Abstract
Objective
To assess the immune competence of patients presenting with septic shock by measuring on-line the production of intracellular cytokines by circulating leukocytes.
Design and setting
Prospective study in a 18-bed medical intensive care unit of a university hospital.
Patients and participants
21 patients with septic shock, and 11 volunteers.
Interventions
Single-step isolation of leukocytes from whole blood obtained within the first 24 h after admission. Leukocytes were fixed immediately or after treatment with lipopolysaccharide (LPS) and/or heterologous plasma.
Measurements and results
Leukocytes were permeabilized, and the intracellular cytokine expression of TNF-α and IL-10 was quantified by immunostaining and flow cytometry. LPS treatment significantly increased monocyte intracellular cytokine TNF-α and IL-10 as well as lymphocyte intracellular cytokine IL-10 in normal leukocytes. Septic monocytes and granulocytes had nonstimulated intracellular cytokine TNF-α concentrations lower than those measured in volunteers and were severely hyporesponsive to LPS. These phenotypic changes were correlated with disease severity and could be reproduced by treatment of normal leukocytes with plasma from patients with septic shock.
Conclusions
Intracellular cytokine staining is a simple and rapid method to assess in situ and on-line the inflammatory balance and responsiveness of leukocyte subpopulations and could therefore represent a useful monitoring tool to assess the immune competence of critically ill patients. This study identifies the cellular source of cytokines in whole blood and confirms prior reports showing that septic phagocytes are characterized by a predominant anti-inflammatory phenotype, with hyporesponsiveness to LPS, depending on a plasma deactivation factor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is the leading cause of death in the intensive care unit, with mortality higher than 40% in patients with septic shock (SS) [1, 2, 3, 4]. The activation of innate biological cascades in response to infection is a key pathogenic element of sepsis [4, 5]. The hypothesis that severe sepsis results from a “malignant systemic inflammation” has been challenged by the failure of clinical trials with cytokine inhibitors [6]. The net inflammatory response was actually found to be anti-inflammatory in the vascular compartment and can lead to immune suppression [4, 5, 7]. This acquired “immunoparalysis” may represent a risk factor for secondary infections and could contribute to the late mortality of sepsis [8, 9]. The assessment of the immunological status of critically ill patients will become increasingly important in the near future with the development of immunomodulatory therapies. There is therefore the need for the development of new laboratory tests that can assess the immune competence of critically ill patients. These tests should ideally reflect leukocyte responsiveness to bacterial products such as endotoxin, be easy to perform, and yield reproducible and rapid results.
The inflammatory activity of biological fluids results from the local interactions between pro- and anti-inflammatory cytokines and their inhibitors. This activity is best quantified using bioassays, and the measurement of cytokine concentrations is usually a poor reflection of their activity [7, 10, 11]. Tumor necrosis factor (TNF) α, a proinflammatory cytokine, plays a role in the early phase of endotoxemia and sepsis. Interleukin (IL)-10, an anti-inflammatory cytokine, is implicated in the monocyte deactivation observed during SS. Both are considered major mediators of the immune response against infection [12, 13, 14, 15, 16]. The plasma TNF-α/IL-10 ratio has been used to estimate the pro-/anti-inflammatory balance and is correlated with outcome in febrile and infected patients [17, 18, 19]. However, the plasma concentrations of these cytokines reflect their global production and metabolism throughout the body but are not indicative of their production in vivo, for example, by circulating leukocyte subpopulations, which is largely unknown [20].
The aims of our study were: (a) to determine the cellular source of TNF-α and IL-10 production in circulating leukocytes and (b) to develop an on-line assay based on intracellular (IC) cytokine measurement in circulating leukocytes that allows one to quantify the immune status of patients with septic shock, without the need to purify leukocyte subpopulations.
Material and methods
Patients and subjects
The study was conducted in the 18-bed medical intensive care unit of a university hospital and included 21 patients with SS [16 men, 5 women; median age 69 years, interquartile range (IQR) 16.5] and 11 healthy subjects (six men, five women; median age 35 years, IQR 3.4; Table 1). The protocol was approved by the ethics committee of our institution. Adult patients were prospectively enrolled after informed consent (obtained from the subjects, the patient or the next of kin) if they were admitted for SS according to criteria described elsewhere [1]. HIV-positive patients or patients receiving immunosuppressive drugs (including steroids) were excluded. We prospectively collected demographic data, Simplified Acute Physiology Score (SAPS) II [21], microbiological data, chemical, hematology and coagulation test results, therapeutic interventions, complications, number of organ dysfunction, length of ICU stay, and outcome. Patients’ median SAPS II was 59 points (IQR 19), and the median length of ICU stay was 9.0 days (IQR 12.5). ICU mortality was 48%.
