients without an associated TBI and a normal CT scan were also used as controls.
Materials and methods
A prospective study was conducted in patients with moderate or severe TBI (Glasgow Coma Scale score ≤ 13), who required ICP monitoring and had an Injury Severity Score (ISS) of < 25 [17, 18]. Exclusion criteria included the presence of severe polytrauma (ISS ≥ 25), any inflammatory or malignant disease, an age of < 18 or over 65 years, patients with bilateral unreactive pupils, and patients admitted to hospital more than 12 h after injury. Traumatic brain lesions were classified according to the Marshall classification [19]. The general management of patients with severe or moderate TBI followed a protocol adapted from the guidelines for the management of severe TBI proposed by the Brain Trauma Foundation [20, 21]. Corticosteroids were not used in the treatment of these patients.
Twenty consecutive patients, 5 with a moderate TBI (GCS 9–13) and 15 with a severe TBI (GCS ≤ 8), were included in the study. Three groups were used as controls: mild polytrauma patients (ISS < 25) without associated TBI and a normal CT scan (15 patients); mild TBI patients with a GCS of 14 or 15 and a normal CT scan (20 patients); and 20 healthy volunteers.
The study received institutional approval. The Ethics Committee of Vall d'Hebron University Hospital waived the requirement for informed consent because blood samples were collected at the same time as routine extraction of blood samples were performed in the ICU, and because of the observational nature of the study. Informed consent was obtained from all volunteers and patients included in control groups.
Microdialysis technique
Brain microdialysis is used routinely in our Neurotraumatology Intensive Care Unit in most severe or moderate TBI patients who require ICP monitoring. The protocol used for implanting probes and the analytes routinely monitored at the bedside has been published elsewhere [22]. In four patients, three of them included in the study group (moderate or severe TBI patients), a high-cut-off (100 kDa) cerebral microdialysis catheter (CMA-71, CMA Microdialysis, Stockholm, Sweden) was inserted in the less damaged hemisphere in a structurally normal area identified by CT scan. The exact situation of the probe was confirmed by a control CT scan. For more detailed information the reader is referred to the electronic supplementary material (ESM).
Extraction and processing of blood samples and brain microdialysates
In the study group, plasma samples from peripheral arterial blood were collected in EDTA-coated plastic tubes at baseline (within the first 12 h from injury) and at 24 h from injury. The tubes were kept at room temperature and centrifuged immediately after extraction (3500 rpm for 15 min at 4°C) and the supernatants stored at −80°C until analysis. In patients monitored by brain microdialysis as well as plasma and microdialysate samples were obtained with the following temporal profile: ≤ 12, 24, 48, 72, and 96 h from injury.
In polytrauma control group, samples were collected using the same time points as in the study group. In mild TBI and healthy volunteers control groups, only a single plasma sample was collected for each patient at one time point per individual and within the first 12 h after injury in mild TBI patients. In all control groups, plasma was obtained from peripheral venous blood.
Zymographic measurement of gelatinase activity
In both plasma and microdialysate samples, MMP-2 and MMP-9 were measured by gelatin-substrate zymography as previously described [23]. In each gel we included a positive control both for MMP-2 and MMP-9 as an internal control that permits the comparison of results among all zymograms. The gelatinolytic zones were quantified using the Kodak 440 imaging system and analyzed using Kodak 1D image analysis software (Kodak, New Haven, CT, USA). The intensity of the bands (measured in arbitrary units) was normalized to that of recombinant human MMP-9 proform control to allow comparisons between gels. This assay is able to detect all gelatinase MMP isoforms. For more detailed information the reader is referred to the ESM.
