Abstract
Objective
The purpose of this study was to determine whether monitoring of respiratory changes in aortic blood flow velocity, recorded by esophageal Doppler, could be used to detect changes in volume depletion.
Design
Animal study.
Animals and interventions
After general anesthesia and tracheotomy, ten New Zealand female rabbits, weighing 4–4.5 kg were studied under mechanical ventilation at a fixed tidal volume; during this time 5-ml blood samples were withdrawn (in increments up to a total of 30 ml) and then retransfused.
Measurements and results
At each step, systolic (SBP), diastolic (DBP), pulse (PP) pressures and maximum descending aortic blood flow (V) were recorded. Respiratory changes of V (ΔV), SBP (ΔSBP) and PP (ΔPP) were calculated as the difference of maximal and minimal values divided by their respective means and expressed as a percentage. The amount of blood withdrawn correlated negatively with SBP, DBP, PP and V and positively with ΔSBP, ΔPP and ΔV. Among these parameters, ΔV correlated best with the amount of blood withdrawn (r=0.89, p<0.001) and it was the most accurate index of volume depletion.
Conclusion
Monitoring of the respiratory variation in V, calculated by esophageal Doppler technique, seems to be a highly accurate index of blood volume depletion and restitution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The diagnosis of inadequate cardiac preload in critically ill patients is difficult to establish at the bedside. Recently, in rabbits receiving mechanical ventilation, the magnitude of stroke volume variation over a respiratory cycle has been shown to detect accurately hypovolemia and preload responsiveness [1]. As a surrogate of stroke volume variation, respiratory variations of arterial systolic pressure [2, 3, 4, 5], arterial pulse pressure [6] and aortic Doppler velocity (using transesophageal echocardiography) [7] have been demonstrated to predict fluid responsiveness in patients with acute circulatory failure. However, the analysis of arterial pressure variation requires an intra-arterial catheter and transesophageal echocardiography cannot be used as a reliable diagnostic technique for long-term hemodynamic monitoring. The esophageal Doppler is a non-invasive technique that has been proposed to monitor descending aortic blood flow in critically patients and in patients undergoing surgery [8]. To our knowledge no animal or clinical study has studied whether monitoring of respiratory variations may be used to detect and to follow volume static changes and responsiveness.
This study was therefore designed to examine the effects of provoked hemorrhage and blood restitution on respiratory variation of the descending aortic flow velocity measured by esophageal Doppler in rabbits receiving mechanical ventilation.
Methods
Animals
Ten New Zealand female rabbits (Charles River Laboratories, 01400 Romans, France), weighing 4–4.5 kg, were used in this study. They were housed in separate cages under controlled ambient conditions (21±1°C; 12:12 h light-dark cycle) for at least 1 week prior to study and then received a standard rabbit chow diet with tap water ad libitum. Initial anesthesia consisted of intravenous midazolam (1 mg/kg) and ketamine (0.75 mg/kg) infused into an ear vein through a catheter. Adequate anesthesia was confirmed by the absence of eyelid reflex, changes in heart rate and arterial pressure, or movement after a tail-clamp application. Tracheostomy was performed, the endotracheal tube was connected to a ventilator and pancuronium bromide (8 mg) was injected. A continuous infusion of midazolam (1 mg/kg per h) maintained anesthesia, 0.9% NaCl (6 ml/kg per h) and intermittent injections of pancuronium were administered throughout the study. Mechanical ventilation was initiated and the tidal volume (VT) was adjusted to obtain a peak respiratory pressure of 20 cmH2O (which was actually almost the same in all rabbits). The ventilation rate and FIO2 were fixed at 40 cycles/min and 21% respectively, and further changes in settings were not permitted during the entire protocol. A catheter (P-50) filled with normal saline was inserted into the right femoral artery to record arterial pressure. Animal care conformed to the Helsinki Declaration and the study was conducted according to the regulations of the French Ministry of Agriculture.