Blood sampling and leukocyte preparation
Blood was collected in patients using an arterial catheter during the first 24 h after admission to the ICU and by direct venous puncture in healthy volunteers, and heparinized. Plasma was isolated by centrifugation (500 g, 10 min) and stored at −70°C until processed. Leukocytes were isolated using gelatin (Physiogel, Braun Medical Switzerland, 1/1 v/v) sedimentation for 30 min, followed by fixation with 1% formaldehyde and osmotic lysis of contaminating erythrocytes (FACS Lysing solution, Becton Dickinson, San Jose, Calif., USA).
Quantification of intracellular cytokine levels by flow cytometry
Fixed leukocytes were permeabilized with IC Perm Buffer (Biosource, Camarillo, Calif., USA) supplemented with 4% bovine serum albumin (Sigma, St. Louis, Mo., USA). Cells were stained with phycoerythrin-labeled monoclonal antibodies against human TNF-α (mouse IgG2a, clone 2B3, Biosource) and against human IL-10 (rat IgG1, clone 9D7, Biosource) or with their corresponding phycoerythrin-labeled isotype controls. Cells were then washed three times with IC Perm, stained with a fluorescein isothiocyanate labeled anti-CD14 monoclonal antibody (mouse IgG2b, clone MÖP9, Becton Dickinson), and analyzed using a FACScan analyzer (Becton Dickinson). Leukocyte subpopulations (monocytes, granulocytes, and lymphocytes) were gated according to their side scatter characteristics and CD14 positive staining. Results were expressed as the mean fluorescence index (MFI), defined as the ratio of the geometric mean fluorescence of the specific antibody over its isotype control. A MFI value greater than 1 was considered as a significant IC expression of the cytokine.
Lipopolysaccharide stimulation
After gelatin sedimentation 106 leukocytes/ml were suspended in culture medium (RPMI supplemented with 10% fetal calf serum, Invitrogen, Carlsbad, Calif., USA). Cells were treated with 200 ng/ml Escherichia coli O111:B4 lipopolysaccharide (LPS; List Biological Laboratories, Campbell, Calif., USA), in the presence of a protein transport inhibitor (Brefeldin A, Sigma). After cell fixation and immunostaining as described above IC TNF-α and IL-10 were measured by flow cytometry. In three volunteers a time course of LPS stimulation (2, 4, and 6 h) was performed using the same protocol. The IC localization of the fluorescence after 6-h of LPS stimulation was also confirmed with confocal microscopy by staining with unconjugated anti-TNF-α or anti-IL-10 monoclonal antibodies and an Alexa 488-conjugated secondary goat anti-murine IgG antibody (Molecular Probes, Eugene, Ore., USA).
Treatment of normal leukocytes with “septic” and “normal” heterologous plasma
To ensure similar experimental conditions leukocytes from a single healthy donor were isolated by gelatin sedimentation and treated with plasma from patients and volunteers in a single experiment. Twenty-four plasma samples (18 patients and 6 volunteers) were randomly selected and tested. Leukocytes (106/ml) were incubated at 37°C for 24 h in RPMI containing 50% of heterologous plasma and a protein transport inhibitor. One-half of the leukocytes were then fixed, and the other one-half was stimulated with LPS (4 h) in the presence of the protein transport inhibitor.
Measurement of plasma cytokine concentrations
TNF-α and IL-10 levels were measured in all plasma samples using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Medgenics, Northridge, Calif., USA).
Statistics
Results are expressed as median values with IQR. Correlations were analyzed by Spearman’s correlation test. The Mann-Whitney U test was used for nonnormally distributed variables. Kinetics experiments results are expressed with mean ±SEM and analyzed by analysis of variance for repeated measures.
Results
Time-course studies of intracellular TNF-α and IL-10 levels in normal leukocytes stimulated with LPS
These experiments were performed with the leukocytes from three volunteers. After LPS treatment there was a progressive time-dependent (Fig. 1, left panels) and LPS dose-dependent (not shown) accumulation of IC TNF-α in monocytes (analysis of variance for repeated measurements, p<0.0001) and to a lesser extent in granulocytes (p<0.0001). The MFI level at the end of the 6-h LPS stimulation period was higher in monocytes (median 3.2, IQR 0.8) than in granulocytes (1.2, IQR 0.2). No significant increase in fluorescence was detected in lymphocytes (Fig. 1A, left panel). After LPS treatment a time-dependent and LPS dose-dependent IC accumulation of IL-10 was detected in monocytes and lymphocytes (p<0.0001). The MFI levels at the end of the stimulation period were higher in lymphocytes (3.1, IQR 0.6) than in monocytes (2.7, IQR 0.5). A small increase in IC levels of IL-10 was also measured in granulocytes but at a nonsignificant MFI ratio less than 1 (0.5 IQR 0.1, Fig. 1B, left panel). These time-course experiments were completed with confocal immunofluorescence microscopy studies (after 6 h of LPS treatment) which showed a characteristic IC distribution of both cytokines in permeabilized leukocytes, predominantly in monocytes (Fig. 1, left panels).
