Abstract
Objective
To evaluate the usefulness of early transcranial Doppler ultrasound (TCD) goal-directed therapy after severe traumatic brain injury initiated before invasive cerebral monitoring is available.
Design
Prospective, observational clinical study.
Setting
Surgical intensive care unit, university hospital.
Patients and participants
Twenty-four severely brain-injured patients.
Interventions
All patients had TCD measurements immediately on admission (T0) and when invasive cerebral monitoring was available (T1). TCD was considered abnormal when two out of three measured values were outside the following limits: Vm < 30 cm/s, Vd < 20 cm/s, PI > 1.4. When admission TCD was abnormal, attending physicians modified treatment to increase cerebral perfusion pressure.
Measurements and results
Admission TCD was performed 18 ± 11 min (T0) after admission, whereas cerebral inasive monitoring was available 242 ± 116 min (T1) after admission. At T0, 11 (46%) patients had abnormal TCD values (group 1) and 13 had normal TCD values (group 2); mean arterial pressure was comparable between groups. All group 1 patients received mannitol and/or norepinephrine. At T1, mean arterial pressure was increased compared to admission in group 1 (105 ± 17 mmHg vs. 89 ± 15 mmHg, p < 0.05) and only two patients had still an abnormal TCD. Although group 1 patients had higher intracranial pressure than those of group 2 (32 ± 13 mmHg vs. 22 ± 10 mmHg, p < 0.01), both cerebral perfusion pressure and jugular venous oxygen saturation were comparable between the groups.
Conclusions
The use of TCD at hospital admission allows identification of severely brain-injured patients with brain hypoperfusion. In such high-risk patients, early TCD goal-directed therapy can restore normal cerebral perfusion and might then potentially help in reducing the extent of secondary brain injury.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Secondary ischemic brain injuries have been extensively shown to be the major prognosis factors after severe traumatic brain injury (TBI) [1], notably during the early post-traumatic period [2, 3, 4]. This period is at particular high risk because of the vulnerability of the traumatized brain to ischemic injuries [5] and the high frequency of arterial hypotension and hypoxemia [2, 3, 4]. Early estimations of cerebral perfusion showed low cerebral blood flow [6] and evidence of brain ischemia [7, 8], which were related to long-term poor outcome.
However, at the scene of the accident and in the hospital before invasive cerebral monitoring is available, cerebral perfusion is not estimated and these episodes of cerebral hypoperfusion are not detected.
International guidelines recommend maintenance of the systolic arterial pressure above 90 mmHg [9, 10]. Fluid loading, but also vasopressors, are frequently required and empirically used to achieve this goal. In a previous study at our institution, we showed that cerebral monitoring was not implemented until a mean 7 h after trauma [11]. At this time, 37% of the patients had low jugular venous oxygen saturation (SjvO2) values in spite of an average mean arterial pressure (MAP) of 80 mmHg. Thus, an unacceptable long delay before diagnosis and treatment of secondary ischemic injuries occurred in one third of patients.
A higher arterial pressure target would be inaccurate and possibly deleterious for the two thirds of severe TBI patients with no impaired cerebral perfusion. Associated bleeding injuries can be worsened by an increase in arterial pressure [12]. Moreover, an important and inappropriate increase in cerebral perfusion pressure (CPP) may induce or increase cerebral vasogenic oedema [13, 14]. If maintaining CPP above 60 mmHg is clearly recommended, many authors also highlight the risks of excessively high CPP values [15, 16].
Transcranial Doppler ultrasound (TCD) is a non-invasive method for the measurement of middle cerebral artery (MCA) blood flow velocity commonly used in standard care of TBI patients. TCD is particularly accurate in detection of episodes of hypoperfusion induced by low CPP [17]. However, there are few reports on the use of TCD in the early post-traumatic period. McQuire et al. reported an 80% rate of abnormal TCD measurements in the very early post-traumatic period and suggested the use of emergency TCD to select a group of patients for higher arterial pressure targets and to help decide the sequence of radiological studies and surgery [18].
