Abstract
Objective
To evaluate the feasibility of implementing a program of controlled non-heart beating organ donation, in patients undergoing the withdrawal of intensive care treatment.
Design and setting
Prospective observational study. Medical and Surgical ICUs in a tertiary university hospital.
Patients
Consecutive patients younger than 70 years dying in the ICU after treatment withdrawal for dire neurological prognosis.
Measurements and results
We analyzed prospectively collected data from the ICU clinical information system. Seventy-three of 516 ICU deaths (13%) were identified, equally distributed among traumatic, stroke, and anoxic brain injury. The management and the course in these three diagnostic categories were similar. All patients underwent withdrawal of mechanical ventilation and half were extubated. Median time to death was of 4.8 h (IQR 1.4–11.5). In 70% of cases the patient received analgesia and 30% sedation. Such treatment was not related to earlier death. Hypotension was observed in 50% of patients during the 30 min preceding cardiac death.
Conclusions
With our current management of terminal patients controlled non-heart beating organ procedure may be difficult due to the duration and variability of the dying process. This observation suggests that we can perform better by evaluating this process moreclosely.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Insufficient procurement of organs from brain-dead donors is the principle factor limiting access to solid organ transplantation. There is evidence that some patients not developing brain death criteria but ending in cardiac death may be suitable for donation of kidney and in certain situations liver or lung [1, 2], a process called non-heart beating donation (NHBD). In the intensive care unit (ICU) when the prognosis (in particular neurological) is unambiguously dire, life-support treatments are futile and should be withdrawn. In such situations controlled NHBD can be considered suitable [2]. This refers to the fact that in such circumstances a treatment plan of palliative care can be carefully explained to the next of kin. Once they have understood and accepted this line of treatment, sufficient time should be given for quiet communication with the relatives so as to seek consent for NHBD before cardiac death, or the relatives may spontaneously propose organ donation. Continuous monitoring enables to pronounce death following the cardiac arrest, in which case a surgical team on standby can retrieve the organs immediately.
Patients presenting catastrophic neurological pathologies are usually perceived as the most suitable for this approach by the healthcare team [3]. Although communication, ethical, and juridical barriers to this approach may appear difficult, and in many countries, including our own, certain of these obstacles have not yet been resolved, there is emerging evidence that in favorable circumstances such a procedure is feasible and can increase the number of available organs for transplantation [4]. From published literature [5, 6], it appears that the timing of the process is vital to limit warm ischemia time. The lack of information regarding the practical aspects of patient management led us to investigate our own data on the process of withdrawing treatment [7].
We studied a group of critically ill, preterminal patients perceived as potential candidates for NHBDs to estimate the feasibility of implementing an NHBD program at our institution. We specifically evaluated the management and the timing of death in patients undergoing the withdrawal of supportive intensive care treatment for dire neurological prognosis.
Method
Patient management
We analyzed all 516 patients suffering catastrophic brain injury, defined as acute brain illness and unresponsive to maximal medical and surgical treatment. Intensive care treatment was withdrawn according to the recommendations of the Swiss Academy of Medical Sciences [8], a national committee issuing bioethical recommendations. Due to patient incompetence the next of kin are informed of the situation, of the focus on palliative care, and of the plan to withdraw life-sustaining therapy. Oral consent by the family is documented in the patient's notes. Firstly, vasoactive agents (if any) are withdrawn, followed by withdrawal of mechanical ventilation, with discretionary extubation. Sedative-analgesic drugs are administered to relieve perceived respiratory distress, vegetative reactions, and disturbing movements if deemed appropriate by the attending physician and nurses. Family members are encouraged to accompany their beloved one during this period and to seek the assistance of a chaplain if required. This procedure reflects current professional conduct and complies with the local ethical and legal standards. There are no existing institutional recommendations for this process at present, apart from the recommendations of the Swiss Academy of Medical Sciences [8].
Study design
In this observational study we considered patients under the age of 70 years who were dying in the ICU between 1 January 2002 and 31 December 2004. The prospective data were entered and analyzed in the computerized clinical information system (CIS) of our medical and surgical ICUs. The CIS provides on-line information on patient management and medical history. Physiological variables from the monitors, ventilators, and laboratory results are automatically entered every minute and validated by the nurses and physicians. During end-of-life in the ICUs the vital signs are usually not displayed on the monitors surrounding the patient but are continuously recorded in the database of the CIS. Administered drugs, procedures and care are selected manually from a predefined scroll down list. The CIS constitutes the nurses' observation chart, and this part of patient's file is paperless.
Our institution's ethics committee waived the requirement of informed consent for analysis of these data and the anonymous publication of results. A nurse certified in intensive care extracted the data while an attending ICU physician validated and analyzed it. For each patient we recorded: the diagnosis, Glasgow Coma Scale (GCS) score at the time of treatment withdrawal, pharmacological treatment, management of mechanical ventilation, and survival time following treatment withdrawal. The total doses of sedation/analgesia administered (intravenous bolus and continuous perfusion) were divided by the duration of the terminal phase and expressed as micrograms per kilogram per hour. Hemodynamic values (mean arterial pressure and heart rate) and SpO2 were recorded.
