Abstract
The object of this study was to evaluate in vitro the influence of various ventilatory parameters on the delivery of synchronized nebulization of terbutaline during mechanical ventilation and to determine a semiempirical model to control the quantity of aerosol delivered into the patient’s lung. An ATOMISOR NL9 M jet nebulizer (La Diffusion Technique Française, France) was filled with terbutaline (Bricanyl, Astra-Zeneca, Sweden) and connected to the inspiratory line of a Horus ventilator (Taema, France). Nebulization was synchronized with the inspiratory phase. We assessed at the end of the endotracheal tube the quantity of terbutaline (terbutaline mass output) and the volume median diameter (VMD) by diffraction-laser method. There was a negative correlation between terbutaline mass output and inspiratory air flow ( r =−0.95, p <0.0001) and between VMD and inspiratory air flow ( r =−0.96, p <0.0001). Moreover, positive end-expiratory pressure levels between 0 cm and 8 cm of water did not significantly change the terbutaline output mass ( p =0.22). Total nebulization time and terbutaline mass output calculated by the mathematical model showed good agreement with experimental data. In conclusion, our semiempirical model allows calculation of the duration of the nebulization required to deliver a given mass of terbutaline into patient lungs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Jet nebulizers are widely used in medicine to deliver solute solutions in the form of aerosols into the respiratory tract. In mechanically ventilated patients, the aerosol is transported throughout a complex circuit consisting of an inspiratory line, a connection for the nebulizer, an interface (endotracheal tube or facial mask) to the patient and an expiratory line. This complex circuit decreases aerosol delivery to the patient, because part of the aerosol produced by the nebulizer is lost into the circuit. On the other hand, nebulization during mechanical ventilation in intubated patients offers the advantage, compared with aerosol delivery in spontaneously ventilated patients, of preventing aerosol deposition in the extrathoracic region and to some extent of standardizing the ventilatory pattern during aerosol delivery.
A number of studies have been performed to characterize and optimize aerosol delivery to the patient during mechanical ventilation, taking into account both the circuit and the ventilatory settings.
The circuit aspect is the easiest part of the problem, as it can be standardized and optimized for any patient’s condition. Connecting the nebulizer to the inspiratory circuit 10—30 cm above the endotracheal tube (ETT) increases aerosol delivery [1, 2]. Reducing ETT diameter to less than 7 mm results in decreasing aerosol delivery due to particle impaction in the ETT, and this is particularly true with pediatric ETT [3]. In comparison with continuous nebulization, nebulization synchronized with the inspiratory phase increases the quantity of aerosol produced, because it limits aerosol loss during expiratory phases [4].
The effects of ventilatory settings on nebulization efficiency have also been extensively studied, but they are more difficult to handle because they depend in part on the patient’s condition. Tidal volume (VT) higher than 500 ml is recommended for adult ventilation to allow aerosol delivery to peripheral airways [5]. A minimum of 0.3 for the ratio between inspiratory time and total time (Ti/Ttot ratio) is recommended [2, 5–8]. Decreasing inspiratory flow (V’) results in increasing aerosol delivery [7, 9]. Heating and humidifying gas in the circuit divides the quantity of aerosol delivered to the patient approximately by a factor of 2 [7, 9, 10].
First in this study, we evaluated in vitro the influence of ventilatory parameters on the efficiency of terbutaline nebulization in a circuit optimized for aerosol delivery. The efficiency of nebulization was expressed as the terbutaline mass output (total mass of terbutaline reaching the extremity of the ETT at the end of the nebulization) and the particle-size distribution. The effects of VT, respiratory rate (f) and Ti/Ttot on these parameters were evaluated. Furthermore, the influence of positive end expiratory pressure (PEEP) and fill volume on aerosol output was assessed. Statistical analyses were performed to determine the relationships between the ventilatory parameters and efficiency in terbutaline nebulization.
Second in this study, we hypothesized that the quantity of aerosol produced at the extremity of the ETT can be predicted and, therefore, targeted for a range of ventilatory parameters (targeted mass of drug). A semiempirical model was then constructed with the aim of determining the nebulization time (targeted nebulization time) required to deliver a given mass of terbutaline (the targeted terbutaline mass) for a known ventilatory condition. This was achieved by applying a statistical approach to the data collected from the first part of this study.
Materials and methods
Experimental model
The influence of ventilatory parameters on terbutaline nebulization during mechanical ventilation was evaluated using an optimized setup, taking into account the parameters described above.