In our institution, we routinely use admission TCD for all trauma patients but also TCD goal-directed therapy in the case of abnormal TCD measurements. Our hypothesis was that admission TCD helps to rapidly identify severe TBI patients with impaired cerebral perfusion and guides the initial phase of brain resuscitation to attenuate the deleterious consequences of secondary brain injury. The aim of this study was to assess the efficacy of this strategy in a preliminary prospective observational study.
Patients and methods
This preliminary, prospective, observational study was conducted in the intensive care unit of Bicêtre Hospital between August 2002 and November 2002. All adult patients with severe TBI, defined by a best Glasgow Coma Scale (GCS) score ≤ 8 before arrival at hospital, were included. Patients with bilateral unreactive mydriasis and/or cardiac arrest were not included. Because no change to our current clinical practice or randomization was performed, the institutional review board waived informed consent.
All severe TBI patients were intubated and ventilated in the field by the pre-hospital medical team. On arrival at hospital, all trauma patients were directly admitted to our surgical intensive care unit. All patients included in this study underwent advanced trauma life resuscitation according to our protocol for severe TBI patients, with or without multiple injuries. All patients received intravenous sedation with midazolam and fentanyl. Mechanical ventilation was adjusted to maintain normocapnia (PaCO2 35–40 mmHg) and arterial oxygen saturation (SaO2) > 95%. Femoral artery catheterization allowed continuous monitoring of arterial pressure and blood sampling. Normal saline and/or colloids and/or blood derivatives were used as needed to maintain the intravascular volume based on heart rate (HR < 120/min), arterial pressure, and estimated blood losses (with a target haemoglobin concentration > 10 g/dl). Norepinephrine infusion was used early to maintain MAP above 75 mmHg.
TCD was performed according to the method described by Aaslid et al. [19]. Right and left MCA were insonated through temporal windows. The depth of insonation giving the highest mean velocity was chosen for recording. Peak systolic (Vs), end-diastolic (Vd) and time-averaged mean (Vm) velocities were measured and the pulsatility index (PI) was calculated as: PI = (Vs – Vd)/Vm. Previous literature showed that early decreased Vm (below 30–35 cm/s) was correlated with poor long-term outcome but also with early death [20, 21]. When CPP decreases, Vd decreases precociously with a Vd/Vm ratio rapidly under approximately 0.6 [22], and the strongest correlation is observed between PI and CPP [17, 23]. Based on these data and our personal experience, TCD was considered abnormal in the present study when two of the three measured values were abnormal using the following thresholds: Vm < 30 cm/s, Vd < 20 cm/s, PI > 1.4. The worst values were considered if the measurements were asymmetric.
The first TCD measurements were performed as soon as possible upon admission (admission TCD). Patients were classified in group 1 if admission TCD was abnormal, in group 2 if admission TCD was normal. In group 1, attending physicians tried to increase cerebral perfusion using vascular loading and/or blood transfusion and/or norepinephrine to increase MAP and/or 20% mannitol (0.7 g/kg) to decrease cerebral oedema. A second TCD was performed at the time invasive cerebral monitoring became available. The same intensivist was responsible over the whole admission period and performed both TCD measurements (T0 and T1).
Invasive cerebral monitoring was performed as soon as possible after hemodynamic stabilization, completion of diagnostic procedures and emergency surgery if needed, and when adequate coagulation parameters were obtained [11]. A 7-Fr catheter (Vigon®, Rouen, France) was placed in the internal jugular vein at the bulb level to monitor SjvO2. The intracranial pressure (ICP) device was an intraparenchymal electric transducer-tipped catheter (Codman®, Mass., USA). CPP was calculated as the difference between MAP and ICP.
Measurements were performed at T0 = admission TCD and at T1 = availability of first ICP and SjvO2 values. The time delays between trauma and arrival at hospital, T0 and T1 were recorded. The Marshall classification [24] was used for cerebral CT scans. Glasgow Outcome Score (GOS) [25] was measured 3 months after trauma.
Data are presented as mean ± SD or median [range]. Continuous variables have been compared between T0 and T1 using a Student paired t-test, and between group 1 and group 2 using a Student t-test. Non-parametric variables were compared using a Mann–Whitney U-test. A p-value < 0.05 was considered statistically significant.
Results
All patients
Twenty-four patients with severe TBI were included. Demographic data are summarized in Table 1.