Experimental protocol
Mild hypovolemia was induced by a stepwise cumulative withdrawal of 5, 10, 15, 20, 25 and 30 ml of blood from the catheter. Blood was withdrawn into a sterile heparinized (5000 µ/l) blood bag and maintained at 37°C by a heated blanket. The time interval between the steps was 10 min. Then all the withdrawn blood was re-injected 10 min after the last bleeding step.
Echocardiographic measurements
A commercially available echocardiograph (Hewlett Packard Sonos 5500, Les Ullis, France) with a 12 MHz pediatric transthoracic transducer was used to measure cardiac output at baseline, at the last withdrawal step and after blood restitution. All measurements were made online using a Sonos 5500 software system. From the parasternal long-axis view, the aortic diameter (D) was measured at the level of the annulus at end-expiration. From the apical five-chamber view, pulsed Doppler aortic flow was recorded at the level of the annulus and the aortic velocity time integral (VTIao) was measured. Stroke volume and cardiac output were determined as follows:
- :
-
Stroke volume = aortic velocity time integral x Π D2/4
- :
-
Cardiac output = stroke volume x heart rate.
Esophageal Doppler examination
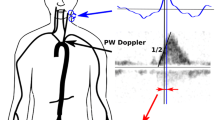
Commercially available Doppler equipment (Dynemo 3000, Arrow, Paris, France) with a 10 MHz pediatric transesophageal probe was used for all studies. This technique has been described extensively elsewhere [9]. In brief, this device uses a pulsed Doppler and allows the recording of blood flow instantaneous velocities at the level of the descending aorta. The probe was introduced into the rabbit esophagus in supine position. The position of the probe was adjusted to obtain the highest aortic blood velocity, the best Doppler and echo signals.
Measurements of blood pressure and Doppler indices
At each step, blood pressure, respiratory movements, airways pressure and esophageal Doppler signals were measured for 7 s and simultaneously recorded through a Hewlett Packard monitor and the Doppler device on a floppy disk. The signal was converted into numbers and transferred to an Excel program. Averaged systolic (SBP), diastolic (DBP) and pulse (PP) pressures (systolic minus diastolic blood pressure) and averaged maximum velocity of the aortic flow (V) were obtained from the 7-s recording. Over a respiratory cycle we measured maximum and minimum systolic (SBPmax and SBPmin), pulse (PPmax and PPmin) pressure and the maximal and minimal velocity of the descending aorta flow (Vmax, Vmin) (Fig. 1). These measurements were performed over three consecutive respiratory cycles and averaged. Relative variation of systolic (ΔSBP) and pulse pressure (ΔPP), and of the velocity of descending aorta blood flow (ΔV) were calculated as follows:
- :
-
ΔSBP (%) = (SBPmax-SBPmin)/ [(SBPmax + SBPmin)/2] x 100
- :
-
ΔPP (%) = (PPmax-PPmin)/[(PPmax + PPmin)/2] x 100
- :
-
ΔV (%) = (Vmax-Vmin)/[(Vmax + Vmin)/2] x 100
In six rabbits, two sets of V, D and VTIao were recorded in order to analyze the intra-observer reproducibility.
Statistical analysis
One-way ANOVA for repeated measurements was performed for statistical analysis. Individual withdrawal curves were studied and linear regressions between each variable and blood withdrawal volume were calculated to analyze the accuracy of Doppler- and blood pressure-derived parameters for assessing blood losses. Individual coefficients of regression (r) were averaged and individual and normalized slopes (divided by the maximal basal value) were recorded and averaged to determine the sensitivity of each Doppler- and blood pressure-derived parameter. In an additional analysis, using all the measurements together (global correlations), we correlated the amount of blood withdrawn with SBP, DBP, PP, ΔV, ΔPP and ΔSBP. A p value of less than 5% was considered statistically significant. All values are presented as means ± one standard error (SEM). The reproducibility of the measurements was assessed as bias ± limits of agreement as described by Bland and Altman [10].
