Abstract
Objective
Colonization of multiple body sites is a leading risk factor for Candida spp. infection in intensive care unit (ICU) patients. We evaluated whether oral nystatin prophylaxis reduces Candida spp. colonization in ventilated ICU patients.
Design and setting
Prospective, randomized, open-label study with blinded assessment of the objective primary evaluation criterion in the medical-surgical ICU of a teaching hospital.
Patients
The study included 98 consecutive patients mechanically ventilated for at least 48 h (mean age 58±19 years; mean SAPS II 40±11), assigned to either treatment group (n=51) or control group (n=47). Study groups were comparable for age, SAPS II, reason for admission, and immune status.
Interventions
Patients were randomized to receive oral nystatin (treatment group; 3×106 U per day) or no nystatin (control group). Multiple body sites (trachea, stomach, rectum, urine, groin, and blood) were tested for Candida spp. on admission and then every 3 days by mycologists blinded to group assignment, and the colonization index was determined.
Results
Colonization by Candida spp. developed in 25% of controls but in none of the treated patients. In multivariate analysis, the absence of nystatin prophylaxis and ICU length of stay were independently associated with Candida spp. colonization. No invasive candidiasis was diagnosed in either study group.
Conclusions
Oral nystatin prophylaxis efficiently prevented Candida spp. colonization in ICU patients at low risk of developing invasive candidiasis. Further studies are needed to determine whether this strategy remains efficient in reducing Candida spp. infections in higher risk ICU patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of nosocomial fungal infections is steadily increasing, especially in severely ill patients. In the United States, a threefold increase in the incidence of sepsis caused by fungal organisms was observed between 1979 and 2000 [1], and Candida spp. was the third most common organism isolated from blood cultures in patients hospitalized in intensive care units (ICUs) from 1995 to 2002 [2]. A European epidemiological study [3] found that invasive candidiasis accounted for 17% of hospital-acquired infections in ICU patients. Candida spp. has thus emerged over the past two decades as a source of severe infections not only in immunocompromised hosts but also in critically ill patients requiring aggressive therapy or invasive procedures [4].
Increasing incidence of severe fungal infections in nonimmunosuppressed patients hospitalized in the ICU is presumably related to the predisposing factors for invasive candidiasis (e.g., Candida spp. colonization, prolonged wide-spectrum antibiotics, mechanical ventilation, multiple invasive devices) that are frequently encountered in this population [4]. Colonization originating from the endogenous flora that develops within the gastrointestinal tract is usually a prerequisite for the development of invasive candidiasis [4, 5, 6, 7, 8]. Invasive candidiasis is a late-onset nosocomial infection associated with a high mortality rate, and its diagnosis remains challenging due to few and nonspecific clinical signs. Since Candida spp. infections are usually preceded by a period of colonization, oral antifungal prophylaxis has been proposed to prevent invasive candidiasis, particularly in immunocompromised hosts (i.e., patients with neutropenia, cancer, or transplant) [9]. In the ICU setting, antifungal prophylaxis has been evaluated mainly in nonimmunosuppressed surgical patients considered at high risk for candidal infection, and this remains controversial [10, 11, 12]. Nevertheless, only few studies have enrolled medical ICU patients [13, 14] and have used oral nystatin for routine prophylaxis in the ICU setting [15, 16, 17]. In addition, the ability of an early prophylaxis to prevent Candida spp. colonization during the ICU stay in consecutive patients admitted for a medical or surgical reason remains to be determined.
We therefore sought to prospectively evaluate the efficacy of a systematic antifungal prophylaxis using oral nystatin, a nonabsorbable antifungal agent, in preventing Candida spp. colonization in a cohort of patients admitted to a medical-surgical ICU. Our study hypothesis was that systematic nystatin prophylaxis would decrease the incidence of Candida spp. colonization, which usually precedes invasive candidiasis [4, 6, 7, 8] during the ICU stay.
Methods and materials
Our institutional review board approved the study, and the patients or their next-of-kin provided informed consent to participation in the study. This was a randomized, open-label, single-center study with blinded assessment of the objective primary evaluation criterion.