Intracellular accumulation of cytokines in normal leukocytes (squares monocytes, circles granulocytes, triangles lymphocytes) stimulated with 200 ng/ml of LPS, and detected by flow cytometry using fluorescent anti-cytokine monoclonal antibodies in permeabilized cells. Results representative of one experiment out of three. A Left Time course of intracellular TNF-α production in monocytes, granulocytes, and lymphocytes stimulated with LPS, expressed as fold-induction of MFI; right confocal microscopy image of leukocytes stained with a fluorescent anti-TNF-αmonoclonal antibody. B Left Time course of intracellular IL-10 production in monocytes, granulocytes, and lymphocytes stimulated with LPS; right confocal microscopy images of leukocytes stained with an anti-IL-10 monoclonal antibody
Intracellular levels of TNF-α and IL-10 in nonstimulated leukocytes (basal level)
A significant basal level of IC TNF-α expression was detected in monocytes and less markedly in granulocytes from the 11 volunteers (Fig. 2A). The IC TNF-α expression was considered not to be significant in lymphocytes. In leukocytes from the same volunteers a significant basal level of IC IL-10 expression was detected in lymphocytes and, less importantly, in monocytes (Fig. 2B). The ratio between basal TNF-α and IL-10 was higher in granulocytes than in monocytes (Fig. 2C). When leukocytes from patients with SS and those from volunteers were compared, the basal IC TNF-α level was found to be significantly lower in monocytes and granulocytes from patients, but not different in lymphocytes (Fig. 2A). The basal IC IL-10 was not significantly different in leukocytes in patients and volunteers (Fig. 2B). Consequently the basal TNF-α/IL-10 ratio was significantly lower in monocytes and granulocytes from patients with SS than in leukocytes from healthy volunteers (Fig. 2C).
LPS responsiveness of circulating leukocytes from patients with SS compared to volunteers
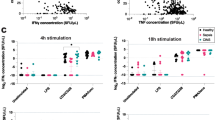
After 4 h of LPS treatment IC TNF-α levels significant increased in monocytes and granulocytes from volunteers but not in leukocytes from patients with septic shock (Fig. 3A). LPS induced a significant increase in the IC expression of IL-10 in monocytes and lymphocytes from volunteers but not in septic leukocytes (Fig. 3B). The LPS-induced TNF-α/IL-10 ratio was significantly lower in monocytes and granulocytes from patients with septic shock than in leukocytes from volunteers (Fig. 3C).
LPS-induced intracellular expression of cytokines of circulating leukocytes in volunteers (n=11) and in patients with septic shock (n=21). A TNF-α. B IL-10. C TNF-α/IL-10 ratio. A, B Results are expressed as percentage increase in MFI, with the basal level of expression before LPS stimulation for each sample as reference. C Results are expression as the MFI ratio of TNF-α/IL-10
Plasma levels of cytokines
The plasma concentration of TNF-α was found to be significantly higher in patients with septic shock than in volunteers (data not shown). Plasma IL-10 levels were elevated in the majority of patients and were undetectable in all volunteers (data not shown). No significant correlation was found between plasma and IC levels for TNF-α and IL-10.
LPS responsiveness of leukocytes of healthy volunteers incubated with heterologous plasma
After a 24-h incubation with plasma the IC levels of TNF-α and IL-10 did not differ significantly between leukocytes cultured in the presence of plasma from patients with SS and cultured in plasma from volunteers (data not shown). LPS treatment of leukocytes incubated with “normal” plasma induced an increase in IC monocyte TNF-α levels similar to that observed in previous experiments with leukocytes from volunteers (Fig. 4A). In sharp contrast, monocytes exposed for 24 h to plasma from patients with SS became unresponsive to the LPS challenge (Fig. 4A). The magnitude of inhibition of “normal monocytes” by “septic” plasma was similar to that observed with freshly isolated monocytes obtained from patients with SS. Granulocytes reacted in a similar way as monocytes and were “deactivated” by plasma from patients with septic shock but not by plasma from healthy subjects (Fig. 4A). No significant effect of plasma was observed on lymphocytes (Fig. 4A). LPS also induced a significantly higher IC IL-10 expression in monocytes and lymphocytes incubated with normal plasma (Fig. 4B).
