Abstract
Objective
To investigate whether mortality in UK intensive care units is higher in winter than in non-winter and to explore the importance of variations in case mix and increased pressure on ICUs.
Design and setting
Cohort study in 115 adult, general ICUs in England, Wales and Northern Ireland.
Patients and participants
113,389 admissions from 1995 to 2000.
Measurements and results
Hospital mortality following admission to ICU was compared between winter (December–February) and non-winter (March–November). The causes of any observed differences were explored by adjusting for the case mix of admissions and the workload of the ICUs. Crude hospital mortality was higher in winter. After adjusting for case mix using the APACHE II mortality probability this effect was reduced but still significant. When additional factors reflecting case mix and workload were introduced into the model, the overall effect of winter admission was no longer significant. Factors reflecting both the case mix of the individual patient and of the patients in surrounding beds were found to be significantly associated with outcome. After adjustment for other factors, the occupancy of the unit (proportion of beds occupied) was not significantly associated with mortality.
Conclusions
The excess winter mortality observed in UK ICUs can be explained by variation in the case mix of admissions. Unit occupancy was not associated with mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that mortality in the United Kingdom is higher during winter than the rest of the year; indeed this winter excess mortality is more severe in the UK than in much of the rest of Europe [1, 2]. The excess deaths have been attributed largely to cardiovascular and respiratory diseases [1, 3, 4], but it is unclear how much this is due to increased incidence of these conditions during winter, and how much is due to worse outcomes in patients with the same severity of disease. It has long been established that case mix, in terms of types of disease, varies at different times of the year. In around 400 BC Hippocrates observed, “All diseases occur at all seasons of the year, but certain of them are more apt to occur and be exacerbated at certain seasons” (Aphorisms 3:19). It may be that the increased mortality in winter is simply a reflection of higher risk conditions during winter. Every year the British media carry stories of operations being cancelled, hospital departments being closed, and patients being transported across the country in search of a free bed during what has popularly become known as the ‘winter crisis’ (‘Hospitals ‘stretched to breaking point’, Sunday Telegraph, 5 Jan. 1997; ‘Consultants fear crisis over NHS bed shortage’, Daily Telegraph, 29 Dec. 1999, ‘Man dies after 200-mile bed hunt’, The Times, 8 Jan. 2000; ‘Urgent cancer operations delayed by epidemic’, The Independent, 12 Jan. 2000). There is an intuitive notion that these increased pressures during winter may lead to worse patient outcomes.
A vital area of the hospital during such crises is the intensive care unit (ICU). Of the 530,870 deaths in England and Wales in the year 2000 among persons aged at least 15 years, 293,177 (55%) occurred in non-psychiatric National Health Service hospitals [5]. The total number of admissions to adult, general ICUs in England and Wales in a single year has been estimated to be 78,000 [6]. With an ultimate hospital mortality of around 32%, this equates to approx. 25,000 deaths per year occurring after admission to ICU and before discharge from hospital. Consequently around 9% of hospital deaths and 5% of all deaths occur in patients treated in ICU. Garfield et al. [7] showed that admissions to intensive care follow a seasonal pattern with a peak in winter. The same seasonality has also been observed in admissions to intensive care with severe sepsis [6]. Studies have shown both directly [8, 9] and through increased pressure for beds leading to more discharges at night [10] that high ICU workload can be associated with increased mortality.
We investigated the existence of winter excess mortality in ICU and the extent to which it can be explained by variation in case mix and workload.
Materials and methods
Data
The data derived from the Intensive Care National Audit and Research Centre’s Case Mix Programme Database (CMPD) covering the period between December 1995 and May 2000. The CMPD is a high-quality clinical database of case mix and outcome data for consecutive admissions to adult critical care units in England, Wales and Northern Ireland [11]. Data were collected prospectively and abstracted by trained data collectors according to precise rules and definitions, and were validated both locally and centrally for completeness and illogical and inconsistent values. The data were truncated at May 2000 to avoid confounding our results with any impact from the Department of Health report ‘Comprehensive Critical Care’ [12] published at that time and accompanied by additional funding of £142.5 million for critical care.