Statistical analysis
All variables were introduced into a database created using Microsoft Excel 2000 (Microsoft, Redmond, WA, USA) and data was imported to the software package SPSS 12.0 for statistical analysis (SPSS, Chicago, IL, USA). Normal distribution was tested using the Kolmogorov–Smirnov test. For testing differences in means among groups the parametric one-way repeated-measures ANOVA was used with the Bonferroni post-hoc test. In not normally distributed data, a repeated measures ANOVA on ranks was used with the Dunn’s post-hoc test. A paired t-test was used to compare the differences between baseline time points and 24-h sample time points when data was normally distributed, whereas a Mann–Whitney rank-sum test was used in not normally distributed data. Statistical significance was established at p ≤ 0.05.
Results
Descriptive data from the TBI study group and the three control groups are shown in Table 1. Only values for each gelatinase (MMP-2 and MMP-9) proforms were statistically analyzed and reported in the text.
MMP-2 and MMP-9 baseline plasma levels
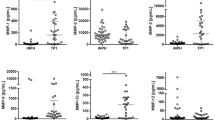
The median MMP-2 baseline plasma levels in the study group was 2.37 units (range 1.45–3.56), 1.26 for polytrauma group (range 0.62–3.2), 1.31 for mild TBI group (range 0.68–2.48), and 1.78 for healthy volunteers (range 0.73–2.7; Fig. 1). The median values of MMP-2 in the study group were significantly higher than median baseline levels in all control groups (p ≤ 0.05). No statistically significant differences were detected in the MMP-2 baseline levels among the three control groups.
Box-and-whisker plots for plasma MMP-2 proform levels at baseline and at 24 h post-injury in the TBI study group and in the polytrauma without TBI control group. Baseline plasma levels in the mild TBI and in the healthy-volunteers control groups are also shown. MMP-2 levels are expressed as ratios that were calculated by dividing the intensity of each band by the MMP-9 proform control in the same zymogram
The median for baseline MMP-9 levels in the study group was 1.68 units (range 0.96–3.54), 1.02 in healthy controls (range 0.58–1.48), 1.25 in patients with mild TBI (range 0.51–2.66), and 1.42 in polytrauma patients (range 0.62–4.24; Fig. 2). The median levels of MMP-9 in the study group were significantly higher than those in the mild TBI group and in healthy volunteers (p ≤ 0.05). Although baseline MMP-9 values in the study group were higher than in polytrauma group, these differences were not statistically significant. Moreover, a statistically significant increase in baseline MMP-9 levels was found in polytrauma patients when compared with healthy volunteers (one-way ANOVA, p = 0.017).
Box-and-whisker plots for plasma MMP-9 proform levels at baseline and at 24 h post-injury in the TBI study group and in the polytrauma without TBI control group. Baseline plasma levels in the mild TBI and in the healthy-volunteers control groups are also shown. MMP-9 levels are expressed as ratios that were calculated by dividing the intensity of each band by the MMP-9 proform control in the same zymogram
Temporal profile of plasma MMP-2 and MMP-9
MMP-2 plasma levels showed a mild but statistically significant decrease in the study group at 24 h after injury compared with baseline levels (p ≤ 0.05); however, the levels at 24 h after injury remained higher than the levels in all the control groups (Fig. 1). The differences found in the levels of MMP-2 at 24 h and the median of the baseline levels in polytrauma patients (1.61) were not statistically significant (p = 0.59).
MMP-9 plasma levels in the study group significantly decreased at 24 h after injury from a median of 1.68 to 1.41 (paired t-test, p < 0.001; Fig. 2); however, these levels still remained elevated at 24 h from injury when compared with healthy volunteers. In the polytrauma group, baseline levels also tended to decrease at 24 h (range 0.56–1.13), but this trend was mild and not statistically significant. Cleaved forms, when detected in plasma samples, showed a similar pattern and time profile to those of proforms for both gelatinases.
Brain extracellular levels of gelatinases
Table 1 summarizes clinical and demographic data of the four patients monitored with brain microdialysis. Microdialysis samples were matched with plasma samples.