Study population
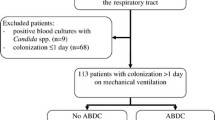
Patients admitted to our ICU between February and July 2002 were eligible if older than 18 years of age and expected to require invasive mechanical ventilation for more than 48 h. Exclusion criteria were: pregnancy, prophylactic or curative antifungal treatment within the last 2 months, contraindication to oral drug administration, known allergy to nystatin or its derivatives, and prior inclusion in the study. In addition, patients who exhibited at baseline a Candida spp. colonization or infection were excluded from the study. Reasons for admission, demographic characteristics, immune status and the Simplified Acute Physiology Score (SAPS) II were recorded on admission. The time to colonization, the duration of mechanical ventilation, the length of stay in the ICU, and ICU mortality were also recorded.
Of the 116 eligible patients 18 were excluded because of the presence of Candida spp. colonization at baseline; the study thus included 98 patients (65 men, 33 women; mean age 58±19 years; mean SAPS II: 40±11). The reason for ICU admission was a medical condition in 45 patients (46%), a surgical procedure in 19 patients (19%), and trauma in 34 patients (35%). In 65% of cases, the patient was hospitalized directly from home, 15% from a medical ward, and 20% from a surgical ward. Immunodeficiency was present in only 12 patients (12%), including diabetes mellitus (n=6), malignancy (n=3), and long-term immunosuppressive therapy (n=3; Table 1). All patients had a central venous catheter and 79 of them (81%) received antibiotics during the ICU stay.
A computer-generated randomization list in balanced blocks of unequal sizes was used and patients were allocated to receive either systematic nystatin prophylaxis (3×106 U per day in three divided oral doses; n=51) or no oral nystatin prophylaxis (n=47; Fig. 1). Baseline characteristics of patients in the two study groups were similar (Table 1). Since randomization was performed on admission, patients of the treatment group received the first dose of nystatin within the first 12 h of hospitalization in the ICU. The 18 excluded patients were evenly distributed between the two study groups and had similar demographic characteristics as the overall study population, with the exception of a greater proportion of immunocompromised hosts.
Mycological studies and definitions
Multiple-site testing for fungi included: tracheal secretions, stomach contents, rectal swab, groin skin fold swab, urine, and blood. These tests were performed in each patient at ICU admission and subsequently every 3 days throughout the ICU stay. The colonization index was calculated for each multiple-site testing as the ratio between the number of distinct body sites colonized by Candida spp. and the total number of sites tested, as previously described [7]. In addition, the need for an antibiotic or corticosteroid therapy, the route of nutrition (i.e., enteral vs. parenteral), and vomiting or the presence of a gastric residual volume greater than 500 ml/24 h were recorded at the time of multiple-site testing. Fungal infections identified during the study period were recorded.
The specimens were placed in a dry medium and taken to the Mycology Laboratory. Group assignment was not indicated on specimens, the mycologists were therefore blinded to treatment allocation. Each specimen was directly microscopically examined and cultured on three media (Chromagar, Sabouraud plus chloramphenicol, and Sabouraud plus actidione). Colonization was assessed for each body site specimen, and yeasts were identified. Fungal colonization was defined as either the presence of the same yeast on two or more of the five distinct body sites tested (blood sample excepted), or on two consecutive specimens from the same body site. Fungal infection was defined as either the presence of a candidemia or the identification of Candida spp. in a normally sterile body site associated with a severe sepsis with negative tests for bacteria or other causes [7].
Statistics
Statistical analysis was performed on the intention-to-treat basis using Statview 5.0 software (SAS Institute, Cary, N.C., USA). The primary evaluation criterion was the development of fungal colonization during ICU stay. Secondary evaluation criteria were the course of the colonization index over time and the occurrence of a fungal infection during the ICU stay. The χ2 test or Fisher’s exact test when indicated were used to compare distributions of qualitative variables between the two patient groups. The Mann-Whitney U test was used for the between-group comparison of quantitative variables. The proportion of positive gastrointestinal sites (i.e., stomach and rectum) over time was compared between the two study groups using the trend χ2 test. To identify independent predictors of fungal colonization in the study population, variables for which the p value was less than 0.20 in the between-group comparison by univariate analysis were entered into a logistic regression model. For an estimated rate of fungal colonization reaching approx. 60% in ICU patients [18], 49 patients per group should have been enrolled in the study to show a 50% reduction in fungal colonization, with an α error of 5% and β error of 20%. Despite a lower incidence of colonization in our study population, a three-member independent data-monitoring institutional committee decided to interrupt the study based on a significant difference in efficacy between study groups for the occurrence of the primary evaluation criterion after having enrolled 98 patients into the trial.