The time between trauma and admission to hospital was 169 ± 110 min [45–450]. Four patients were secondarily referred, three in group 1 and one in group 2. For these four patients the delay between trauma and admission was 390 ± 74 min, compared with 125 ± 45 min for the patients primarily referred.
The first TCD measurements were performed 18 ± 11 min after hospital admission (T0), whereas the first ICP and SjvO2 measurements were available 242 ± 116 min after hospital admission (T1).
Pre-treatment group 1 versus group 2 (T0)
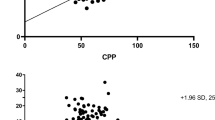
At T0, 11 patients (46%) had abnormal TCD values (group 1) and 13 (54%) had normal TCD values (group 2) using our criteria. Accordingly, blood flow velocities were significantly lower and PI significantly higher in group 1 than in group 2 (Table 2). In group 1, all patients had abnormal Vd values, 10 had abnormal PI values and 4 had abnormal Vm values. In group 2, all patients had normal Vm, Vd and PI values except two with high PI values (Fig. 1).
Individual values of Vm, Vd and PI at admission to hospital (T0) and at availability of invasive cerebral monitoring (T1) in patients with abnormal TCD (filled squares) and in those with normal TCD (open squares) at admission. Dashed lines indicate normal predefined values for each TCD parameter measured
Age, Injury Severity Score (ISS), Marshall CT scan classification, time from trauma to hospital admission and time from admission to T0 were not statistically significantly different between the two groups. GCS was significantly lower in group 1 than in group 2 (5 [3–7] vs. 7 [3–8], p < 0.01). Pre-hospital treatments included norepinephrine infusion for three patients (one in group 1, two in group 2) and mannitol bolus for one patient (group 2) (Tables 1 and 2).
At T0, MAP, temperature, haemoglobin, PaO2 and SaO2 were not significantly different between the two groups. PaCO2 was significantly lower (40 ± 5 vs. 45 ± 6 mmHg, p < 0.05) and pH significantly higher (7.39 ± 0.04 vs. 7.32 ± 0.06, p < 0.05) in group 1 than in group 2 (Table 2).
Treatment group 1 (abnormal admission TCD)
Between T0 and T1, all patients in group 1 received a treatment to improve cerebral perfusion: nine, norepinephrine infusion, five, a mannitol bolus and three, an emergency neurosurgical procedure.
MAP significantly increased from 89 ± 15 mmHg to 105 ± 17 mmHg (p < 0.05), blood flow velocities significantly increased, and PI significantly decreased from T0 to T1 (Table 2). At the same time, temperature, haemoglobin, pH, PaO2, PaCO2 and SaO2 did not vary significantly.
At T1, all patients but two had normalized TCD measurements.
Treatment group 2 (normal admission TCD)
In group 2, no patient received mannitol or underwent a neurosurgical procedure before T1. Four patientsreceived a norepinephrine infusion. Between T0 and T1, MAP, TCD measurements, PaO2, SaO2, haemoglobin and temperature did not vary significantly. PaCO2 decreased significantly (45 ± 6 vs. 41 ± 6 mmHg, p < 0.05) and pH increased significantly (7.32 ± 0.06 vs. 7.36 ± 0.07, p < 0.05) between T0 and T1.
Post-treatment group 1 versus group 2 (T1)
Time from admission to T1 was not statistically different between the groups. At T1, there was still a statistically significant difference for Vd and PI between groups, with a lower Vd and a higher PI in group 1 than in group 2.
ICP was significantly higher in group 1 than in group 2 (32 ± 13 vs. 22 ± 10 mmHg, p < 0.01) but CPP was comparable between the groups. All patients but one in each group had the first measured CPP value above 60 mmHg. SjvO2 measurements were not significantly different between groups, and all patients but one in each group had a SjvO2 value above 55% (Table 2).
Outcome
Three patients (13%) died and four more patients (17%) had an unfavourable outcome (GOS 3–4). Seventeen patients (70%) had a moderate to good outcome (GOS 1–2).
Three-month GOS was not correlated to admission GCS or ISS, but was significantly poorer in group 1 than in group 2 (3 [1–5] vs. 1 [1–2], p < 0.006) (Fig. 2).