Effect of 24-h preincubation of leukocytes from one volunteer with heterologous plasma from healthy subjects (n=6) and plasma from patients with septic shock (n=18) on the LPS-induced intracellular production of cytokines. A TNF-α. B IL-10. The results are expressed as percentage increase in MFI with the basal level of expression before LPS stimulation for each sample as reference. Septic Leukocytes incubated with plasma from patients with septic shock; volunteers leukocytes incubated with plasma from healthy volunteers
Correlation between biological and clinical variables
The IC TNF-α/IL-10 ratio after LPS treatment was lower in granulocytes from patients who died than in patients who survived in the ICU (p=0.02). A high SAPS II score was correlated with a low LPS-induced TNF-α/IL-10 ratio in monocytes (ρ=−0.580, p=0.035) and in granulocytes (ρ=−0.622, p=0.037). A significant correlation was also found between a high SAPS II score and a low LPS-induced IC TNF-α levels in monocytes (ρ=−0.345, p=0.0099) and between a high SAPS II score and a low basal IC granulocytes IC TNF-α levels (ρ=−0.526, p=0.0083). No other significant correlation was found between IC cytokine levels and clinical variables, in particular when considering the nature of the micro-organism or the focus of infection.
Discussion
Using flow cytometry with cell permeabilization, IC immunostaining, and ex vivo LPS stimulation, we showed that: (a) Monocytes are the main source of TNF-α among circulating leukocytes in normal subjects, and that IL-10 is produced by monocytes and lymphocytes. (b) Significantly basal IC TNF-α levels are lower in circulating monocytes and granulocytes from patients with SS than in cells from healthy subjects. (c) Leukocytes from patients with SS are largely unresponsive to LPS stimulation ex vivo. (d) The exposure of leukocytes from healthy subjects to plasma from patients with SS reproduces these phenotypic changes, suggesting the presence of a deactivating factor in the “septic” plasma. Several studies have reported a low IC expression of cytokines in circulating leukocytes of trauma and surgical patients [22, 23, 24]. Here we report the results of the first application of this technique to patients admitted in a medical ICU for a septic shock. In contrast to other studies, we rigorously analyzed all leukocyte subtypes in the same blood sample, measured basal and LPS-stimulated IC cytokines and compared them to those from healthy volunteers. Flow cytometry has been used to detect IC cytokines in various experimental and clinical conditions [25, 26, 27, 28, 29]. With this technique the precise cytokine production by leukocyte subpopulations can be investigated without the need of specific isolation steps. This “direct” and single-step assay greatly reduces the risk of poor purification yields and of artifactual cell activation. It is also a unique method for measuring basal levels of cytokine expression by leukocytes in situ. Finally, keeping all circulating leukocytes populations interacting together in LPS stimulation experiments is more relevant to the in vivo situation. For all these reasons we chose to measure IC TNF-α and IL-10 in circulating leukocytes from patients with SS to quantify their production and assess the balance between a pro- and anti-inflammatory response.
To obtain reproducible results we first had to optimize some important methodological aspects, such as protocols for cell fixation and permeabilization, choice of antibodies working intracellularly, protein transport inhibition, immunostaining, and washing steps [30]. A “positive” IC level of an antigen measured by flow cytometry reflects the specific binding of the anti-cytokine antibody compared to its nonspecific isotype control. This level can be quantified by the MFI, a ratio of fluorescences emitted by the two antibodies. MFI is not an absolute value but only reflects a relative abundance of the IC or cell-associated antigen. Ideally flow cytometry results should be confirmed by another technique. In our experimental conditions we measured the IC cytokine concentration in leukocyte lysates by ELISA, but failed to detect significant levels. This is probably due to the limit of detection of the assay under these conditions, flow cytometry being a more sensitive method. The use of an inhibitor of protein transport during LPS stimulation experiments also made the comparison between IC production detected by flow cytometry and extracellular secretion measured ELISA in the same experiment impossible. However, the results of the time-course and dose response studies performed by flow cytometry are in accordance with ELISA studies measuring the whole-blood production of cytokines after treatment with LPS [31, 32, 33]. Finally, the IC localization of the cytokine stained by the antibody was also confirmed by confocal fluorescence microscopy. Therefore despite these limitations flow cytometry seems to be a reliable and convenient method to quantify the IC expression of cytokines in leukocytes.