Results
In the treatment group, no Candida spp. colonization occurred whereas 12 patients (25%) from the control group exhibited a fungal colonization (p<0.001). In the latter subset of patients, the mean time lag between ICU admission and the diagnosis of fungal colonization was 8.4±5.2 days (range 2–17 days; Fig. 2), and mean number of positive body sites was 2.2 (range 2–4). During hospitalization in the ICU, the mean colonization index was higher in the control group than in the treatment group (0.19±0.20 vs. 0.06±0.13; p<0.0001). This difference persisted over time (Fig. 3). In the treatment group, the proportion of positive gastrointestinal sites (i.e., stomach and rectum) tended to decrease during the ICU stay (p=0.02), as opposed to the control group (p=0.20; data not shown). No Candida spp. infection was diagnosed in the two study groups during the ICU stay. The Candida species isolated in the 12 patients were: C. albicans (n=9), C. tropicalis (n=2) and C. krusei (n=1). No clinically detectable adverse effect of nystatin therapy was recorded. Specifically, none of the patients from the treatment group suffered from repeated vomiting impeding oral administration of nystatin.
In the univariate analysis, risk factors for fungal colonization included the ICU length of stay, the duration of antibiotic therapy, and the absence of nystatin prophylaxis (Table 2). In the multivariate analysis, both the absence of nystatin prophylaxis (odds ratio not computable) and ICU length of stay (odds ratio 1.05, p=0.02, 95% confidence interval 1.01–1.10 per additional day of hospitalization) were independent factors associated with fungal colonization during the ICU stay.
Discussion
The main finding of the present study is that systematic nystatin oral prophylaxis initiated upon admission in patients expected to be mechanically ventilated for more than 48 h efficiently prevents the development of Candida spp. colonization without noticeable adverse effects. In our low-risk patients, however, we failed to demonstrate any effect on the occurrence of invasive candidiasis since no case of Candida spp. infection was recorded in the two study groups.
The reported incidence of Candida spp. colonization in medical-surgical ICUs varies greatly according to study populations. In the current trial, it reached 25% in the absence of nystatin prophylaxis, similar to that reported in a recent multicenter survey [19]. Using similar diagnostic criteria, Garbino et al. [14] found an overall incidence of 65% of fungal colonization during the ICU stay. In this trial, however, the proportion of patients admitted from other wards or having immunodeficiency was substantially larger than that of the current study.
In patients who were admitted to our ICU without fungal colonization, the mean time to colonization was 8 days, ranging from 2 to 17 days (Fig. 2). Similarly, a recent study showed that in the absence of antifungal prophylaxis, Candida spp. colonization developed rapidly, within a few days after hospitalization in the ICU [14]. None of the studied variables allowed us to accurately distinguish patients who developed early Candida spp. colonization (<5 days) from those who had late positive tests (>8 days) (data not shown).
When initiated systematically on the first day of ICU hospitalization, oral nystatin was consistently effective in preventing Candida spp. colonization in our ICU patients. Accordingly, the absence of nystatin prophylaxis was an independent predictor of Candida spp. colonization, in conjunction with ICU length of stay. This finding was presumably related to the efficacy of nystatin administered orally to significantly reduce the proportion of positive gastrointestinal sites (e.g., stomach and rectum), as shown in the treatment group. Accordingly, the colonization index previously defined by Pittet et al. [7] regularly decreased over time in patients receiving nystatin, whereas it tended to increase in controls (Fig. 3), as recently observed in high-risk medical patients evaluated serially throughout their ICU stay [20].