Exclusion criteria were selected to satisfy the conditions for the Acute Physiology and Chronic Health Evaluation II (APACHE II) model [13] to be used for case mix adjustment. Admissions were excluded from the primary analyses if they met any of the following criteria: age less than 16 years, length of stay in ICU less than 8 h, readmission of a previous ICU admission within the same hospital stay, transfer in from another ICU, admission following coronary artery bypass graft, and admission for burns. A sensitivity analysis, including these admissions, was undertaken to check that the results were not unduly influenced by these exclusions.
We elected a priori to define winter as the period from December to February, inclusive, with non-winter defined as March to November, inclusive. This definition was based on consultation with clinicians regarding an appropriate definition of winter from their experiences of critical care in the UK and was consistent with definitions used elsewhere in the UK medical literature [14, 15, 16, 17]. The Christmas and New Year period has been shown to be associated with highly significant variations in the patterns of admissions to various specialties [18]; however, this period was not excluded from the analyses as such variations in admission patterns may be the cause of any variation in mortality. Mortality for a critical care admission was measured by the ultimate hospital mortality, defined as the status (alive/dead) at ultimate discharge from hospital following admission to ICU.
The CMPD contained validated data from 113,389 admissions to 115 critical care units during the specified time period. In total 23,932 (21.1%) admissions were excluded as a result of the APACHE II exclusion criteria. A further 9,807 (11.0%) admissions were excluded as they were missing either the outcome variable of ultimate hospital mortality or one or more of the modelling covariates. Following these exclusions 79,650 admissions remained, of which 19,329 (24.3%) were admitted between December and February (winter), and 60,321 (75.7%) were admitted between March and November (non-winter). The numbers of admissions excluded as a result of the APACHE II exclusion criteria for winter and non-winter are summarised in Table 1. The exclusions in winter and non-winter were broadly similar, with the largest difference being in admissions transferred in from another ICU (4.7% in winter vs. 3.8% in non-winter).
Statistical methods
The data were analysed according to the following analysis plan, agreed a priori: (a) Crude mortality in winter and non-winter were compared to establish the existence of an association. (b) If mortality was found to be higher in winter, we attempted to explain this association by case mix (risk) adjustment using the APACHE II mortality probability and looked for any interaction between the effects of winter admission and the APACHE II mortality probability. The APACHE II score is composed of points for age, severe conditions in the medical history, and acute severity defined by derangement from the normal range for 12 physiological variables in the first 24 h in the ICU [13]. The APACHE II predicted probability of hospital mortality is derived from the APACHE II score, a diagnostic category based on the reason for admission to ICU, and an indicator for admissions following emergency surgery. Coefficients for the predicted mortality were taken from the published UK APACHE II model as this is better calibrated to a UK intensive care population [19]. Case mix adjustment was performed by including the APACHE II predicted log odds of mortality (the logit of the APACHE II mortality probability) as a linear term in the logistic regression models. (c) If the association still remained, we sought to explain it by adjusting for additional covariates in a multivariate model to account for residual case mix (not explained by APACHE II), ICU workload as measured by the case mix of concurrent ICU admissions, and direct measures of ICU workload.
The covariates selected for the multivariate analysis were as follows:
Residual case mix
Number of organ system failures assessed physiologically according to the definitions of Knaus et al. [20]. Cardiopulmonary resuscitation (CPR) within 24 h prior to ICU admission. Mechanical ventilation at any time during the first 24 h in ICU (or entire stay if less than 24 h). Surgical status classified as admissions direct to ICU following elective or scheduled surgery, admissions direct to ICU following emergency or urgent surgery, and non-surgical admissions. Admissions direct to ICU following surgery included admissions direct from theatre and admissions from theatre passing through a transient location of recovery, radiography, endoscopy, computed tomography or similar, or ‘accident and emergency’. Non-surgical admissions were admissions to ICU from all other sources. Night admission defined as admission to ICU between 00:00 and 04:59 hours, based on the definition used for evaluating times of discharge from ICU by Goldfrad and Rowan [10]. Length of hospital stay prior to ICU admission categorised as 0 days (hospital and ICU admission on the same date), 1 day, 2 days, or 3+ days.
Case mix of concurrent admissions
Median total length of ICU stay of all admissions whose ICU stay overlapped with the first 24 h in ICU for the index admission (or the entire stay if less than 24 h). Median APACHE II mortality probability from the first 24 h in ICU of all admissions whose ICU stay overlapped with the first 24 h in ICU for the index admission (or the entire stay if less than 24 h).