Gelatinase levels in ECF samples presented a time profile similar to that of plasma samples both for MMP-2 and MMP-9, with higher values at the first evaluation and a spontaneous decrease over time, except for patient 2 (Figs. 3, 4). In this patient, brain ECF MMP-2 levels at 96 h where close to those of baseline. Moreover, an increase in plasma MMP-9 levels was observed at 48 h post-TBI. Due to the small sample size, no definite conclusions can be extracted from these data.
The CT scan 24 h after injury and the MMP-2 and MMP-9 time profiles in both brain microdialysates and plasma in patient 2. Values are shown superimposed on bands. Molecular weights are shown on the left side of the zymogram. Plasma and microdialysate extraction hours from injury are shown at the top of the zymograms. proMMP-9, MMP-9 proform form; proMMP-2, MMP-2 proform form; cleMMP-9, MMP-9 cleaved form
Discussion
Emerging evidence suggests that neuroinflammation, traditionally considered a secondary factor, actually plays a major role in the pathophysiology of TBI, and when upregulated it might be a secondary insult that occurs at the very early stages after injury. There is robust evidence that protease systems become dysregulated after stroke and TBI. In the introduction to their seminal work on MMPs, Parks and Meacham stated that “...aberrant regulation of MMP production is thought to be a primary mechanism contributing to disease progression and injury” [24]. Gelatinases target mainly the BBB, damaging its structural integrity, and altering its permeability, allowing leukocytes to migrate from the blood to the brain tissue and facilitating the formation of brain edema [25–27].
MMP-9 is increased in the plasma of acute stroke patients and its levels have shown a good correlation with neurological outcome [28, 29]; however, the role of gelatinases in TBI is still relatively unexplored. We observed a significant increase in plasma levels in both gelatinase proforms (MMP-2 and MMP-9) in the very acute phase of moderate and severe TBI. Gelatinase levels decreased during the first 24 h after injury, suggesting a burst of early systemic inflammatory response induced by the traumatic event. MMP-9 levels remained elevated at 24 h post-TBI when compared with healthy controls, although a clear trend towards normalization was observed. Moderate polytrauma also induced an early systemic overexpression of gelatinases, but not so marked as in moderate and severe TBI. A similar pattern was found in the MMP-2 proform, but our data also suggest that MMP-2 plays a more selective role than MMP-9 in the acute phase of TBI. MMP-9 levels decreased at 24 h from injury, both in polytrauma patients without brain injury and in TBI patients; however, MMP-2 levels remained elevated only in the study group. Whether or not this overexpression could follow a biphasic pattern, with later increases after the early decrease, is not known yet and will require further studies; however, in patients in whom the plasma levels were monitored for 96 h, this was not the general tendency (Figs. 3, 4).
The initial increase of both gelatinase levels and the spontaneous reduction found in our study is an intriguing phenomenon. Suehiro et al. observed a similar pattern and attributed it to the use of moderate hypothermia in their patients [16]. We did not induce hypothermia in any of our patients, but the observed temporal MMP decline was similar to that shown by Suehiro’s group [16].
The most relevant information provided by our study is that both gelatinases could be detected by high-cut-off microdialysis membranes in the brain ECF, showing that gelatinase release occurs not only systemically but also locally in the brain. The present study also shows, for the first time to our knowledge, an increased level of MMP-2 and MMP-9 in brain ECF following human TBI. The time profile of the brain ECF followed a similar pattern to that of plasma; thus, an increase of gelatinase levels in the early phase of TBI was found with a spontaneous decrease 24 h after injury. However, MMP-9 appeared to be more overexpressed than MMP-2. This information is important because in our study we targeted structurally normal brain tissue. Consequently, it can be expected that probes placed close to damaged brain (contusions, ischemic lesions, etc.) can have a higher overexpression of both gelatinases. This information cannot be neglected because it shows that even macroscopically normal areas of the brain have an inflammatory response to injury that can contribute to the pathophysiology of both focal and diffuse brain lesions.