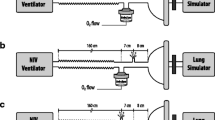
A Horus ventilator (Taema, France) was used with the ATOMISOR NL9 M nebulizer (Diffusion Technique Française, Saint Etienne, France) connected to its inspiratory line via a T-piece, 20 cm upstream from the Y-piece.
An 8 mm ETT was connected downstream to the Y-piece (Fig. 1). The nebulizer was filled with terbutaline (Bricanyl, Astra-Zeneca, Sweden) and fed by air at a 6 l/min flow rate. Nebulization was synchronized with the inspiratory phase of the mechanical breath. The Horus ventilator delivers a square wave-flow pattern. V’ is compensated for the addition of gas coming though the nebulizer. The gas mixture (fraction of inspired oxygen [FiO2] 21%) delivered through the circuit was neither heated nor humidified.
Experimental steps
-
1.
The effects of V’ on terbutaline mass output and particle-size distribution were assessed using eight different ventilatory settings (Table 1). At this step, the PEEP was 0 cm H2O, and the nebulizer was filled with 10 mg/4 ml of terbutaline.
Table 1 Influence of tidal volume ( VT), respiratory rate ( f) and duty cycle ( Ti/Ttot) on the terbutaline output fraction and the particle-size distribution ( VMD volume median diameter) -
2.
The effect of PEEP on terbutaline mass output was assessed at four levels of PEEP (0 cm, 4 cm, 6 cm, 8 cm H2O). The ventilator was set at VT of 600 ml, f of 12/min, and Ti/Ttot of 0.5, and the nebulizer was filled with 10 mg/4 ml of terbutaline.
-
3.
The effect of nebulizer fill volume on terbutaline mass output was assessed with two different terbutaline charges (10 mg/4 ml and 10 mg/8 ml). The ventilator was set at VT of 600 ml, f of 12/min, and Ti/Ttot of 0.5. To increase the amount of data for statistical testing of PEEP effect and nebulizer fill-volume effect, each terbutaline charge was performed at PEEP 0 cm, 4 cm, 6 cm and 8 cm H2O.
Terbutaline mass output measurement
An absolute filter (A/E, Gelman, Ann Arbor, USA) was placed between the end of the ETT and the lung model whose compliance was set at 24±3 ml/cm H2O (Fig. 1). Filters were changed every 6 min until the beginning of the sputtering. Another filter was used from the beginning of sputtering to the end of the nebulization (no aerosol production by visual and aural examination). The duration of nebulization until no aerosol production defines the total nebulization time (TNT). A final filter was used for 6 min after the end of the nebulization to verify the absence of aerosol production. After nebulization, the filters were placed in 30 ml NaOH at 0.1 N and centrifuged for 15 min at 4,500 g. The desorbed terbutaline mass was determined using a spectrophotometer at 243 nm.
The terbutaline mass output was calculated by summing the terbutaline mass collected on each of the filters until the end of the aerosol production. The terbutaline output fraction was calculated by the ratio between the terbutaline output mass and the terbutaline nebulizer charge. The terbutaline output flow rate was the ratio between the terbutaline mass collected on one filter and the filtering time for this same filter.
Particle size distribution measurement
The particle size distribution was measured with a laser diffraction method (Mastersizer X, Malvern, UK). The end of the ETT was placed 1 cm above the laser beam and 1 cm to the side of the lens. The aerosol produced at the end of the ETT was collected by a vacuum pump placed 1 cm from the laser beam. Particle size distribution was therefore measured in an open model, which differs from the closed model made by the ETT introduced into the bronchial tree of a patient in clinical practice. Indeed, we hypothesized that the particle size distribution is not influenced by the pressure in the circuit.
The volume median diameter (VMD) of the aerosol produced at the end of the ETT was measured continuously during consecutive time intervals of 6 min until the end of nebulization.
Semiempirical model for aerosol delivery
The aim of this semiempirical model was to be able to determine the nebulization time (the targeted nebulization time) to administer a certain mass of terbutaline (the targeted terbutaline mass) for any given ventilatory condition. This was achieved by applying a statistical approach to the data collected from the experiments described above (Table 2).
The semiempirical model was based on the two following equations:
The targeted nebulization time was modeled with the experimental data. The targeted terbutaline mass that depends on the clinical condition was chosen a priori. Determination of terbutaline output flow rate was based on the correlation between terbutaline output flow rate experimental data and the ratio (Ti/Ttot)/V’.
We also verified that the model was accurate in boundary conditions, i.e., the targeted nebulization time was less than or equal to the total nebulization time (targeted nebulization time ≤ total nebulization time).