An important finding of our study is the lower “basal” and LPS-induced IC expression of TNF-α in monocytes and granulocytes from patients with SS compared than those from volunteers, with a relative preservation of the IL-10 expression. Although the difference was statistically significant, the overlap of values between volunteers and patients precludes the use of this test as a diagnostic parameter for patients with SS. The detection of a basal IC expression of TNF-α and IL-10 in nonstimulated circulating leukocytes from healthy subjects confirms previous reports, and may reflect the observed low plasma TNF-α (approx. 2.5 pg/ml) and IL-10 (approx. 1 pg/ml) levels measured in normal subjects [34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45]. In patients with septic shock the lower level of expression of TNF-α resulted in a lower “basal” TNF-α/IL-10 ratio in monocytes and granulocytes. Moreover, septic monocytes and granulocytes were found to be severely hyporesponsive to an LPS challenge with no significant increase in IC TNF-α expression. This resulted in a markedly lower LPS-induced TNF-α/IL-10 ratio in phagocytes from patients with SS than those from volunteers. Together with a decreased expression of antigen presentation molecules this marked anti-inflammatory phenotype of circulating monocyte is now recognized as a hallmark of the “immune paralysis” described in patients with severe sepsis and septic shock [15, 16, 46].
An “anti-inflammatory” phenotype has also been described in granulocytes isolated from critically ill patients [47, 48, 49, 50, 51]. Although these changes are less marked than in monocytes, they could play a significant role in the sepsis-associated immune dysfunction due to the abundance of granulocytes and their primary role as phagocytes. Interestingly, in our experiments plasma from patients with septic shock rendered normal monocytes and granulocytes hyporesponsive to LPS, confirming the “immunosuppressive” properties of plasma [52]. Many circulating mediators could play a role in this phenomenon and the bioassay described here could be a valuable tool to identify such mediators. Previous reports have shown that the phenotypic changes in monocytes induced by sepsis are partially linked to bioactive circulating IL-10 [15, 16]. Our data show that IL-10 expression is relatively preserved in cells from patients with SS, and therefore it could exert locally its immunosuppressive and anti-inflammatory activity on circulating leukocytes. The low TNF-α/IL-10 ratio in the plasma was previously shown to be a useful prognostic factor in patients with severe infections and sepsis [17, 18, 53]. Our data extend these findings by showing that both basal and LPS-induced IC TNF-α/IL-10 ratio of monocytes and granulocytes are lower in patients with septic shock than that found in healthy subjects, with a correlation with disease severity.
Several hypotheses can be raised to explain the phenotypic changes affecting monocytes and granulocytes of patients with SS. First, it has been shown that apoptosis could be increased during sepsis [54]. Apoptotic monocytes or granulocytes could represent the majority of circulating cells, and this may explain their low level of cytokine production. Further studies with concomitant determination of markers of apoptosis and IC cytokines may unravel such a phenomenon. It is also possible that the majority of circulating leukocytes sampled in our studies represented a subset of cells with decreased migrating capacities. We always observed a flow cytometric homogeneous, unimodal pattern of IC cytokine expression within a given leukocyte population, arguing against such a hypothesis. Circulating phagocytes rapidly transmigrate to infected and inflamed tissues, but it is unknown whether the tissue leukocytes retain their “circulating” phenotype of immune suppression, or whether the latter phenotype is reversible depending on the surrounding milieu.
The important observed interindividual variation in IC contents of TNF-α and IL-10 parallels that which has been described with ELISA measurements of cytokine levels in plasma. This can be linked at least partially to genetic polymorphisms in the regulatory regions of these cytokine genes [44, 55, 56]. Interestingly, plasma and IC TNF-α and IL-10 were not correlated. This is in accordance with the work from Muñoz et al. [20] who have shown a dissociation between plasma and monocyte-associated cytokines during sepsis.
The patients studied were older than the control subjects, and this may partially account for the differences observed in IC cytokine levels. However, despite the general assumption that aging is associated with a lower inflammatory response, several investigators have reported that old patients have an increased type 1 and type 2 cytokine reactions, and that their leukocyte response to LPS was stronger than in younger subjects [57, 58, 59]. This was also the case when cytokines were measured intracellularly by flow cytometry [28, 29]. Finally, we did not observe a correlation between age and IC levels of both cytokines, as previously shown with plasma levels of cytokines in septic patients [60].
The quantitative assessment of IC cytokine levels measured by flow cytometry is an easy and rapid test that can provide the investigator and the clinician with information on basal cell cytokine levels and on the reactivity of circulating leukocytes. It also offers direct information on leukocyte function and their inflammatory response capacity without the need to purify leukocyte subpopulations, thereby avoiding artifacts due to isolation and cell purification steps. The usefulness of IC cytokine measurement by flow cytometry as a monitoring tool and its comparison with plasma cytokines should now be tested with a reproducible technique in a large cohort of patients with varying degrees of sepsis severity. Such studies should determine precisely the accuracy and the precision of this technique using a standardized protocol as well as set up normal values. This seems mandatory to investigate whether intraleukocyte cytokine determination has a future as a diagnostic and/or a monitoring tool. The use of sequential measurements of cell-associated cytokines, leukocyte responsiveness, and HLA-DR expression during the course of sepsis could shed light on mechanisms of the inflammatory and immune response and be used for the immune monitoring of such patients [61, 62]. This may prove useful in the near future for selecting patients for immunotherapy as well as to improve our understanding of the pathophysiology of this deadly syndrome.