Descriptive statistics were calculated using JMP Statistical software version 5.1.2 (SAS Institute, Cary, N.C., USA). Data are presented as a mean ± SD or as median (interquartile range, IQR). Comparisons between diagnostic groups and between patients receiving or not receiving analgesia or sedation, extubated or not were carried out by the Wilcoxon test. Differences at the level of p < 0.05 were considered statistically significant.
Results
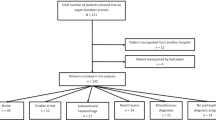
Of the 516 patients dying in the ICU 73 fulfilled the above criteria (Fig. 1); their characteristics are presented in Table 1. There were 21 (29%) with traumatic brain injury, 25 (37%) anoxia, and 25 (34%) stroke (either ischemic, subarachnoidial hemorrhage or cerebral hematoma). In addition to the fact that head trauma patients were significantly younger, there were no differences between the diagnostic groups. At the time of treatment withdrawal, a GCS of 3 was recorded in 7 patients, 4 in 55, and 5 in 11, confirming the severity of the neurological disorder.
All patients were mechanically ventilated. Four patients were treated with norepinephrine, which was interrupted as the first measure. Mechanical ventilation was withdrawn in all the other patients, followed by extubation of 53%. Overall survival was 4.8 h (IQR 1.4–11.5). In 22% of cases the patient died during the first hour and in 31% after 2 h. Extubated patients survived significantly longer, 9.4 h (3.3–15.3) vs. 3.1 h (1.2–6.6; p < 0.01; see Electronic Supplementary Material, S.F1). These patients died 7.6 h (1.5–13.6) after extubation (S.F2).
Analgesia was administered to 70% of the patients, mainly as a morphine drip at an hourly dose of 75 μg/kg (32–134). Sedation was administered to 26%, principally midazolam at 81 μg/kg per hour (54–145). Analgesia was administered to patients with a more protracted course, and these patients therefore survived significantly longer [6.6 h (2.8–13.2) vs. 1.9 h ([0.6–5.0), p < 0.04], but survival time did not differ between patients receiving or not receiving sedation [6.1 h (3.3–9.9) vs. 3.8 h (1.2–11.8), p = 0.6].
Hemodynamic instability was frequent in all three groups. Hypotension, defined as having a mean arterial pressure lower than 60 mmHg, was observed in 80% of patients 10 min before death. One-half of patients (52%) were already hypotensive 30 min before death, and one-third (37%) during their last hour of life (S.T1, S.F3). Likewise, hypoxia defined as SpO2 below 90% was present in 67% of patients 10 min before death, 71% at time minus 30 min, and 75% 1 h before (S.T1, S.F4). We could not identify factors statistically correlated with the occurrence of hypotension, hypoxia, or the duration of agony.
Discussion
Withdrawal of life-support treatment in patients presenting an unequivocally poor prognosis is considered a duty for intensive care practitioners in most countries. This not only allows the patient to die with dignity but also promotes efficient ICU bed use [9]. This approach is well established and has been socially accepted in our country for many years [8]. Harvesting organs from NHBD is a far more complex problem. This approach is based on a utilitarian attitude, believing that organ procurement, a good and useful action, enables some aspects of the death process to be manipulated. Ethical issues have been discussed in detail [1, 10], some aspects of which are still controversial. Policies and regulations vary from country to country and institution to institution depending on the emphasis put on respecting the integrity of a dying patient or on maximizing organ procurement.
The time course of withdrawing life-sustaining treatment and cardiac arrest is entirely in accord with the result of a large European study on unselected ICU patients [9] and those of a study in the United States on patients suffering neurological disaster [11]. We therefore believe that a median survival time of several hours should be expected in such circumstances, and we were not able to identify patient characteristics that would predict the precise time of death, in particular with the use of sedation and analgesia, or tracheal extubation. During agony, hypotension and hypoxia were frequently observed, although the tolerability for transplanted organ viability is not settled yet.
Such an extent and variability in the time while awaiting death can raise problems. It is difficult to establish a NHBD program with a complete surgical team waiting on standby, in some situations up to 1 day, for the cardiac death of a potential organ donor. Hemodynamic instability and hypoxia were frequently observed during agony, although their effect on organ function could not be evaluated. To overcome this problem and have a better control of the process one could decide for example, limiting the time between treatment withdrawal and cardiac death to 1 h. Such a decision, from our analysis would result in a decrease in this donor pool by 80%. It is equally worth weighing the arguments for and against the setting up of such a program for which there is only a 20% chance of it actually happening. In addition to ethical, legal, and logistical aspects, family care and communication related to organ donation request should be considered, as well as the negative consequences of a failed NHBD. Recent evidence shows that the death of a family member is a major determinant of posttraumatic stress disorder for the next of kin, and that sharing end-of-life decisions further increases the risk of anxiety and depression [12]. The impact of controlled NHBD on relatives of the deceased patient should hence be carefully evaluated.