We know that both intra- and extracellular edema coexist in different phases of TBI, but the nature of the alterations in the BBB permeability after injury and the timing of such a disorder is still a mystery. Several experimental studies have shown a close relationship between high MMP levels in brain parenchyma and increased BBB permeability in brain ischemia [30–34]. Furthermore, this association has been found recently in experimental TBI models. Shigemori et al. showed a strong association among MMP-9 levels, the BBB opening, and brain edema formation after cortical contusion in rats [35].
An additional question to answer is which cells produce gelatinases in the injured brain. Endothelial cells, microglial cells, astrocytes, and even neurons can secrete gelatinases after simulated mechanical injury in vitro [12]. Another potential source for MMPs are leukocytes entering the brain across the BBB [36]. In tissue samples obtained from patients who died from ischemic stroke, Rosell et al. found that MMP-9 was mainly located around blood vessels together with the presence of perivascular immunoreactive neutrophils [23].
From a therapeutic perspective, emerging preliminary results in experimental models have shown that pharmacological blockage of the extracellular regulated protein kinase can reduce MMP-9 levels and attenuate brain edema and tissue damage in mice [9]. Moreover, in a rat model of TBI it has been shown that hyperbaric oxygen reduces the expression of MMP-9 in the acute phase of TBI [37]. If these data were confirmed in humans, the opportunity for pharmacological blocking of this pathway involved in protease upregulation would exist.
Limitations of the study
There are two main limitations to our study: (a) its sample size, and the reduced number of samples obtained by high-resolution microdialysis; and (b) the pure observational nature of the study that prevented any correlation analysis from being conducted between the levels of plasma gelatinases and patient outcome. The goal of the study was not to predict outcome, and therefore it was underpowered in establishing this type of correlation. A further limitation is that our study was focused on understanding the early dysregulation of gelatinases after TBI. It is generally accepted that gelatinases also have an important role in repairing brain damage; in fact, upregulation late after injury can be a necessary response and has a beneficial effect on the natural healing process of the injured brain [38]. In addition, TIMPs were not evaluated. In future studies it would be necessary to study the balance between MMPs and their inhibitors to establish whether the increased levels of gelatinases depend on gelatinase production alone or are also influenced by the modulation of their activity by TIMPs.
Conclusion
In conclusion, high levels of MMP-2 and MMP-9 in plasma and in brain ECF were detected in the very early phase of TBI in the non-damaged brain. This suggests an early upregulation of both systemic and brain gelatinases after mechanical brain injury. Gelatinase overexpression seems to be an unspecific reaction to injury and might be implicated in the disruption of the BBB and in the production of brain swelling after human TBI. If our preliminary findings are confirmed in larger studies, these results may open a whole new avenue for TBI patients for whom modulation of both local and systemic trauma-induced early upregulation of gelatinases could be a future therapeutic target.