References
American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 20:864–874
Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29:1303–1310
Martin GS, Mannino DM, Eaton S, Moss M (2003) The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348:1546–1554
Hotchkiss RS, Karl IE (2003) The pathophysiology and treatment of sepsis. N Engl J Med 348:138–150
Cohen J (2002) The immunopathogenesis of sepsis. Nature 420:885–891
Abraham E (1999) Why immunomodulatory therapies have not worked in sepsis. Intensive Care Med 25:556–566
Munford RS, Pugin J (2001) Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am J Respir Crit Care Med 163:316–321
Volk HD, Reinke P, Docke WD (2000) Clinical aspects: from systemic inflammation to ‘immunoparalysis.’ Chem Immunol 74:162–177
Haveman JW, Muller Kobold AC, Tervaert JW, van den Berg AP, Tulleken JE, Kallenberg CG, The TH (1999) The central role of monocytes in the pathogenesis of sepsis: consequences for immunomonitoring and treatment. Neth J Med 55:132–141
Pugin J, Ricou B, Steinberg KP, Suter PM, Martin TR (1996) Proinflammatory activity in bronchoalveolar lavage fluids from patients with ARDS, a prominent role for interleukin-1. Am J Respir Crit Care Med 153:1850–1856
Pugin J, Verghese G, Widmer MC, Matthay MA (1999) The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit Care Med 27:304–312
Tracey KJ, Cerami A (1993) Tumor necrosis factor: an updated review of its biology. Crit Care Med 21:S415–S422
Randow F, Syrbe U, Meisel C, Krausch D, Zuckermann H, Platzer C, Volk HD (1995) Mechanism of endotoxin desensitization: involvement of interleukin 10 and transforming growth factor beta. J Exp Med 181:1887–1892
Brandtzaeg P, Osnes L, Ovstebo R, Joo GB, Westvik AB, Kierulf P (1996) Net inflammatory capacity of human septic shock plasma evaluated by a monocyte-based target cell assay: identification of interleukin-10 as a major functional deactivator of human monocytes. J Exp Med 184:51–60
Sfeir T, Saha DC, Astiz M, Rackow EC (2001) Role of interleukin-10 in monocyte hyporesponsiveness associated with septic shock. Crit Care Med 29:129–133
Fumeaux T, Pugin J (2002) Role of interleukin-10 in the intracellular sequestration of human leukocyte antigen-DR in monocytes during septic shock. Am J Respir Crit Care Med 166:1475–1482
Gogos CA, Drosou E, Bassaris HP, Skoutelis A (2000) Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis 181:176–180
Dissel JT van, van Langevelde P, Westendorp RG, Kwappenberg K, Frolich M (1998) Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet 351:950–953
Park WY, Goodman RB, Steinberg KP, Ruzinski JT, Radella F 2nd, Park DR, Pugin J, Skerrett SJ, Hudson LD, Martin TR (2001) Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 164:1896–1903
Munoz C, Misset B, Fitting C, Bleriot JP, Carlet J, Cavaillon JM (1991) Dissociation between plasma and monocyte-associated cytokines during sepsis. Eur J Immunol 21:2177–2184
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Cabioglu N, Bilgic S, Deniz G, Aktas E, Seyhun Y, Turna A, Gunay K, Esen F (2002) Decreased cytokine expression in peripheral blood leukocytes of patients with severe sepsis. Arch Surg 137:1037–1043
Mueller A, Kreuzfelder E, Nyadu B, Lindemann M, Rebmannn V, Majetschak M, Obertacke U, Schade UF, Nast-Kolb D, Grosse-Wilde H (2003) Human leukocyte antigen-DR expression in peripheral blood mononuclear cells from healthy donors influenced by the sera of injured patients prone to severe sepsis. Intensive Care Med 29:2285–2290
Spolarics Z, Siddiqi M, Siegel JH, Garcia ZC, Stein DS, Denny T, Deitch EA (2003) Depressed interleukin-12-producing activity by monocytes correlates with adverse clinical course and a shift toward Th2-type lymphocyte pattern in severely injured male trauma patients. Crit Care Med 31:1722–1729
Maino VC, Picker LJ (1998) Identification of functional subsets by flow cytometry: intracellular detection of cytokine expression. Cytometry 34:207–215