The present trial found no deep-seated or systemic fungal infections in either of the two study groups during the ICU stay, whereas Garbino et al. [14] previously reported a 16% rate of Candida spp. infection in ICU patients who did not receive an antifungal prophylaxis. Jacobs et al. [13] observed the development of a local fungal infection in 5% of patients and a systemic candidiasis in 1% of patients admitted in the ICU for septic shock in the absence of antifungal prophylaxis. Charles et al. [20] reported one case of disseminated candidiasis in 92 nonneutropenic ICU patients hospitalized for more than 7 days. Interestingly, the mean colonization index in our colonized patients (n=12; none of them receiving nystatin prophylaxis) was 0.31±0.13, markedly lower than that reported by Garbino et al. [14] and Charles et al. [20]. This discrepancy is due to our recruiting patients at low risk for invasive candidiasis; 65% of them were hospitalized directly from home and only 12% were immunocompromised. Despite the presence of other risk factors for Candida spp. infection in our study population (e.g., central venous catheter, severity of illness, prior abdominal surgery) [4, 19], the clinical relevance of routine antifungal prophylaxis in our patients was retrospectively questionable with regard to the absence of documented Candida spp. infection in the control group. Recent guidelines for antifungal prophylaxis either do not include nonimmunocompromised patients [9] or fail to recommend routine oral prophylaxis in ICU patients [21] due to the absence of published studies with sufficient statistical power to demonstrate the benefit of this approach on large groups of critically ill patients [11, 12]. In addition, fluconazole, the most frequently used agent for antifungal prophylaxis, may lead to selection of resistant organisms and substantial cost [11]. In contrast, minimally absorbed agents from the gastrointestinal tract such as nystatin may prevent the emergence of resistant fungal strains, and has the advantages of its low cost (approx. €1 per day) and absence of side effects, as shown in the present study. Although questioned in immunocompromised patients [22], the efficacy of nystatin prophylaxis to reduce Candida spp. infection rate has been shown in burned patients and in surgical ICU settings [15, 16].
To determine the efficacy of routine nystatin prophylaxis in preventing Candida spp. colonization in our medical-surgical ICU, we purposely excluded from the current study all patients who presented with positive sites for Candida spp. on admission. This presumably selected a subgroup of patients at low risk for invasive candidiasis, as reflected by the absence of Candida spp. infection in the control group and did not allow us to evaluate the efficacy of nystatin to decrease the number of positive sites over time in colonized patients. Since the present study was not designed to evaluate the ability of nystatin prophylaxis to significantly decrease candidal infection rate, the positive results obtained to prevent Candida spp. colonization in our patients cannot be extrapolated to the prevention of fungal infection. Further studies are needed to evaluate the ability of oral nystatin to reduce Candida spp. infection rate in ICU patients at higher risk of sustaining deep-seated fungal infections or candidemia (e.g., higher colonization index), as recently emphasized [10, 12]. The present results underline the utmost importance of inclusion criteria in future clinical trials to carefully select ICU patients who are at high risk of developing Candida spp. infection.
Conclusions
In the present study Candida spp. colonization was efficiently and safely prevented in a selected group of low-risk critically ill patients by a low-cost systematic nystatin prophylaxis initiated upon admission to the ICU. Although our patients were not heavily colonized, they had other significant risk factors for Candida spp. infection and were representative of the population routinely admitted to a medical-surgical ICU. As far as we know, this is the first trial that demonstrates that early nystatin prophylaxis efficiently prevents the occurrence of Candida spp. colonization in critically ill, yet nonimmunocompromised patients during the ICU stay. Further studies are needed to determine whether this strategy is efficient in reducing the rate of invasive candidiasis in patients at higher risk for Candida spp. infection who are admitted to medical-surgical ICUs.
