Abstract
Objective
This study investigated the potential benefits of combination therapy using antithrombin (AT) with danaparoid sodium (DA) compared with the use of AT with unfractionated heparin (UFH) in the treatment of sepsis.
Methods
Rats infused with lipopolysaccharide were treated with either DA alone, AT alone, AT plus DA, AT plus UFH, or human serum albumin as controls. AT (125 U/kg) was injected into the AT group immediately after lipopolysaccharide infusion. The AT/DA and AT/UFH groups received the same dose of AT in conjunction with either DA (400 U/kg) or UFH (400 U/kg). The status of the mesenteric microcirculation was examined by intra-vital microscopy and the laboratory indices of coagulation, inflammation, and organ dysfunction were measured.
Results
The coagulation markers were improved following the administration of DA or UFH. The decreases in the WBC counts were significantly suppressed in the AT/DA group. The elevation of IL-6 decreased in the AT, DA, and AT/DA groups (all p<0.01) but not in the AT/UFH group. The prostaglandin I2 levels were significantly elevated only in the AT/DA group (p<0.05). The WBC adhesion was significantly suppressed in the DA, AT/UFH, and AT/DA groups (p<0.05), and the RBC velocity was best maintained in the AT/DA group with no associated increase in capillary hemorrhage. The elevation of ALT and BUN significantly improved only in the AT/DA group.
Conclusion
Organ dysfunction can thus be alleviated by even moderate doses of AT replacement when co-administered with DA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The activation of coagulation and the generation of inflammatory mediators by the coagulation system, resulting in disturbance of the microcirculation, are both important factors involved in the pathogenesis of organ dysfunction during sepsis [1]. It was recently demonstrated that specific receptors for clotting factors, such as thrombin and activated Factor X (FXa), are present on the cell membrane [2]. Subsequent to this finding, the role of clotting factors as inflammatory mediators began to attract close attention. At the same time, the treatment of severe sepsis with anticoagulants, which are now known to not only exert an anticoagulant activity, but also modulate inflammatory reactions, also became the focus of considerable interest [3, 4]. Following these findings, recombinant human activated protein C was approved by the U.S. Food and Drug Administration as the first drug of choice for severe sepsis in the wake of a successful clinical trial [5].
Similar to activated protein C, antithrombin (AT) inhibits the inflammatory reactions by binding to glycosaminoglycan on the vascular endothelial cell membrane and suppressing the activation of NF-κB [6]. Antithrombin has also been reported to suppress the adhesion of leukocytes to the vascular endothelium, thus resulting in the amelioration of organ failure and thereby achieving an increased survival rate [7]; however, a large-scale clinical trial [8] failed to demonstrate sufficient efficacy. Since a pre-specified subgroup analysis of antithrombin-treated patients without concomitant heparin administration revealed a favorable trend in terms of outcome when compared with the control group, we speculate that a major reason for the failure of the trial was the concomitant use of heparin [9]. One of the aims of this study is thus to confirm the adverse effects of heparin on AT action.
A number of studies have shown the antiinflammatory effects of AT to only be achieved when its activity is elevated to supranormal levels (>150%) [10, 11]; however, to obtain such levels of AT in a clinical setting would be costly while also limiting its utility based on an unfavorable cost-benefit ratio.
The second aim of this study was to determine whether or not an adequate response may be observed when AT is administered at a moderate dosage, to achieve an estimated post-transfusion recovery concentration of 100%, in combination with danaparoid sodium (DA).
Danaparoid sodium is a low molecular weight heparinoid with a mean molecular weight of approximately 6000 daltons. It consists mainly of heparin sulfate (83%) and dermatan sulfate (12%). The high-affinity fraction of heparan sulfate inhibits factor F Xa by catalyzing its binding to AT. In addition to maximizing the anti-F Xa effect of AT, DA has been shown to have a lower binding affinity for AT in comparison with unfractionated heparin (UFH) [12]; thus, DA is expected not to abolish the anti-inflammatory effects of AT [13].