Discussion
Using our criteria, 11 (46%) of 24 patients with severe TBI had an abnormal admission TCD and received specific treatment to increase cerebral blood flow. After this treatment TCD measurements were normalized for all but two patients. When invasive cerebral monitoring was available, ICP was higher in patients with abnormal admission TCD than in patients with normal admission TCD. However CPP and SjvO2 were comparable between the groups, suggesting that TCD goal-directed therapy actually targeted patients with the most severely compromised cerebral perfusion and reduced the duration of secondary ischemic injuries.
Only a few data were available on admission TCD in severe TBI patients when this study was undertaken. In a series of 121 patients with minor to severe TBI, admission velocities were related to GCS, and admission Vm < 28 cm/s correctly predicted 80% of early deaths [20]. Van Santbrink et al. reported a good correlation between Vm and brain tissue pO2, with the lowest velocities observed in the first 8 h after trauma and associated with severity of injury and outcome [21].
The most reliable indicators of low cerebral perfusion are probably low Vd with high PI. In our study, Vm was abnormal in only 4 of the 11 patients in group 1 and Vd and PI were concordant (both normal or both abnormal) in nearly all patients (21/24 at T0 and 22/24 at T1). Within autoregulation range, TCD velocities are poorly correlated with cerebral blood flow [23, 26]. Below autoregulation range, however, experimental [27] and clinical [22, 23] studies showed that Vd decreases with CPP more rapidly than Vm and Vs, with the strongest correlation observed between CPP and PI [23]. In brain-dead patients, the first step in cerebral circulatory arrest is a decrease in Vd toward a zero value without significant variation of Vs [28]. In addition, TCD recorded velocities vary with the real blood velocity according to the cosine of the angle of insonation, but PI is dimensionless and therefore independent of sampling techniques [17]. An increase in PI confirms that low velocities are related to an increase in pulse amplitude, not to a high insonation angle. Thus, the use of a low threshold for Vd associated with an increased PI is highly predictive of compromised cerebral perfusion.
Recent studies confirmed the value of early PI and Vd measurements after severe TBI. Trabold et al. reported that admission Vd < 25 cm/s and PI > 1.3 were associated with a poor outcome [29]. Voulgaris et al. found a strong correlation between PI and ICP for ICP values > 20 mmHg, and between PI and CPP for CPP values < 70 mmHg [30]. Finally, in a series of 78 patients with mild to moderate TBI, Jaffres et al. observed a significantly higher admission PI in the subgroup of patients suffering a secondary neurological deterioration [31].
Patients with abnormal TCD measurements at admission (group 1) using our definition comprised patients with a more severe TBI than patients with normal TCD values (group 2). Group 1 patients had an ICP 10 mmHg higher than group 2 patients despite the previous use of mannitol in five of them. Group 1 patients also had a significantly lower initial GCS, and six patients in group 1 compared to only one patient in group 2 had an unfavourable outcome. Thus, admission TCD, using our thresholds, identifies patients with an impaired cerebral perfusion associated with a poorer outcome.
TCD goal-directed therapy permitted improvement of cerebral perfusion in patients presenting abnormal admission TCD (group 1). After treatment, all group 1 patients but two had normalized TCD measurements. Moreover, all group 1 patients but one had appropriate CPP or SjvO2 values despite an ICP 10 mmHg higher than in group 2. Because these patients received norepinephrine (with a significant effect on MAP) and/or mannitol, it can be assumed that without these treatments CPP and SjvO2 values would have been much lower.
We previously reported a group of 27 severe TBI patients managed without admission TCD [11]. The time before invasive monitoring was approximately the same as in the present study. Seven hours after trauma, 37% of these patients had SjvO2 values below 55%, and 63% had CPP values below 60 mmHg. In the present study, when invasive cerebral monitoring was available only 2/24 patients (8.3%) had low SjvO2 values and 2/24 (8.3%) had low CPP, suggesting a major improvement in our management due to the early use of TCD. Regarding the time between admission and availability of invasive cerebral monitoring in our hospital, the use of TCD at admission permitted the diagnosis and guided treatment of impaired cerebral perfusion 3 h before invasive cerebral monitoring became available.