The model of the total nebulization time (TNT) was obtained as follows: For a nebulization lasting until the end of aerosol production, the duration of the aerosol generation (Tgeneration) is the product of TNT and Ti/Ttot (Tgeneration = TNT(Ti/Ttot)), thus TNT = Tgeneration / (Ti/Ttot). For a jet nebulizer, the aerosol drug-flow output of the nebulizer (Qneb) is the ratio between the drug mass produced (Mdrug) and Tgeneration (Qneb = Mdrug / Tgeneration), thus, Tgeneration = Mdrug/Qneb. For a nebulization lasting until the end of aerosol production, Mdrug is the difference between nebulizer charge (Mcharge) and the residual mass in the nebulizer (Mresidual) (Mdrug = Mcharge −Mresidual). Thus, TNT in mechanical ventilation can be calculated with this equation:
Qneb given by the manufacturer of the ATOMISOR NL9 M jet nebulizer (VMD=3.9 µm and output rate=0.26 ml/min for operating continuously at 6 l/min) amounted to 0.65 mg of terbutaline/min. Mresidual for 10 mg/4 ml terbutaline nebulizer charge measured by spectrophotometry amounted to 3.3 mg. The numerical application to calculate TNT was, therefore, TNT =10.3/(Ti/Ttot).
Statistical analysis
Values were expressed as median. Correlations between terbutaline output fraction and V’, terbutaline output flow rate and Ti/Ttot, terbutaline output flow rate and V’, VMD and V’, and TNT and Ti/Ttot were tested using Spearman’s correlation test. Statistical differences between the terbutaline output fractions among the different PEEP and between the terbutaline output fractions among the different nebulizer charges were tested by the analysis of variance (ANOVA) exact test for general scores. Linear regression analysis was made using least-square fitting. A p value less than 0.05 was considered statistically significant. The statistical analysis was performed with the StatXact software program (Cytel Software, USA, v. 3.0.2, 1995).
Results
Effects of inspiratory flow (Table 1) (step 1)
There was a negative correlation between the terbutaline output fraction and V’ ( r =−0.75, p <0.0001, Spearman correlation exact test). The optimal terbutaline output fraction was obtained with the lowest V’ obtained with VT=600 ml, f=12/min, Ti/Ttot=0.5.
There was a positive correlation between terbutaline output flow rate and Ti/Ttot ( r =0.96, p <0.0001, Spearman correlation exact test). There was a negative correlation between terbutaline output flow rate and V’ ( r =−0.95, p < 0.0001, Spearman correlation exact test).
There was a negative correlation between the VMD and V’ (r =−0.96, p <0.0001, Spearman correlation exact test). The largest VMD was obtained with the lowest V’ obtained using VT =600 ml, f=12/min, Ti/Ttot=0.5.
There was a negative correlation between TNT and Ti/Ttot ( r =−0.96, p <0.0001, Spearman correlation exact test).
Effects of PEEP (step 2)
For 10 mg/4 ml of terbutaline filled in the nebulizer, terbutaline output fractions were 44%, 44%, 44%, and 46% for PEEP levels at 0 cm, 2 cm, 4 cm, and 6 cm water, respectively (VT 600 ml and f 12/min, Ti/Ttot 0.5). There were no statistical differences between the terbutaline output fractions for the different PEEP ( p =0.66, n =12, ANOVA exact test for general scores).
For 20 mg/8 ml of terbutaline filled in the nebulizer, terbutaline output fractions were 52%, 50%, 49%, and 52% for PEEP levels at 0 cm, 2 cm, 4 cm, 6 cm water, respectively. There were no statistical differences between the terbutaline output fractions for the different PEEP levels ( p =0.22, n =12, ANOVA exact test for general scores).
Effects of terbutaline volume charge (step 3)
The median terbutaline output fractions of terbutaline were 45% and 51% for 10 mg/4 ml and 20 mg/8 ml terbutaline filled in the nebulizer, respectively. There was a statistical difference between the terbutaline output fractions for the two different volume charges ( p =0.0002, n =12, permutation test for paired samples).
Terbutaline mass output semiempirical model
There was a strong correlation between the cumulative inhaled mass and the nebulization time for each V’ ( y = ax, r >0.90, the constant being governed by V’). By linear regression analysis, there was a strong correlation between terbutaline output flow rate ( y in the equation) and the ratio (Ti/Ttot)/V’ ( x in the equation) ( y =6.28 x +0.03, r =0.97, p <0.00001) (Fig. 2).
Furthermore, TNT calculated by the mathematical model (TNT=10.3/(Ti/Ttot)) showed good agreement with the experimental data ( r =0.98, p <0.00001) (Fig. 3).