Materials and methods
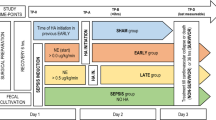
Ten-week-old Wistar rats were used in this study. All experimental procedures were conducted after obtaining the approval of the Ethical Committee for Animal Experiments of Juntendo University. All rats were provided standard rat chow and water ad libitum. The rats were anesthetized with sodium pentobarbital (40 mg/kg, intraperitoneally), and systemic inflammation was induced by administering a single injection of LPS (E. coli O55-B5, Difco) via the caudal vein at a dose of 5.0 mg/kg. The dose of AT (Antithrombin P, Aventis Behring GmbH, now ZLB Behring GmbH, Marburg, Germany) used throughout the study and administered immediately after the LPS injection was 125 U/kg. The animals were divided into four treatment groups (n=15/group): including the AT group; intravenous (i.v.) administration of AT alone: DA group; i.v. administration of 400 U/kg of DA (Orgaran, Nippon Organon Co., Osaka, Japan) alone. The AT/UFH group had concurrent i.v. administration of AT and 400 U/kg of UFH (Heparin, Mitsubishi Pharma Co., Osaka, Japan): and the AT/DA group had concurrent i.v. administration of AT and 400 U/kg of DA. A fifth group of 15 animals was given 5.0% human serum albumin (HSA) i.v. (Albumin-Behring, ZLB Behring GmbH, Marburg, Germany) immediately after the injection of LPS at a volume equal to that of the AT used in the active treatment groups. These animals served as negative controls.
In 5 animals from each treatment and control group, the mesenteric microcirculation was examined using intra-vital microscopy as previously described [14]. In brief, the abdomen was opened under anesthesia by a median incision and the mesentery was displayed and immobilized on a special stand. The microcirculation was observed using the Optiphoto microscopic system (Nikon, Tokyo, Japan). Each field was recorded for 3 min at 30 frames/s using a video system (DXC-950, Sony, Tokyo, Japan). In each animal, six consecutive fields were randomly selected. The images obtained were used to document and analyze the number of adherent WBC, the frequency of capillary bleeding using an image-analyzing system (Image Tracker PIV, Digimo, Osaka, Japan) to determine the RBC velocity using an original program (Well System, Tokyo, Japan).
Three hours after the LPS injection, blood samples were obtained under anesthesia from the inferior vena cava of the remaining 10 animals not subjected to intra-vital microscopy from each group. These samples were used for the measurement of WBC, the platelet counts, and to determine the levels of AT activity, FDP, fibrinogen, interleukin (IL) 6, 6-keto-PGF1α (a stable metabolite of PG I2), alanine aminotransferase (ALT), and blood urea nitrogen (BUN). IL-6 was measured using an ELISA kit (ELISA kit for rat IL-6, TFB Co., Tokyo, Japan). In addition, 6-keto-PGF1α was measured by radioimmunoassay (Mitsubishi BCL, Tokyo, Japan).
Statistical analysis
All data are expressed as the means±SD. A statistical analysis was performed using one-way analysis of variance with the Stat View II statistical software package for Macintosh. Statistical differences were deemed significant at p<0.05.
Results
In the control group, AT activity (normal range: 100–120%) decreased to below 70% of normal at 3 h after the infusion of LPS. In the AT and AT/UFH groups, however, AT activity was maintained at approximately 100% of normal [p<0.01 (AT); p<0.05 (AT/UFH)]. In contrast, in the AT/DA group, AT activity actually increased substantially to approximately 50% above normal levels (p<0.01; Fig. 1, left panel). The activation of coagulation was monitored by measuring the FDP (normal range <5 μg/dl) and fibrinogen concentrations (normal range 200–350 mg/dl) at 3 h after LPS infusion. The FDP levels in the AT group were not statistically different from those found in the control group; however, this response was significantly suppressed by concurrent anticoagulation with either UFH or DA (p<0.01; Fig. 1, middle panel). The decrease in the fibrinogen level in the controls was significantly suppressed in the DA and AT/DA groups (p<0.01, respectively) but not in the AT (p=0.45) and AT/UFH (p=0.05) groups (Fig. 1, right panel); however, no significant differences in the FDP and fibrinogen levels were observed at 3 h between the AT/UFH group and the AT/DA groups.