ICU workload
Unit occupancy defined as the mean proportion of beds in the ICU occupied during the first 24 h in ICU for the index admission (or the entire stay if less than 24 h). The number of beds in the ICU was taken to be the number of beds registered with the Case Mix Programme and therefore did not fluctuate on a short-term basis with changes in staffing. Unit occupancy greater than 100% was therefore possible during times of high demand when additional beds may be temporarily staffed. Throughput measured as the number of new admissions to the ICU during the first 24 h in ICU for the index admission (or the entire stay if less than 24 h) divided by the number of available staffed beds (not counting the bed occupied by the index admission). The resulting value was scaled to represent a measurement over 24 h for admissions whose length of stay was less than 24 h. All continuous variables were modelled as having a linear effect on the log odds of hospital mortality (predicted probabilities were modelled as linear in the predicted log odds). All analyses were performed in Stata 8.0 (Stata, College Station, Tex., USA).
Results
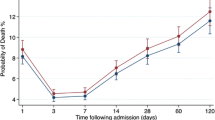
The crude ultimate hospital mortality for ICU admissions in winter was 32.2% and that in non-winter was 29.3% (Table 2). This corresponds to an odds ratio (OR) for winter compared to the reference period of 1.14 [95% confidence interval (CI) 1.11–1.19; Table 3]. There were also significant differences in case mix for winter compared to non-winter (Table 2), with higher APACHE II score (mean 18.0 vs. 17.5), more organ system failures (mean 1.02 vs. 0.94), a higher percentage of admissions following CPR (8.6% vs. 7.8%), a higher percentage of mechanically ventilated admissions (69.2% vs. 65.0%), and a higher percentage of non-surgical (medical) admissions (54.7% vs. 49.5%). There was a clear seasonal pattern in the ultimate hospital mortality by month, although the numbers of admissions per unit remained approximately constant throughout the year (Fig. 1), suggesting that the higher occupancy rates in winter were the result of longer lengths of stay rather than higher rates of admission.
After adjusting for APACHE II mortality probability, the effect of winter admission was still significant although somewhat reduced (OR 1.10, 95% CI 1.05–1.14; Table 3). When an interaction between APACHE II mortality probability and winter admission was included in the model the effect of winter admission was found to be most pronounced at intermediate predicted mortalities, with little or no effect in the lower and upper quartiles of predicted mortalities.
The results of the multivariate model are shown in Table 4. All the covariates classified as case mix of index or concurrent admissions were found to be highly significant predictors of ultimate hospital mortality. Ultimate hospital mortality increased with increasing APACHE II score, number of organ system failures, and length of hospital stay prior to ICU admission (Table 4). It was higher in admissions receiving CPR within 24 h prior to admission, and in those ventilated during the first 24 h in ICU, and was higher in non-surgical admissions than in emergency and elective surgery admissions (Table 4). Although crude mortality was worse for patients admitted at night, after adjusting for the other factors these admissions were found to have significantly better outcomes (Table 4). Increased odds of hospital mortality were associated with increasing length of stay and decreasing APACHE II mortality probability of the patients in surrounding beds (Table 4). Crude mortality did not increase with increasing unit occupancy beyond the level of 70% occupancy, and there was no significant effect of occupancy on outcome after adjusting for the other factors. Increased throughput of cases conveyed a very slight reduction in odds of hospital mortality (Table 4).
After adjusting for all of the above factors, the overall effect of winter admission was no longer significant (P=0.09; Table 3). Discrimination of the full model was good (area under the receiver operating characteristic curve 0.831, 95% CI 0.828–0.833). The Hosmer-Lemeshow calibration statistic showed a statistically significant lack of calibration. However, this measure is sensitive to sample size and our model was estimated on a very large sample. The deviations from perfect calibration, while significant, were small in magnitude and with no evident systematic pattern (Electronic Supplementary Material, Table S1). In a secondary analysis, including those admissions excluded by APACHE II gave similar results, although the effect of throughput was no longer significant (Electronic Supplementary Material, Tables S2, S3).