Discussion
In this study, we have: (1) characterized the effect of the most relevant ventilatory parameters on aerosol delivery in an in vitro model of mechanical ventilation using a synchronized jet nebulizer; and (2) described a semiempirical model allowing the targeting of nebulization time to deliver a known amount of terbutaline with a nebulizer characterized in terms of its residual volume and output rate. Furthermore, the semiempirical model allowed us to validate some hypotheses about the effect of ventilatory parameters on aerosol kinetics.
The optimal terbutaline mass output was obtained with the lowest V’ that amounted to 0.20 l/s. But low flow may not be readily tolerated by non-sedated patients. Moreover, increasing terbutaline output does not always support the use of lowest inspiratory flows (+13% of terbutaline output for inspiratory flow decreasing from 36 l/min to 14.4 l/min). This result is consistent with previous studies suggesting that V’ may partly explain the particle impaction into the ventilatory circuit. The proportion of aerosol lost in the circuit is composed of the largest particles, and this proportion increases when inspiratory air flow increases. But particle size measurements at the extremity of the ETT appear to be only slightly correlated with terbutaline output. As particle size decreased from 2.4 µm to 1.1 µm, there was only a small, 1–2%, change in terbutaline output. This example shows that the factor-2 decreasing of particle size at the end of the ETT is not a consequence of terbutaline lost by impaction. The mathematical relationship between particle size and mass (mass ≈ρ (4/3)πVMD3, with ρ the density equal to 1 for terbutaline solution) indicates that a particle size decreasing from 2.4 µm to 1.1 µm has as consequence a terbutaline mass output decreasing from 55% to 4%. Thus, particle decreasing at the extremity of the ETT is not only due to particles lost in the circuit but perhaps also to particle evaporation in the circuit (as, conversely, the effect of humidity on particle size growth).
PEEP set between 0 cm and 8 cm H2O did not change terbutaline output fraction nor particle size distribution in our study. This conclusion confirms previous results obtained with an ultrasonic nebulizer used at different PEEP levels [11].
Increasing terbutaline fill volume from 4 ml to 8 ml tended to increase terbutaline output fraction (45% vs 51%) ( p =0.0002, n =12, permutation test for paired samples). These results could be explained by the terbutaline residual mass in the nebulizer. In vitro results in our study showed a lower residual mass in percentage of terbutaline charge for 20 mg/8 ml than for 10 mg/4 ml (22% vs 34%).
Terbutaline output flow rate was constant during nebulization ( r >0.90, p <0.0001 by linear regression). This result is consistent with previous studies [9]. The terbutaline flow output positively correlated with Ti/Ttot ratio ( r =0.96, p <0.0001, Spearman correlation exact test) and negatively correlated with V’ ( r =−0.95, p <0.0001). The positive correlation between terbutaline output flow rate and Ti/Ttot reflects the impact of synchronizing nebulization with inspiratory phase, whereas the negative correlation between terbutaline output flow rate and V’ is explained by the loss of terbutaline into the circuit.
In summary, terbutaline output flow rate can be calculated by the correlation obtained with Ti/Ttot ratio corrected by the aerosol transport factor (V’) (terbutaline output flow rate =6.28 [(Ti/Ttot)/V’] +0.03, r =0.97, n =146, p <0.0001) (Fig. 2). Because this model is based on data analysis, the model is only validated in our experimental conditions, i.e., for terbutaline aerosol generated by the synchronized ATOMISOR NL9 M jet nebulizer in our optimized circuit. However, TNT can be calculated by the mathematical model, which shows good agreement with experimental data (TNT =[(Mcharge −Mresidual) / Qneb] × [1/(Ti/Ttot)], r =0.98, p <0.00001, n =24) (Fig. 3). This mathematical determination of the total nebulization time for nebulization synchronized with the inspiratory phase should therefore be robust enough to be applied with other jet nebulizers, ventilatory patterns and circuits.
Our semiempirical model allows calculation of the duration of the nebulization required to deliver a given mass of terbutaline (terbutaline targeted mass). The equation “terbutaline output flow rate =6.28 [(Ti/Ttot)/V’] +0.03” allows calculation of the terbutaline output flow rate for known ventilatory settings. The targeted nebulization time is the ratio between the terbutaline targeted mass and the calculated terbutaline output flow rate. Table 3 is an example of this application. For example, to administer 2 mg of terbutaline to a patient ventilated with VT of 500 ml, f of 15/min and Ti/Ttot of 0.4, the nebulization time must be 12 min.