Comparison of the antithrombin activity, FDP and fibrinogen levels. The antithrombin (AT) activity (left panel), fibrin/fibrinogen degradation products (FDP; middle panel) and fibrinogen levels (right panel) at 3 h after lipopolysaccharide (LPS) treatment are shown for the five groups. The controls received 5.0% albumin at the same volume as that of the AT given to the remaining group. The four remaining groups received danaparoid sodium (400 U/kg) alone (DA group), or antithrombin (125 U/kg) either alone (AT group) or in combination with unfractionated heparin (400 U/kg; AT/UFH group) or danaparoid sodium (400 U/kg; AT/DA group) immediately after the administration of LPS. * p<0.05, ** p<0.01
The serum IL-6 levels, used as an indicator of the inflammatory response, rose to 56,300±26,800 pg/ml in the control group at 3 h after LPS infusion. All four treatment groups demonstrated a decrease in this response and the differences were significant for the AT, DA, and AT/DA groups (11,700±8200 pg/ml; p<0.01) but did not reach significance in the AT/UFH group (33,200±32,400 pg/ml; Fig. 2, left panel). The levels of 6-keto-PGF1α increased in all four treatment groups in comparison with the control group, but the difference only reached statistical significance in the AT/DA group (p<0.05; Fig. 2, right panel).
Comparison of the IL-6 and 6-keto-PG F1α levels. The IL-6 (left panel) and 6-keto-PG F1α (right panel) levels at 3 h after LPS treatment are shown. The controls received 5.0% albumin at the same volume as that of the AT given to the remaining groups. The four remaining groups received danaparoid sodium (400 U/kg) alone (DA group), or antithrombin (125 U/kg) either alone (AT group) or in combination with UFH (400 U/kg) (AT/UFH group) or danaparoid sodium (400 U/kg; AT/DA group) immediately after the administration of lipopolysaccharide.
In the control group, the WBC count (normal range 8000–12,000/mm3) markedly decreased to 1900±755/mm3 at 3 h after LPS injection. This decrease was significantly suppressed only in the AT/DA group (p<0.05; Fig. 3, left panel). The platelet count (normal range >120×104/mm3) also markedly decreased in the control group (44.2±21.0×104/mm3). This decrease was significantly suppressed in the DA (p<0.05), AT/UFH (p<0.05), and AT/DA (p<0.01), but not in the AT group. The platelet count in the AT/DA group remained more than double (95.5±32.1×104/mm3) that of the control group (Fig. 3, right panel).
Comparison of the white blood cell (WBC) and platelet counts. The WBC (left panel) and platelet counts (right panel) at 3 h after LPS treatment are shown. The controls received 5.0% albumin at the same volume as that of the AT given to the remaining groups. The four remaining groups received danaparoid sodium (400 U/kg) alone (DA group), or AT (125 U/kg) either alone (AT group) or in combination with unfractionated heparin (400 U/kg; AT/UFH group) or danaparoid sodium (400 U/kg; AT/DA group) immediately after administration of LPS.
Observations of the mesenteric microcirculation by intra-vital microscopy showed the frequency of leukocyte adhesion in the venules in the control group to be 31.5±8.90/min at 1 h after LPS administration. This level was significantly less than that (by about two-thirds) in the control group of each treatment group (p<0.05). The number of adhesive leukocytes increased over time in the controls, to reach 54.0±20.9/min at 3 h after the LPS injection. This number was significantly lower in the DA, AT/UFH, and AT/DA group (p<0.05, respectively), but not in the AT alone group at 3 h (Fig. 4, left panel). The frequency of bleeding tended to increase over time in all the test and control groups; however, the frequency of bleeding at 3 h in the AT/DA group (1.60±2.51/field) was less than half that observed in the HSA control group (3.28±2.61/field), but this difference was not statistically significant. The frequency of bleeding at 3 h was the highest in the AT/UFH group (3.83±2.48/field; Fig. 4, right panel).