This study had several limitations. Firstly, at admission, mean PaCO2 was significantly higher in group 2 than in group 1. This difference could partly explain the higher velocities in group 2 than in group 1. However, at T1, mean PaCO2 was normalized in group 2 without significant variation of TCD measurements. Secondly, the thresholds used to define abnormal TCD in this study were based on few data from the literature and mainly on our own clinical experience. Treatment TCD threshold values must be defined by larger-scale studies. Thirdly, compared with our previous study [11], we observed an important improvement in first measured invasive cerebral parameters with the use of admission TCD. However, a randomized study with a non-intervention group would be necessary to definitely prove the usefulness of TCD goal-directed therapy but also to evaluate its impact on outcome.
Conclusion
Cerebral perfusion should be estimated as soon as possible after severe TBI to limit the burden of secondary ischemic brain injury. TCD is a simple and non-invasive method for cerebral perfusion evaluation and is particularly accurate to detect hypoperfusion. Our study confirms that early TCD permits to identify high-risk patients with impaired cerebral perfusion and poor outcome. Moreover, our study suggests that TCD goal-directed therapy improves cerebral perfusion before availability of invasive cerebral monitoring and reduces the duration of secondary brain injuries. Further studies are needed to define optimal treatment TCD threshold values for the initial management of TBI, and its effect on outcome.
References
Jones PA, Andrews PJD, Midgley S, Anderson SI, Piper IR, Tocher JL, Housley AM, Corrie JA, Slattery J, Dearden NM, Miller JD (1994) Measuring the burden of secondary insults in head-injured patients during intensive care. J Neurosurg Anesthesiol 6:4–14
Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, Jane JA, Marmarou A, Foulkes MA (1993) The role of secondary brain injury in determining outcome from severe head injury. J Trauma 34:216–222
Stocchetti N, Furlan A, Volta F (1996) Hypoxemia and arterial hypotension at the accident scene in head injury. J Trauma 40:764–767
Rouxel JP, Tazarourte K, Le Moigno S, Ract C, Vigue B (2004) [Medical prehospital rescue in head injury]. Ann Fr Anesth Reanim 23:6–14
DeWitt DS, Jenkins LW, Prough DS (1995) Enhanced vulnerability to secondary ischemic insults after experimental traumatic brain injury. New Horiz 3:376–383
Bouma GJ, Muizelaar JP, Stringer WA, Choi SC, Fatouros P, Young HF (1992) Ultra-early evaluation of regional cerebral blood flow in severely head-injured patients using xenon-enhanced computerized tomography. J Neurosurg 77:360–368
Gopinath SP, Robertson CS, Contant CF, Hayes C, Feldman Z, Narayan RK, Grossman RG (1994) Jugular venous desaturation and outcome after head injury. J Neurol Neurosurg Psychiatry 57:717–723
Coles JP, Fryer TD, Smielewski P, Chatfield DA, Steiner LA, Johnston AJ, Downey SP, Williams GB, Aigbirhio F, Hutchinson PJ, Rice K, Carpenter TA, Clark JC, Pickard JD, Menon DK (2004) Incidence and mechanisms of cerebral ischemia in early clinical head injury. J Cereb Blood Flow Metab 24:202–211
[No authors listed] (1999) [What are the treatment modalities of severe head injuries in the prehospital phase?]. Ann Fr Anesth Reanim 18:36–46
Gabriel EJ, Ghajar J, Jagoda A, Pons PT, Scalea T, Walters BC (2002) Guidelines for prehospital management of traumatic brain injury. J Neurotrauma 19:111–174
Vigué B, Ract C, Benayed B, Zlotine N, Leblanc PE, Samii K, Bissonnette B (1999) Early SjvO2 monitoring in patients with severe brain trauma. Intensive Care Med 25:445–451
Revell M, Porter K, Greaves I (2002) Fluid resuscitation in prehospital trauma care: a consensus view. Emerg Med J 19:494–498