Discussion
The excess winter mortality observed in ICUs in England, Wales and Northern Ireland can be explained by variation in the case mix of admissions. The outcome for a particular admission was associated not only with their own severity of illness but also with that of concurrent admissions to the same ICU. However, these associations were not in the direction expected: longer lengths of stay and lower APACHE II probabilities of other patients were associated with worse risk-adjusted outcome whereas we expected these patients to be less resource intensive resulting in better outcomes for the index patient. After adjusting for other factors the occupancy of the unit did not have a significant effect on patient outcome. Throughput (number of new admissions per bed-day) showed a small but significant effect, with slightly improved risk-adjusted outcomes at busier times.
One could take these results regarding both throughput and case mix of concurrent admissions as evidence of improved performance of ICUs under pressure. We think it more likely, however, that these measures reflect not only workload but also the general case mix of patients. By failing adequately to adjust for case mix in the index case these measures may be indicative of residual confounding and should not be interpreted at face value. In any case, the observed effects, while statistically significant, were very small.
While crude mortality was higher for patients admitted at night, after adjustment for all the other factors, the opposite effect was found. A more in depth study of the day and time of admission to intensive care found no effect of these factors on hospital mortality after case mix adjustment [21]. It is reasonable to suppose that the higher mortality among admissions at night would be explained by these being emergency admissions of very sick patients. This hypothesis is also supported by another study considering night admissions to ICU [22].
The great strength of this study lies in being based on a large representative sample of admissions to a high proportion of UK ICUs, recorded in a high quality clinical database. Case mix adjustment methods in intensive care are relatively sophisticated compared with other areas, and the APACHE II method is a well accepted and widely used approach. It does, however, have its drawbacks. Of all the admissions 21% were excluded as a result of the APACHE II exclusion criteria, although these exclusions were reasonably balanced between winter and non-winter and repeating the analyses including the excluded cases did not materially change the results. The APACHE II risk adjustment method uses the worst value of physiological variables recorded during the first 24 h following admission to intensive care. Consequently a patient who was poorly managed after admission, possibly due to high workload, and whose severity of illness worsened rapidly would be indistinguishable from a patient with worse severity at the time of admission. This has been noted previously as a drawback to the APACHE method and to other risk adjustment methods that utilise data after the point of admission [23, 24, 25, 26]. It is possible that this masked some effect of workload on outcome in our study. The UK APACHE II model was fitted on data from 1988 to 1990, and therefore the coefficients are likely to be somewhat out of date. This may explain some of the need for additional adjustment for residual case mix factors. Work is currently nearing completion at the Intensive Care National Audit and Research Centre to investigate optimal methods for risk adjustment, including the gains that can be made by updating the coefficients of existing models including APACHE II.
Ultimate hospital mortality was chosen as the outcome measure as it is the longest follow-up routinely available in the CMPD. Hospital mortality is also the outcome on which the UK APACHE II model was developed, and it is therefore not clear that this model would predict as well if a different outcome variable were used. Hospital mortality following intensive care has also been found to be very similar to 90-day mortality in the UK [19]. Of 41,650 survivors with data available on their destination following ultimate hospital discharge only 122 (0.3%) were discharged to a hospice or equivalent, and hence there is no evidence to suggest that a large number of hospital survivors would have died shortly after discharge.
Tarnow-Mordi and colleagues [8] have stated, “On the assumption that the APACHE II equation adjusted adequately for risk, we conclude that variations in hospital mortality may be partly explained by excess ICU workload.” In this study we found that risk adjustment by APACHE II alone was not adequate, and, after accounting for a number of additional case mix factors, that there is no significant effect of occupancy on mortality. It may be noted that unit occupancy was high at all times, with median occupancy over 80% even in the reference period of March to November. While our results suggest that variations in case mix are the principal cause of winter excess mortality, the limitations of the observational design and risk adjustment methods mean that an effect of workload cannot be ruled out. We would recommend further prospective research with more detailed measurement of workload for a better understanding of this problem.