Frequency of WBC adhesion and bleeding events. The levels of leukocyte adhesion and frequency of bleeding at 1, 2, and 3 h after LPS treatment are shown for the five groups. The controls received 5.0% albumin at the same volume as that of the AT given to the remaining groups. The DA group received 400 U/kg of danaparoid sodium alone. The three remaining groups received AT (125 U/kg) either alone (AT group) or in combination with unfractionated heparin (400 U/kg; AT/UFH group) or danaparoid sodium (400 U/kg; AT/DA group) immediately after the administration of LPS. Open circles: control group, open squares: AT group; triangles: DA group; solid circles: AT/UFH group; solid squares: AT/DA group. Asterisk: p<0.05 compared with the control group
The RBC velocity (in millimeters per second) in the arterioles decreased over time in the control group, with the velocity at 3 h being less than half of that observed at 1 h. The administration of AT alone was associated with a well maintained flow during the first 2 h and thereafter the flow decreased. The velocity in the AT/DA group was significantly greater than the HSA controls at 1 h (p<0.05) and this velocity was maintained throughout the experimental period, and remained at a significantly higher level at 3 h (p<0.01; Fig. 5, left panel). In contrast, although the AT/UFH group demonstrated a significantly higher RBC velocity than the HSA controls for the full 3-h period of observation (p<0.05), there was a progressive reduction in the velocity over this period. Very similar trends were seen regarding the RBC velocity in the venules (Fig. 5, right panel).
The RBC velocity in the arterioles and venules The red blood cell (RBC) velocity in the arterioles (left panel) and venules (right panel) at 1, 2, and 3 h after LPS treatment are shown for the five groups. The controls received 5.0% albumin at the same volume as that of the AT given to the remaining groups. The DA group received 400 U/kg of danaparoid sodium alone. The three remaining groups received AT (125 U/kg) either alone (AT group) or in combination with unfractionated heparin (400 U/kg; AT/UFH group) or danaparoid sodium (400 U/kg; AT/DA group) immediately after the administration of LPS. Open circles: control group; triangles: DA group; open squares: AT group; solid circles: AT/UFH group; solid squares: AT/DA group; * p<0.05, ** p<0.01 compared with the control group
The ALT (normal range <30 IU/l) levels were significantly elevated in the control animals. AT/UFH (p<0.05) and AT/DA (p<0.01) modified the ALT response and the change was more prominent in AT/DA group. Similarly, the controls exhibited a significant rise in the BUN following LPS treatment (normal range <10 mg/dl). All four treatments showed a tendency to modify this response, but it only reached significance in the AT/DA group (p<0.01; Fig. 6, right panel).
Comparison of the alanine aminotransferase (ALT) and blood urea nitrogen (BUN) levels. The ALT and BUN (right panel) levels at 3 h after lipopolysaccharide treatment are shown. The controls received 5.0% albumin at the same volume as that of the AT given to the remaining groups. The four remaining groups received danaparoid sodium (400 U/kg) alone (DA group), or AT (125 U/kg) either alone (AT group) or in combination with unfractionated heparin (400 U/kg; AT/UFH group) or danaparoid sodium (400 U/kg; AT/DA group) immediately after the administration of LPS.
Discussion
Disturbances in the microcirculation system are closely associated with the progression and poor prognosis of severe sepsis. Activation in coagulation and enhanced inflammation play major roles in this mechanism [15]. Enhanced clotting begins with the expression of tissue factor, which is followed by the activation of the extrinsic coagulation pathway. In the progress of inflammation, activated neutrophil plays a central role and the damage to the vascular endothelium is the main feature in this reaction [16]. Enhanced coagulation and inflammatory reactions are additive and act cooperatively in promoting a progressive failure of the microcirculation eventually resulting in organ failure and multiple organ dysfunction syndrome (MODS).