Estcourt C, Rousseau Y, Sadeghi HM, Thieblemont N, Carreno MP, Weiss L, Haeffner-Cavaillon N (1997) Flow-cytometric assessment of in vivo cytokine-producing monocytes in HIV-infected patients. Clin Immunol Immunopathol 83:60–67
Berg AP van den, Twilhaar WN, van Son WJ, van der Bij W, Klompmaker IJ, Slooff MJ, The TH, de Leij LH (1998) Quantification of immunosuppression by flow cytometric measurement of intracellular cytokine synthesis. Transpl Int 11 [Suppl 1]:S318–S321
McNerlan SE, Rea IM, Alexander HD (2002) A whole blood method for measurement of intracellular TNF-alpha, IFN-gamma and IL-2 expression in stimulated CD3+ lymphocytes: differences between young and elderly subjects. Exp Gerontol 37:227–234
Pietschmann P, Gollob E, Brosch S, Hahn P, Kudlacek S, Willheim M, Woloszczuk W, Peterlik M, Tragl KH (2003) The effect of age and gender on cytokine production by human peripheral blood mononuclear cells and markers of bone metabolism. Exp Gerontol 38:1119–1127
Prussin C, Metcalfe DD (1995) Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods 188:117–128
Desch CE, Kovach NL, Present W, Broyles C, Harlan JM (1989) Production of human tumor necrosis factor from whole blood ex vivo. Lymphokine Res 8:141–146
Finch-Arietta MB, Cochran FR (1991) Cytokine production in whole blood ex vivo. Agents Actions 34:49–52
De Groote D, Zangerle PF, Gevaert Y, Fassotte MF, Beguin Y, Noizat-Pirenne F, Pirenne J, Gathy R, Lopez M, Dehart I et al (1992) Direct stimulation of cytokines (IL-1 beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSF) in whole blood. I. Comparison with isolated PBMC stimulation. Cytokine 4:239–248
Desfaits AC, Serri O, Renier G (1998) Normalization of plasma lipid peroxides, monocyte adhesion, and tumor necrosis factor-alpha production in NIDDM patients after gliclazide treatment. Diabetes Care 21:487–493
Elborn JS, Norman D, Delamere FM, Shale DJ (1992) In vitro tumor necrosis factor-alpha secretion by monocytes from patients with cystic fibrosis. Am J Respir Cell Mol Biol 6:207–211
Anand M, Chodda SK, Parikh PM, Nadkarni JS (1998) Dysregulated cytokine production by monocytes from chronic lymphocytic leukemia patients. Cancer Biother Radiopharm 13:43–48
Granchi D, Ciapetti G, Stea S, Savarino L, Filippini F, Sudanese A, Zinghi G, Montanaro L (1999) Cytokine release in mononuclear cells of patients with Co-Cr hip prosthesis. Biomaterials 20:1079–1086
Peters AM, Jager FS, Warneke A, Muller K, Brunkhorst U, Schedel I, Gahr M (1991) Cytokine secretion by peripheral blood monocytes from human immunodeficiency virus-infected patients is normal. Clin Immunol Immunopathol 61:343–352
Shinohara K, Ayame H, Tanaka M, Matsuda M, Ando S, Tajiri M (1991) Increased production of tumor necrosis factor-alpha by peripheral blood mononuclear cells in the patients with aplastic anemia. Am J Hematol 37:75–79
Dubravec DB, Spriggs DR, Mannick JA, Rodrick ML (1990) Circulating human peripheral blood granulocytes synthesize and secrete tumor necrosis factor alpha. Proc Natl Acad Sci U S A 87:6758–6761
Dibbs Z, Thornby J, White BG, Mann DL (1999) Natural variability of circulating levels of cytokines and cytokine receptors in patients with heart failure: implications for clinical trials. J Am Coll Cardiol 33:1935–1942
Vassilakopoulos T, Karatza M-H, Katsaounou P, Kollintza A, Zakynthinos S, Roussos C (2003) Antioxidants attenuate the plasma cytokine response to exercise in humans. J Appl Physiol 94:1025–1032
Godot V, Harraga S, Deschaseaux M, Bresson-Hadni S, Gottstein B, Emilie D, Vuitton DA (1997) Increased basal production of interleukin-10 by peripheral blood mononuclear cells in human alveolar echinococcosis. Eur Cytokine Netw 8:401–408
Suarez A, Castro P, Alonso R, Mozo L, Gutierrez C (2003) Interindividual variations in constitutive interleukin-10 messenger RNA and protein levels and their association with genetic polymorphisms. Transplantation 75:711–717
Kilpinen S, Huhtala H, Hurme M (2002) The combination of the interleukin-1alpha (IL-1alpha-889) genotype and the interleukin-10 (IL-10 ATA) haplotype is associated with increased interleukin-10 (IL-10) plasma levels in healthy individuals. Eur Cytokine Netw 13:66–71