References
Curwen M (1990/91) Excess winter mortality: a British phenomenon? Health Trends 4:169–175
Laake K, Sverre JM (1996) Winter excess mortality: a comparison between Norway and England plus Wales. Age Ageing 25:343–348
Kendrick S, Frame S, Povey C (1997) Beds occupied by emergency patients: long-term trends in patterns of short-term fluctuation in Scotland. Health Bull (Edinb) 55:167–175
Keatinge WR (2002) Winter mortality and its causes. Int J Circumpolar Health 61:292–299
Office for National Statistics (2002) Mortality statistics: general. Review of the Registrar General on deaths in England and Wales, 2000. Series DH1 no.33. Office for National Statistics, London
Padkin A, Goldfrad C, Brady AR, Young D, Black N, Rowan K (2003) Epidemiology of severe sepsis occurring in the first 24 hrs in intensive care in England, Wales, and Northern Ireland. Crit Care Med 31:2332–2338
Garfield M, Ridley S, Kong A, Burns A, Blunt M, Gunning K (2001) Seasonal variation in admission rates to intensive care units. Anaesthesia 56:1136–1140
Tarnow-Mordi WO, Hau C, Warden A, Shearer AJ (2000) Hospital mortality in relation to staff workload: a 4-year study in an adult intensive care unit. Lancet 356:185–189
UK Neonatal Staffing Study Group (2002) Patient volume, staffing, and workload in relation to risk-adjusted outcomes in a random stratified sample of UK neonatal intensive care units: a prospective evaluation. Lancet 359:99–107
Goldfrad C, Rowan K (2000) Consequences of discharges from intensive care at night. Lancet 355:1138–1142
Rowan K, Black N (2000) A bottom-up approach to performance indicators through clinician networks. Health Care UK, Spring:42–46
Department of Health (2000) Comprehensive critical care: a review of adult critical care services. Department of Health, London
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Leach CE, Blair PS, Fleming PJ, Smith IJ, Platt MW, Berry PJ, Golding J (1999) Epidemiology of SIDS and explained sudden infant deaths. CESDI SUDI Research Group. Pediatrics 104:e43
Pell JP, Sirel J, Marsden AK, Cobbe SM (1999) Seasonal variations in out of hospital cardiopulmonary arrest. Heart 82:680–683
Atkinson W, Harris J, Mills P, Moffat S, White C, Lynch O, Jones M, Cullinan P, Newman Taylor AJ (1999) Domestic aeroallergen exposures among infants in an English town. Eur Respir J 13:583–589
Sandberg S, Paton JY, Ahola S, McCann DC, McGuinness D, Hillary CR, Oja H (2000) The role of acute and chronic stress in asthma attacks in children. Lancet 356:982–987
Fullerton KJ, Crawford VLS (1999) The winter bed crisis: quantifying seasonal effects on hospital bed usage. Q J Med 92:199–206
Rowan KM (1992) Outcome comparison of intensive care units in Great Britain and Ireland using the APACHE II method. Thesis, University of Oxford
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) Prognosis in acute organ system failure. Ann Surg 202:685–693
Wunsch H, Mapstone J, Brady T, Hanks R, Rowan K (2004) Hospital mortality associated with day and time of admission to intensive care units. Intensive Care Med 30:895–901
Morales IJ, Peters SG, Afessa B (2003) Hospital mortality rate and length of stay in patients admitted at night to the intensive care unit. Crit Care Med 31:858–863
Selker HP (1993) Systems for comparing actual and predicted mortality rates: characteristics to promote cooperation in improving hospital care. Ann Intern Med 118:820–822
Boyd O, Grounds RM (1993) Physiological scoring systems and audit. Lancet 341:1573–1574
Rutledge R (1996) The Injury Severity Score is unable to differentiate between poor care and severe injury. J Trauma 40:944–950
Shann F, Pearson G, Slater A, Wilkinson K (1997) Paediatric index of mortality (PIM): a mortality prediction model for children in intensive care. Intensive Care Med 23:201–207
Acknowledgements
We thank all the staff in the ICUs participating in the Case Mix Programme and those responsible for local funding.
Author information
Authors and Affiliations
Corresponding author
Additional information
This research was supported by: Intensive Care National Audit and Research Centre, Ramathibodi Hospital and Medical School, and ESRC Research Methods (grant H 333 25 0047)
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Harrison, D.A., Lertsithichai, P., Brady, A.R. et al. Winter excess mortality in intensive care in the UK: an analysis of outcome adjusted for patient case mix and unit workload. Intensive Care Med 30, 1900–1907 (2004). https://doi.org/10.1007/s00134-004-2390-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-004-2390-6