The LPS challenged rat model has been widely used to examine the effect of AT, and we previously studied the effects of AT and other anticoagulants [17, 18]. In addition, intra-vital microscopy is the best modality for observing various phenomena in the microvasculature such as leukocyte-endothelial interaction and microbleeding. This study examined the potential benefits of using moderate doses of AT plus concurrent anticoagulation with either UFH or DA. The UFH serves as a cofactor for AT and increases the anticoagulant activity of AT 1000-fold or more [19]. It inhibits both thrombin and F Xa at a molar ratio of 1:1. In contrast, DA acts specifically on F Xa and this is 20 times more selective for F Xa than UFH [20]. Following the recent discovery of protease-activated receptors (PAR) on the cell surface, coagulation factors, such as thrombin and F Xa have been highlighted as not only key enzymes in the clotting system, but also as major mediators of inflammation. PAR-1 has been reported to be activated by thrombin, whereas PAR-2 is activated by F Xa and tissue factor/F VIIa complex [21]. Since the activation of these receptors modulates the leukocyte–endothelial interaction [2, 22], the shift of the target from thrombin to F Xa is of interest. We previously reported the beneficial effects of a synthetic low molecular weight inhibitor of F Xa [17] and DA [23]. In the present study, F Xa inhibition was achieved using moderate dose of AT and DA.
The difference between UFH and DA depends not only on their specificities for F Xa, but also on their binding affinities to AT. The AT bound to heparin cannot bind to glycosaminoglycans on the vascular endothelium, and this induces the synthesis of prostaglandin I2 (PG I2) [24, 25]. The PG I2 has both anti-inflammatory and anti-platelet effects, including the inhibition of WBC adhesion and the suppression of platelet aggregation [26]; thus, it is possible that the anti-inflammatory activity of AT is partially attributable to the induction of PG I2 synthesis. Using a similar model, Hoffman et al. [27] also demonstrated that UFH completely abolishes AT’s effects of preserving mesenteric microcirculation. Furthermore, in a large-scale recently completed clinical study of the value of AT in patients with severe sepsis, no significant survival benefit was observed [8]. As many of the patients included in that study received UFH, the possibility that the bioavailability of PG I2 may have been compromised has been suggested as one explanation for the study’s failure [28]. In contrast to UFH, since the binding affinity of DA to AT is lower because of its structure, it has been suggested that DA binding to AT may not interfere with the initiation of PG I2 synthesis [12], and this hypothesis is supported by the findings of the present study.
The decrease in the WBC count in this model of sepsis reflects the adhesion and sequestration of WBC to the vascular endothelium [28]. In this study, as expected, the WBC in the control group decreased markedly to below 25% of the normal range. This response was suppressed in the AT/DA group with the WBC being maintained at twice the level observed in the control group, whereas the other groups showed no significant differences. Similarly, a decrease in the platelet count reflects the adhesion of platelets to the endothelium and intra-vascular aggregation [29]. The fall in the platelet count in the controls significantly decreased in the DA, AT/UFH, and AT/DA groups but not in the animals receiving AT alone. The overall coagulation and inflammatory responses seen in this study suggest that the combination with DA appears to be associated with consistently favorable results. These conclusions were further confirmed by studies of the microcirculation and the influence on the hepatic and renal function that may be expected from a microcirculatory dysfunction.
Using intra-vital microscopy, it was found that following LPS administration, the frequency of leukocyte adhesion in the post capillary venules increased over time. In previous studies, we demonstrated that, for AT to exert its anti-inflammatory activity, a dose of 500 U/kg was required [7]. In the findings reported herein, a much lower dose of 125 U/kg failed to have a significant anti-inflammatory effect when given alone; however, when this dose was given in combination with either UFH or DA, a significant suppression of leukocyte adhesion was observed in comparison with the controls and those animals given AT alone. These results lead us to conclude that the concurrent use of heparin or heparinoids augments the adhesion-suppressive effect of AT. In this regard, the report that heparinoids inhibit P-selectin and may thus inhibit leukocyte adhesion is an interesting finding [30]. Adherent activated leukocytes or platelets damage the vascular endothelium, activate coagulation, and significantly inhibit the blood flow [28]. In the present study, the RBC velocity was best maintained in the AT/DA group.