References
Sahuquillo J, Poca MA, Amoros S (2001) Current aspects of pathophysiology and cell dysfunction after severe head injury. Curr Pharm Des 7:1475–1503
Nagase H, Woessner JF (1999) Matrix metalloproteinases. J Biol Chem 274:21491–21494
Cunningham LA, Wetzel M, Rosenberg GA (2005) Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia 50:329–339
Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T (2003) Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem 253:269–285
Fatar M, Stroick M, Griebe M, Hennerici M (2005) Matrix metalloproteinases in cerebrovascular diseases. Cerebrovasc Dis 20:141–151
Horstmann S, Kalb P, Koziol J, Gardner H, Wagner S (2003) Profiles of matrix metalloproteinases, their inhibitors, and laminin in stroke patients: influence of different therapies. Stroke 34:2165–2170
Vilalta A, Rosell A, Poca MA, Sahuquillo J, Montaner J, de los Rios J, Riveiro M (2006) Increased levels of matrix metalloprotease-9 in plasma and brain extracellular fluid in the early phase of traumatic brain injury. J Neurotrauma 23:772–773 (Abstract)
Jaworski DM (2000) Differential regulation of tissue inhibitor of metalloproteinase mRNA expression in response to intracranial injury. Glia 30:199–208
Mori T, Wang X, Aoki T, Lo EH (2002) Downregulation of matrix metalloproteinase-9 and attenuation of edema via inhibition of ERK mitogen activated protein kinase in traumatic brain injury. J Neurotrauma 19:1411–1419
Morita-Fujimura Y, Fujimura M, Gasche Y, Copin JC, Chan PH (2000) Overexpression of copper and zinc superoxide dismutase in transgenic mice prevents the induction and activation of matrix metalloproteinases after cold injury-induced brain trauma. J Cereb Blood Flow Metab 20:130–138
Rosenberg GA (1995) Matrix metalloproteinases in brain injury. J Neurotrauma 12:833–842
Wang X, Mori T, Jung JC, Fini ME, Lo EH (2002) Secretion of matrix metalloproteinase-2 and -9 after mechanical trauma injury in rat cortical cultures and involvement of MAP kinase. J Neurotrauma 19:615–625
Vecil GG, Larsen PH, Corley SM, Herx LM, Besson A, Goodyer CG, Yong VW (2000) Interleukin-1 is a key regulator of matrix metalloproteinase-9 expression in human neurons in culture and following mouse brain trauma in vivo. J Neurosci Res 61:212–224
von Gertten C, Holmin S, Mathiesen T, Nordqvist AC (2003) Increases in matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-1 mRNA after cerebral contusion and depolarisation. J Neurosci Res 73:803–810
Wang X, Jung J, Asahi M, Chwang W, Russo L, Moskowitz MA, Dixon CE, Fini ME, Lo EH (2000) Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J Neurosci 2000 20:7037–7042
Suehiro E, Fujisawa H, Akimura T, Ishihara H, Kajiwara K, Kato S, Fujii M, Yamashita S, Maekawa T, Suzuki M (2004) Increased matrix metalloproteinase-9 in blood in association with activation of interleukin-6 after traumatic brain injury: influence of hypothermic therapy. J Neurotrauma 2004 21:1706–1711
Teasdale G, Jennett B (1974) Assessment of coma and impaired consciousness. A practical scale. Lancet 2:81–84
Baker SP, O'Neill B, Haddon W Jr, Long WB (1974) The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma 14:187–196
Marshall SB, Klauber MR, Van Berkum Clark M, Eisenberg HM, Jane J, Luerssen TG, Marmarou A, Foulkes MA (1991) A new classification of head injury based on computerized tomography. J Neurosurg 75:S14–S20
Sahuquillo J, Biestro A, Mena MP, Amoros S, Lung M, Poca MA, De Nadal M, Baguena M, Panzardo H, Mira JM, Garnacho A, Lobato RD (2002) First tier therapeutic measures in the management of high intracranial pressure after severe head injury. Rationale and proposal for a protocol. Neurocirugia 13:78–100
Bullock RM, Chesnut RM, Clifton GL, Ghajar J, Marion DW, Narayan RK, Newell DW, Pitts LH, Rosner MJ, Walters BC, Wilberger JE, Chesnut RM, Maas AIR, Servadei F, Teasdale G, Untenberg A, von Holst H, Walters BC, Contant C, Florin R, Kelly JP, Marmarou A, Queen PC, Rosenberg J, Valadka AB, Dearden M, Miller JD, Stocchetti N (2000) Management and prognosis of severe traumatic brain injury. Intracranial pressure treatment threshold. J Neurotrauma 17:493–495