Actively accelerating the dying process could conceivably resolve many issues. This would simplify the organization and minimize hemodynamic instability before death. High doses of opiates and sedation would most likely accomplish this goal. Outside the context of NHBD this practice was in fact documented in a European observational study, with a significant shortening of the dying process in 6.5% of ICU end-of-life situations [9]. The use of high doses of sedation for the purpose of “improving” NHBD goes far beyond the classical “double-effect” in the use of medication to alleviate suffering. Such practice has been proposed in an opinion paper highlighting the limited harm to the dying patient compared to the benefit for the receiver [3]. It has even been suggested, along these lines, that the perfect process for controlled NHBD would be to harvest organs without even bothering to wait for cardiac arrest, since the patient is dying anyway and that he had given his consent.
There are obviously numerous objections to this approach, based on the principle of beneficence. The use of terminal sedation to deliberately accelerate death is explicitly condemned by the ethical recommendations in our country [8]. Indeed, in our setting analgesia or sedation was administered only when the family or the healthcare workers felt uneasy, in particular when the patient presented signs of dysautonomy that suggest suffering or abnormal movements that were distressing. If terminal sedation is necessary to facilitate controlled NHBD, this issue should be openly addressed and debated by ethics committees. It is necessary to reach ethical, legal, and social consensus before this approach can be implemented.
With our current management of terminal patients controlled NHBD organ procedure may be difficult due to the duration and variability of the dying process. This observation suggests that we can perform better by evaluating this process more close. On the other hand, actions aiming to accelerate dying, such as the administration of high doses of sedation or analgesia, raise vast ethical questions.
References
Daar AS (2004) Non-heart-beating donation: ten evidence-based ethical recommendations. Transplant Proc 36:1885–1887
Kootstra G (1995) Statement on non-heart-beating donor programs. Transplant Proc 27:2965
Truog RD, Robinson WM (2003) Role of brain death and the dead-donor rule in the ethics of organ transplantation. Crit Care Med 31:2391–2396
Brook NR, Waller JR, Richardson AC, Andrew Bradley J, Andrews PA, Koffman G, Gok M, Talbot D, Nicholson ML (2004) A report on the activity and clinical outcomes of renal non-heart beating donor transplantation in the United Kingdom. Clin Transpl 18:627–633
Bell MDD (2005) Non-heartbeating organ donation: clinical process and fundamental issues. Br J Anaesth 94:474–478
Sudhindran S, Pettigrew GJ, Drain A, Shrotri M, Watson CJ, Jamieson NV, Bradley JA (2003) Outcome of transplantation using kidneys from controlled (Maastricht category 3) non-heart-beating donors. Clin Transpl 17:93–100
Revelly JP, Imperatori L, Maravic P, Schaller MD, Chioléro RL (2005) Are terminally ill patients dying in the ICU suitable for non-heart beating organ donation? Intensive Care Med 31:S156
Vallotton M (2004) Care of patients in the end of life. Medical-ethical guidelines of the Swiss Academy of Medical Sciences. http://www.samw.ch/content/Richtlinien/e_RL_Lebensende.pdf
Sprung CL, Cohen SL, Sjokvist P, Baras M, Bulow HH, Hovilehto S, Ledoux D, Lippert A, Maia P, Phelan D, Schobersberger W, Wennberg E, Woodcock T, Group Ethicus Study (2003) End-of-life practices in European intensive care units: the Ethicus Study. JAMA 290:790–797
Bell MDD (2003) Non-heart beating organ donation: old procurement strategy-new ethical problems. J Med Ethics 29:176–181
Mayer SA, Kossoff SB (1999) Withdrawal of life support in the neurological intensive care unit. Neurology 52:1602–1609
Azoulay E, Pochard F, Kentish-Barnes N, Chevret S, Aboab J, Adrie C, Annane D, Bleichner G, Bollaert PE, Darmon M, Fassier T, Galliot R, Garrouste-Orgeas M, Goulenok C, Goldgran-Toledano D, Hayon J, Jourdain M, Kaidomar M, Laplace C, Larché J, Liotier J, Papazian L, Poisson C, Reignier J, Saidi F, Schlemmer B (2005) Risk of post-traumatic stress symptoms in family members of intensive care unit patients. Am J Respir Crit Care Med 171:987–994
Author information
Authors and Affiliations
Corresponding author
Additional information
René Chioléro and Luca Imperatori are members of the SwissFoundation to Support Organ Donation.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Revelly, JP., Imperatori, L., Maravic, P. et al. Are terminally ill patients dying in the ICU suitable for non-heart beating organ donation?. Intensive Care Med 32, 708–712 (2006). https://doi.org/10.1007/s00134-006-0116-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-006-0116-7