The administration of anticoagulant therapy in patients with severe sepsis raises concerns that such treatment may either promote or exacerbate a hemorrhagic tendency. This possibility was investigated during an examination of the mesenteric circulation by intra-vital microscopy. The bleeding observed was not significantly different in the four treatment groups in comparison with the control animals; however, although the difference was not statistically significant, the AT/UFH group had a higher frequency of bleeding, namely more than double that seen in the AT/DA group, and this finding was closer to that observed in the control group.
The primary objective of this study was to test the potential benefits of combination AT/anticoagulant therapy in preventing major organ dysfunction. Regarding organ damage, AT alone at a dose of 125 U/kg was found to be ineffective in comparison with the treated controls; however, in combination with both UFH and DA, a significant degree of protection of the hepatic function was observed, and it was significantly greater in the AT/DA group. Moreover, regarding renal function, a significant degree of protection was only seen when AT was combined with DA. These results suggest that when AT is used, even at a moderate dosage, it might be possible to prevent the occurrence of organ dysfunction in patients with severe sepsis but only when used in combination with DA; however, the question whether this treatment improves the survival was remained to be answered, this important query should be examined in the next step.
In conclusion, these findings based on an experimental animal model of severe sepsis suggest that DA, a highly specific AT-dependent inhibitor of FXa, in combination with AT, at a moderate and cost-effective dosage, may have a synergistic effect in protecting major organ function without increasing the risk of bleeding.
References
Iba T, Kidokoro A, Yagi Y (1998) The role of the endothelium in changes in procoagulant activity. J Am Coll Surg 87:321–329
Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R (2001) Proteinase-activated receptors. Pharmacol Rev 53:245–282
Freeman BD, Zehnbauer BA, Buchman TG (2003) A meta-analysis of controlled trials of anticoagulant therapies in patients with sepsis. Shock 20:5–9
Matthay MA (2001) Severe sepsis: a new treatment with both anticoagulant and anti-inflammatory properties. N Engl J Med 344:759–762
Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ Jr (2001) Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 344:699–709
Oelschlager C, Romisch J, Staubitz A, Stauss H, Leithauser B, Tillmanns H, Holschermann H (2002) Antithrombin III inhibits nuclear factor k B activation in human monocytes and vascular endothelial cells. Blood 99:4015–4020
Iba T, Kidokoro A (2002) High dose antithrombin therapy for sepsis: mechanism of action. Shock 18:389–394
Warren BL, Eid A, Singer P, Pillay SS, Carl P, Novak I, Chalupa P, Atherstone A, Penzes I, Kubler A, Knaub S, Keinecke HO, Heinrichs H, Schindel F, Juers M, Bone RC, Opal SM, KyberSept Trial Study Group (2001) High-dose antithrombin III in severe sepsis. A randomized controlled trial. J Am Med Assoc 286:1869–1878
Iba T, Kidokoro A (2004) What can we learn from the three mega-trials using anticoagulants in severe sepsis? Shock 22:508–512
Dickneite G, Leithauser (1999) Influence of antithrombin III on coagulation and inflammation in porcine septic shock. Arterioscler Thromb Vasc Biol 19:1566–1572
Uchiba M, Okajima K, Murakami K (1998) Effects of various doses of antithrombin III on endotoxin-induced endothelial cell injury and coagulation abnormalities in rats. Thromb Res 89:233–241