Tuor UI, Edvinsson L, McCulloch J (1986) Catecholamines and the relationship between cerebral blood flow and glucose use. Am J Physiol 251:H824–H833
Kroppenstedt SN, Kern M, Thomale UW, Schneider GH, Lanksch WR, Unterberg AW (1999) Effect of cerebral perfusion pressure on contusion volume following impact injury. J Neurosurg 90:520–526
Hlatky R, Furuya Y, Valadka AB, Robertson CS (2001) Management of cerebral perfusion pressure. Semin Respir Crit Care Med 22:3–12
Steiner LA, Czosnyka M, Piechnik SK, Smielewski P, Chatfield D, Menon DK, Pickard JD (2002) Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med 30:733–738
Kirkpatrick PJ, Chan KH (1997) Transcranial Doppler. In: Reilly P, Bullock MR (eds) Head injury. Chapman & Hall, London, pp 243–259
McQuire JC, Sutcliffe JC, Coats TJ (1998) Early changes in middle cerebral artery blood flow velocity after head injury. J Neurosurg 89:526–532
Aaslid R, Markwalder TM, Nornes H (1982) Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg 57:769–774
Chan KH, Miller JD, Dearden NM (1992) Intracranial blood flow velocity after head injury: relationship to severity of injury, time, neurological status and outcome. J Neurol Neurosurg Psychiatry 55:787–791
van Santbrink H, Schouten JW, Steyerberg EW, Avezaat CJ, Maas AI (2002) Serial transcranial Doppler measurements in traumatic brain injury with special focus on the early posttraumatic period. Acta Neurochir (Wien) 144:1141–1149
Czosnyka M, Matta BF, Smielewski P, Kirkpatrick PJ, Pickard JD (1998) Cerebral perfusion pressure in head-injured patients: a noninvasive assessment using transcranial Doppler ultrasonography. J Neurosurg 88:802–808
Chan KH, Miller JD, Dearden NM, Andrews PJ, Midgley S (1992) The effect of changes in cerebral perfusion pressure upon middle cerebral artery blood flow velocity and jugular bulb venous oxygen saturation after severe brain injury. J Neurosurg 77:55–61
Marshall LF, Marshall SB, Klauber MR, van Berkum Clark M (1991) A new classification of head injury based on computerized tomography. J Neurosurg 75(Suppl):S14–S20
Jennett B (1997) Outcome after severe head injury. In: Reilly P, Bullock MR (eds) Head injury. Chapman & Hall, London, pp 439–461
Bishop CC, Powell S, Rutt D, Browse NL (1986) Transcranial Doppler measurement of middle cerebral artery blood flow velocity: a validation study. Stroke 17:913–915
Czosnyka M, Richards H, Kirkpatrick P, Pickard J (1994) Assessment of cerebral autoregulation with ultrasound and laser doppler wave forms—an experimental study in anesthetized rabbits. Neurosurgery 35:287–293
Ducrocq X, Hassler W, Moritake K, Newell DW, von Reutern GM, Shiogai T, Smith RR (1998) Consensus opinion on diagnosis of cerebral circulatory arrest using Doppler sonography: Task Force Group on cerebral death of the Neurosonology Research Group of the World Federation of Neurology. J Neurol Sci 159:145–150
Trabold F, Meyer PG, Blanot S, Carli PA, Orliaguet GA (2004) The prognostic value of transcranial Doppler studies in children with moderate and severe head injury. Intensive Care Med 30:108–112
Voulgaris SG, Partheni M, Kaliora H, Haftouras N, Pessach IS, Polyzoidis KS (2005) Early cerebral monitoring using the transcranial Doppler pulsatility index in patients with severe brain trauma. Med Sci Monit 11:CR49–52
Jaffres P, Brun J, Declety P, Bosson JL, Fauvage B, Schleiermacher A, Kaddour A, Anglade D, Jacquot C, Payen JF (2005) Transcranial Doppler to detect on admission patients at risk for neurological deterioration following mild and moderate brain trauma. Intensive Care Med 31:785–790
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ract, C., Le Moigno, S., Bruder, N. et al. Transcranial Doppler ultrasound goal-directed therapy for the early management of severe traumatic brain injury. Intensive Care Med 33, 645–651 (2007). https://doi.org/10.1007/s00134-007-0558-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-007-0558-6