Volk HD, Thieme M, Heym S, Docke WD, Ruppe U, Tausch W, Manger D, Zuckermann S, Golosubow A, Nieter B et al (1991) Alterations in function and phenotype of monocytes from patients with septic disease: predictive value and new therapeutic strategies. Behring Inst Mitt 88:208–215
Schultz MJ, Olszyna DP, de Jonge E, Verbon A, van Deventer SJ, van der Poll T (2000) Reduced ex vivo chemokine production by polymorphonuclear cells after in vivo exposure of normal humans to endotoxin. J Infect Dis 182:1264–1267
Schleiffenbaum B, Fehr J (1990) The tumor necrosis factor receptor and human neutrophil function. Deactivation and cross-deactivation of tumor necrosis factor-induced neutrophil responses by receptor down-regulation. J Clin Invest 86:184–195
McCall CE, Grosso-Wilmoth LM, LaRue K, Guzman RN, Cousart SL (1993) Tolerance to endotoxin-induced expression of the interleukin-1 beta gene in blood neutrophils of humans with the sepsis syndrome. J Clin Invest 91:853–861
Stephan F, Yang K, Tankovic J, Soussy CJ, Dhonneur G, Duvaldestin P, Brochard L, Brun-Buisson C, Harf A, Delclaux C (2002) Impairment of polymorphonuclear neutrophil functions precedes nosocomial infections in critically ill patients. Crit Care Med 30:315–322
Marie C, Muret J, Fitting C, Losser MR, Payen D, Cavaillon JM (1998) Reduced ex vivo interleukin-8 production by neutrophils in septic and nonseptic systemic inflammatory response syndrome. Blood 91:3439–3446
Cavaillon JM (2002) “Septic plasma”: an immunosuppressive milieu. Am J Respir Crit Care Med 166:1417–1418
Taniguchi T, Koido Y, Aiboshi J, Yamashita T, Suzaki S, Kurokawa A (1999) Change in the ratio of interleukin-6 to interleukin-10 predicts a poor outcome in patients with systemic inflammatory response syndrome. Crit Care Med 27:1262–1264
Oberholzer C, Oberholzer A, Clare-Salzler M, Moldawer LL (2001) Apoptosis in sepsis: a new target for therapeutic exploration. FASEB J 15:879–892
Westendorp RG, Langermans JA, Huizinga TW, Elouali AH, Verweij CL, Boomsma DI, Vandenbroucke JP, Vandenbrouke JP (1997) Genetic influence on cytokine production and fatal meningococcal disease. Lancet 349:170–173
Stüber F (2000) Impact of genomic variation on inflammatory processes and sepsis. In: Eichacker PQ, Pugin J (eds) Evolving concepts in sepsis and septic shock. Kluwer, Boston
Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R (2003) Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci USA 100:9090–9095
Zanni F, Vescovini R, Biasini C, Fagnoni F, Zanlari L, Telera A, Di Pede P, Passeri G, Pedrazzoni M, Passeri M (2003) Marked increase with age of type 1 cytokines within memory and effector/cytotoxic CD8+ T cells in humans: a contribution to understand the relationship between inflammation and immunosenescence. Exp Gerontol 38:981–987
Gabriel P, Cakman I, Rink L (2002) Overproduction of monokines by leukocytes after stimulation with lipopolysaccharide in the elderly. Exp Gerontol 37:235–247
Marik PE, Zaloga GP (2001) The effect of aging on circulating levels of proinflammatory cytokines during septic shock. Norasept II Study Investigators. J Am Geriatr Soc 49:5–9
Volk HD, Reinke P, Docke WD (1999) Immunological monitoring of the inflammatory process: which variables? When to assess? Eur J Surg Suppl 584:70–72
Heagy W, Hansen C, Nieman K, Cohen M, Richardson C, Rodriguez JL, West MA (2000) Impaired ex vivo lipopolysaccharide-stimulated whole blood tumor necrosis factor production may identify ‘septic’ intensive care unit patients. Shock 14:271–276
Acknowledgements
The authors acknowledge the financial support of the Stanley Thomas Johnson Foundation and the Institutional Fund Projet de Recherche et Développement of the University Hospital of Geneva.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fumeaux, T., Dufour, J., Stern, S. et al. Immune monitoring of patients with septic shock by measurement of intraleukocyte cytokines. Intensive Care Med 30, 2028–2037 (2004). https://doi.org/10.1007/s00134-004-2429-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-004-2429-8