Danhof M, de Boer A, Magnani HN, Stiekema JC (1992) Pharmacokinetic considerations on orgaran (ORG10172) therapy. Haemostasis 22:73–78
Iba T, Kidokoro S, Fukunaga M, Ogasawara T, Kato H (2004) Antithrombin/danaparoid sodium combination therapy is effective for septic organ dysfunction. J Jpn Soc Intensive Care Med 11:193–199
Iba T, Kidokoro A, Fukunaga M, Fuse S, Suda M (2003) Antithrombin modulates the leukocyte-endothelial cell interaction in the staphylococcal enterotoxin B-challenged mouse. J Trauma 55:546–550
Wenzel RP (2003) Treating sepsis. N Engl J Med 347:966–967
Bauer PR (2002) Microvascular responses to sepsis: clinical significance. Pathophysiology 8:141–148
Iba T, Kidokoro A, Fukunaga M, Fuse S, Suda M, Kunitada S, Hara T (2002) Factor Xa-inhibitor (DX-9065a) modulates the leukocyte-endothelial cell interaction in endotoxemic rat. Shock 17:159–162
Iba T, Kidokoro A, Fukunaga M, Nagakari K, Shirahama A, Ida Y (2005) Activated protein C improves the visceral microcirculation by attenuating the leukocyte-endothelial interaction in a rat LPS model. Crit Care Med 33:368–372
Buller HR, ten Cate JW (1989) Acquired antithrombin III deficiency: laboratory diagnosis, incidence, clinical implications and treatment with antithrombin III concentrate. Am J Med (Suppl 3B):44s–48s
Meuleman DG (1992) Orgaran (Org10172): its pharmacological profile in experimental models. Haemostasis 22:58–65
Camerer E, Huang W, Coughlin SR (2000) Tissue factor-and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc Natl Acad Sci USA 97:5255–5260
Kanke T, Macfarlane SR, Seatter MJ, Davenport E, Paul A, McKenzie RC, Plevin R (2001) Proteinase-activated receptor-2-mediated activation of stress-activated protein kinases and inhibitory kappa B kinases in NCTC 2544 keratinocytes. J Biol Chem 276:31657–31666
Iba T, Kidokoro A, Fukunaga M, Ogasawara T, Kato H (2004) Antitirombin/danaparoid sodium combination therapy is effective for organ dysfunction induced by endotoxin. J Jpn Soc Intensive Care Med 11:193–199
Pulletz S, Lehmann C, Volk T, Schmutzler M, Ziemer S, Kox WJ, Scherer RU (2000) Influence of heparin and hirudin on endothelial binding of antithrombin in experimental thrombinemia. Crit Care Med 28:2881–2886
Yamauchi T, Umeda F, Inoguchi T, Nawata H (1989) Antithrombin III substitutes prostacyclin production by cultured aortic endothelial cell. Biochem Biophys Res Commun 163:1404–1411
Kozek-Langenecker SA, Spiss CK, Michalek-Sauberer A, Felfernig M, Zimpfer M (2003) Effect of prostacyclin on platelets, polymorphonuclear cells, and heterotypic cell aggregation during hemofiltration. Crit Care Med 31:988–989
Hoffmann JN, Vollmar B, Laschke MW, Inthorn D, Kaneider NC, Dunzendorfer S, Wiedermann CJ, Romisch J, Schildberg FW, Menger MD (2002) Adverse effect of heparin on antithrombin action during endotoxemia: microhemodynamic and cellular mechanisms. Thromb Haemost 88:242–252
Iba T, Kidokoro A, Fukunaga M (2001) Leukocyte and platelet adhesion induce microcirculatory disturbance in endotoxemic organ dysfunction. Jpn J Thromb Hemost 12:32–38
Iba T, Kidokoro A, Fukunaga M (2003) The mechanism of thrombocytopenia during sepsis; from the site? of microcirculatory disturbance. J Jpn Biomed Forum 13:43–46
Ley K, Cerrito M, Arfors KE (1991) Sulfated polysaccharides inhibit leukocyte rolling in rabbit mesentery venules. Am J Physiol 260:H1667–H1673
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iba, T., Kidokoro, A., Fukunaga, M. et al. Antithrombin ameliorates endotoxin-induced organ dysfunction more efficiently when combined with danaparoid sodium than with unfractionated heparin. Intensive Care Med 31, 1101–1108 (2005). https://doi.org/10.1007/s00134-005-2707-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-005-2707-0