References
Martin GS, Mannini DM, Eaton S, Moss M (2003) The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348:1546–1554
Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB (2004) Nosocomial bloodstream infections in US hospitals: analysis of 24, 179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:309–317
Vincent JL, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas-Chanoin MH, Wolff M, Spencer RC, Hemmer M (1995) The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) study. JAMA 274:639–644
Eggimann P, Garbino J, Pittet D (2003) Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect Dis 3:685–702
Nucci M, Anaissie E (2001) Revisiting the source of candidemia: skin or gut? Clin Infect Dis 33:1959–1967
Voss A, Hollis RJ, Pfaller MA, Wenzel RP, Doebbeling BN (1994) Investigation of the sequence of colonization and candidemia in nonneutropenic patients. J Clin Microbiol 32:975–980
Pittet D, Monod M, Suter PM, Frenk E, Auckenthaler R (1994) Candida colonization and subsequent infections in critically ill surgical patients. Ann Surg 220:751–758
Solomkin JS, Flohr AB, Quie PG, Simmons RL (1980) The role of Candida in intraperitoneal infections. Surgery 88:524–530
Rex JH, Walsh TJ, Sobel JD, Filler SG, Pappas PG, Dismukes WE, Edwards JE (2000) Practice guidelines for the treatment of Candidiasis. Clin Infect Dis 30:662–678
Eggimann P, Garbino J, Pittet D (2003) Management of Candida species infections in critically ill patients. Lancet Infect Dis 3:772–785
Rex JH, Sobel JD (2001) Prophylactic antifungal therapy in the intensive care unit. Clin Infect Dis 32:1191–1200
Azoulay E, Schlemmer B (2004) Faut-il proposer une prophylaxie antifungique aux patients non neutropéniques de réanimation? Réanimation 13:87–92
Jacobs S, Price Evans DA, Tariq M, Al Omar NF (2003) Fluconazole improves survival in septic shock: a randomized double-blind prospective study. Crit Care Med 31:1938–1946
Garbino J, Lew DP, Romand JA, Hugonnet S, Auckenthaler R, Pittet D (2002) Prevention of severe Candida infections in nonneutropenic, high-risk, critically ill patients: a randomized, double-blind, placebo-controlled trial in patients treated by selective digestive decontamination. Intensive Care Med 28:1708–1717
Desai MH, Rutan RL, Heggers JP, Herndon DN (1992) Candida infection with and without nystatin prophylaxis: a 11-year experience with patients with burn injury. Arch Surg 127:159–162
Cerra FB, Maddaus MA, Dunn DL, Wells CL, Konstantinides NN, Lehmann SL, Mann HJ (1992) Selective gut decontamination reduces nosocomial infections and length of stay but not mortality or organ failure in surgical intensive care unit patients. Arch Surg 127:163–167
Savino JA, Agarwal N, Wry P, Policastro A, Cerabona T, Austria L (1994) Routine prophylactic antifungal agents (clotrimazole, ketoconazole, and nystatin) in nontransplant/nonburned critically ill surgical and trauma patients. J Trauma 36:20–25
Petri MG, König J, Moecke HP, Gramm HJ, Barkow H, Kujath P, Dennhart R, Schäfer H, Meyer N, Kalmar P, Thülig P, Müller J, Lode H, Paul-Ehrlich Society for Chemotherapy, Divisions of Mycology and Pneumonia Research (1997) Intensive Care Med 23:317–325
McKinnon PS, Goff DA, Kern JW, Devlin JW, Barletta JF, Sierawski SJ, Mosenthal AC, Gore P, Ambegaonkar AJ, Lubowski TJ (2001) Temporal assessment of Candida risk factors in the surgical intensive care unit. Arch Surg 136:1401–1408
Charles PE, Dalle F, Aube H, Doise JM, Quenot JP, Aho LS, Chavanet P, Blettery B (2005) Candida spp. colonization significance in critically ill medical patients: a prospective study. Intensive Care Med 31:393–400
Régnier B, Attal M, Bezie Y, Blanc V, Buzyn A, Choutet P, Mimoz O, Papazian L, Pialoux G, Pottecher T, Poyart C, Tazi A, Tilleul P (2004) Prise en charge des aspergilloses et candidoses invasives de l’adulte. Réanimation 13:5–13
Gotzsche PC, Johansen HK (2002) Nystatin prophylaxis and treatment in severely immunodepressed patients. Cochrane Database Syst Rev 4:CD002033
Acknowledgements
We gratefully thank Pr. Didier Pittet for his expertise and constructive critique of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is discussed in the editorial available at: http://dx.doi.org/10.1007/s00134-005-2806-y
Rights and permissions
About this article
Cite this article
Normand, S., François, B., Dardé, ML. et al. Oral nystatin prophylaxis of Candida spp. colonization in ventilated critically ill patients. Intensive Care Med 31, 1508–1513 (2005). https://doi.org/10.1007/s00134-005-2807-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-005-2807-x