Poca MA, Sahuquillo J, Vilalta A, de los Rios J, Robles A, Exposito L (2006) Percutaneous implantation of cerebral microdialysis catheters by twist-drill craniostomy in neurocritical patients: description of the technique and results of a feasibility study in 97 patients. J Neurotrauma 23:1510–1517
Rosell A, Ortega-Aznar A, Alvarez-Sabin J, Fernandez-Cadenas I, Ribo M, Molina CA, Lo EH, Montaner J (2006) Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke 2006 37:1399–1406
Parks WC, Mecham RP (1998) Matrix metalloproteinases. Academic Press, San Diego
Morganti-Kossmann MC, Rancan M, Stahel PF, Kossmann T (2002) Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr Opin Crit Care 8:101–105
Schmidt OI, Heyde CE, Ertel W, Stahel PF (2005) Closed head injury: An inflammatory disease? Brain Res Rev 48:388–399
Stamatovic SM, Dimitrijevic OB, Keep RF, Andjelkovic AV (2006) Inflammation and brain edema: new insights into the role of chemokines and their receptors. Acta Neurochir Suppl 96:444–450
Montaner J, Molina CA, Monasterio J, Abilleira S, Arenillas JF, Ribo M, Quintana M, Alvarez-Sabin J (2003) Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation 107:598–603
Ning M, Furie KL, Koroshetz WJ, Lee H, Barron M, Lederer M, Wang X, Zhu M, Sorensen AG, Lo EH, Kelly PJ (2006) Association between tPA therapy and raised early matrix metalloproteinase-9 in acute stroke. Neurology 66:1550–1555
Gasche Y, Soccal PM, Kanemitsu M, Copin JC (2006) Matrix metalloproteinases and diseases of the central nervous system with a special emphasis on ischemic brain. Front Biosci 11:1289–1301
Mun-Bryce S, Rosenberg GA (1998) Gelatinase B modulates selective opening of the blood-brain barrier during inflammation. Am J Physiol 274:R1203–R1211
Pfefferkorn T, Rosenberg GA (2003) Closure of the blood-brain barrier by matrix metalloproteinase inhibition reduces rtPA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke 34:2025–2030
Rosenberg GA, Estrada EY, Dencoff JE (1998) Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke 29:2189–2195
Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA (2007) Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab 27:697–709
Shigemori Y, Katayama Y, Mori T, Maeda T, Kawamata T (2006) Matrix metalloproteinase-9 is associated with blood-brain barrier opening and brain edema formation after cortical contusion in rats. Acta Neurochir Suppl 96:130–133
Sellebjerg F, Sorensen TL (2003) Chemokines and matrix metalloproteinase-9 in leukocyte recruitment to the central nervous system. Brain Res Bull 61:347–355
Vlodavsky E, Palzur E, Soustiel JF (2006) Hyperbaric oxygen therapy reduces neuroinflammation and expression of matrix metalloproteinase-9 in the rat model of traumatic brain injury. Neuropathol Appl Neurobiol 32:40–50
Agrawal SM, Lau L, Yong VW (2008) MMPs in the central nervous system: where the good guys go bad. Semin Cell Dev Biol 19:42–51
Acknowledgements
This study was supported by Fondo de Investigaciones Sanitarias de la Seguridad Social (FIS) grant number 05/1092, by the Commission of the European Communities, specific RTD programme ‘Quality of Life and Management of Living Resources’ project QLK6-CT-2002-02583, and by Fundación Mapfre Medicina grant 2004-2005. A Vilalta is the recipient of a pre-doctoral grant from the Institut Fundació de Recerca, Vall d’Hebron University Hospital, Universitat Autònoma de Barcelona, Barcelona, Spain. We are grateful to M. Quintana for statistical advice and to S. Voss for her assistance in translating the manuscript into English.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was presented, in part, at the 8th International Neurotrauma Symposium, Rotterdam, The Netherlands, 21–25 May 2006.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Vilalta, A., Sahuquillo, J., Rosell, A. et al. Moderate and severe traumatic brain injury induce early overexpression of systemic and brain gelatinases. Intensive Care Med 34, 1384–1392 (2008). https://doi.org/10.1007/s00134-008-1056-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-008-1056-1