This study shows that it is possible to control the drug mass administrated into a model of mechanical ventilation with a known aerosolized drug. A recent study has demonstrated the clinical relevance of such an in vitro approach of aerosol delivery in mechanical ventilation conditions [12]. For one circuit type, one jet nebulizer type, and one type of drug, we have demonstrated that the quantity of aerosol produced at the extremity of the ETT can be predicted and, therefore, targeted for a range of ventilatory parameters. The relevance of our mathematical model has been established in specific conditions in terms of ETT size, flow, temperature and humidification. It could be tested in other conditions to determine the impact of these parameters on the terbutaline output and particle size distribution.
The potential of our semiempirical approach to predict aerosol delivery is easy enough to be tested with any other combination ventilation / nebulizer.
Abbreviations
- VMD :
-
Volume median diameter
- ETT :
-
Endotracheal tube
- f :
-
Respiratory rate
- Mcharge :
-
Drug mass charged in the nebulizer
- Mdrug :
-
Aerosol drug mass output of the nebulizer
- Mresidual :
-
Residual mass in the nebulizer
- PEEP :
-
Positive end expiratory pressure
- Qneb :
-
Aerosol drug flow mass output of the nebulizer
- Tgeneration :
-
Duration of the effective aerosol generation
- Ti/Ttot :
-
Ratio between inspiratory time and total time
- TNT :
-
Total nebulization time until at the absence of aerosol production
- V’ :
-
Inspiratory flow
- VT :
-
Tidal volume
References
Harvey CJ, O’Doherty MJ, Page CJ, Thomas SH, Nunan TO, Treacher DF (1995) Effect of a spacer on pulmonary aerosol deposition from a jet nebuliser during mechanical ventilation. Thorax 50:50–53
O’Doherty MJ, Thomas SH, Page CJ, Treacher DF, Nunan TO (1992) Delivery of a nebulized aerosol to a lung model during mechanical ventilation. Effect of ventilator settings and nebulizer type, position, and volume of fill. Am Rev Respir Dis 146:383–388
Ahrens RC, Ries RA, Popendorf W, Wiese JA (1986) The delivery of therapeutic aerosols through endotracheal tubes. Pediatr Pulmonol 2:19–26
Pelkonen AS, Nikander K, Turpeinen M (1997) Jet nebulization of budesonide suspension into a neonatal ventilator circuit: synchronized versus continuous nebulizer flow. Pediatr Pulmonol 24:282–286
Dhand R, Tobin MJ (1997) Inhaled bronchodilator therapy in mechanically ventilated patients. Am J Respir Crit Care Med 156:3–10
Fink JB, Tobin MJ, Dhand R (1999) Bronchodilator therapy in mechanically ventilated patients. Respir Care 44:53–69
Fink JB, Dhand R, Grychowski J, Fahey PJ, Tobin MJ (1999) Reconciling in vitro and in vivo measurements of aerosol delivery from a metered-dose inhaler during mechanical ventilation and defining efficiency-enhancing factors. Am J Respir Crit Care Med 159:63–68
Hess DR, Dillman C, Kacmarek RM (2003) In vitro evaluation of aerosol bronchodilator delivery during mechanical ventilation: pressure-control vs volume control ventilation. Intensive Care Med 29:1145–1150
Diot P, Morra L, Smaldone GC (1995) Albuterol delivery in a model of mechanical ventilation. Comparison of metered-dose inhaler and nebulizer efficiency. Am J Respir Crit Care Med 152:1391–1394
O’Riordan TG, Greco MJ, Perry RJ, Smaldone GC (1992) Nebulizer function during mechanical ventilation. Am Rev Respir Dis 145:1117–1122
Williams L, Fletcher GC, Daniel M, Kinsella J (1999) A simple in vitro method for the evaluation of an ultrasonic nebulizer for drug delivery to intubated, ventilated patients and the effect of nebulizer and ventilator settings on the uptake of fluid from the nebulizer chamber. Eur J Anaesthesiol 16:479–484
Miller DD, Amin MM, Palmer LB, Shah AR, Smaldone GC (2003) Aerosol delivery and modern mechanical ventilation: in vitro/in vivo evaluation. Am J Respir Crit Care Med 168:1205–1209
Acknowledgment
We wish to thank John Fonseca (Pulmomed, France) for reviewing the English
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vecellio, L., Guérin, C., Grimbert, D. et al. In vitro study and semiempirical model for aerosol delivery control during mechanical ventilation. Intensive Care Med 31, 871–876 (2005). https://doi.org/10.1007/s00134-005-2594-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-005-2594-4