Results
Doppler- and pressure-derived measurements
The heart rate remained unchanged throughout the protocol (baseline: 279±14 beats/min; last blood withdrawal step: 255±14 beats/min). Cardiac output (and stroke volume) decreased during the hemorrhage period from 0.52±0.1 l/min (2.0±0.1 ml) to 0.34±0.06 l/min (1.4±0.1 ml) (baseline versus last blood withdrawal p=0.05) and returned to baseline value 0.55±0.1 l/min (2.0±0.1 ml). The SBP and DBP (Fig. 2) significantly decreased from the baseline to the 30 ml blood withdrawal step (from 125±3.6 to 100±7 mmHg, p=0.004, and from 87±4 to 63±5 mmHg, p=0.001, respectively). These pressure measurements returned to control values after blood restitution. The ΔPP and ΔV progressively increased during the hemorrhage period, returning to baseline values after blood volume restitution (Fig. 3). Similarly, ΔSBP increased from 5.9±0.6% at the baseline to 11.9±1.6% at the last blood withdrawal step (p=0.001) before returning to baseline values after blood restitution. After 15 ml of blood withdrawal, which represented 10% variation of rabbit blood volume, the respiratory variation of descending aorta blood flow velocity was always equal to or higher than 19%. In other words, the presence of a ΔV of 19% or more means that more than 10% of the blood volume was lost (with a sensitivity of 100%).
Correlations
Table 1 presents the averaged individual correlation coefficients between Doppler- and blood pressure-derived indices of blood volume withdrawn. Concerning global correlations, the amount of blood withdrawn correlated directly with ΔPP and ΔSBP (r=0.50, p<0.01 and r=0.53, p<0.01, respectively) and most strongly with ΔV (%) (r=0.89, p<0.0001) (Fig. 4). A fair correlation was demonstrated between ΔV (%) at 30 ml withdrawn and stroke volume changes after blood restitution (r=0.71, p<0.01).
Slopes
The slopes and normalized slopes of the relationships between the volume of blood withdrawn and Doppler- and blood pressure-derived parameters are presented in Table 1. The ΔV appeared to be the most sensitive parameter for detecting blood volume losses.
Reproducibility
When expressed as bias ± precision, the reproducibility of echocardiographic (D =0.00±0.004 cm) and Doppler (VTIao =0.11±0.66 cm, V =0.00±0.14 m/s) parameters was excellent.
Discussion
The results of this study demonstrated that ΔV, obtained using esophageal Doppler, was an index highly sensitive to graded hemorrhage in rabbits. The esophageal Doppler is a non-invasive monitoring technique introduced in 1970, in patients receiving general anesthesia [11]. Using a small probe with an M-mode echocardiography to measure aortic diameter and pulsed Doppler to record descending aorta blood velocity, esophageal Doppler provides an accurate aortic blood flow measurement [9, 12, 13]. In patients, good correlations were found between cardiac output measured using esophageal Doppler and thermodilution [9, 14]. Therefore, esophageal Doppler appears to be a suitable technique for monitoring the respiratory variation of aortic blood flow reflecting the respiratory changes in stroke volume.
The magnitude of respiratory variation of stroke volume has been demonstrated to be a marker of preload-dependence and, thus, of hypovolemia and preload fluid responsiveness [1]. By decreasing the venous return pressure gradient, mechanical insufflation decreases the right ventricular stroke volume if the right ventricle is sensitive to changes in preload. In this condition, the subsequent decrease in left ventricular filling may result in a decrease in left ventricular stroke volume. Therefore, the magnitude of the left ventricular stroke volume respiratory changes and, thus, the descending aorta blood flow should reflect the sensitivity of the heart to changes in preload induced by mechanical insufflation. Some experimental [1, 15] and clinical [3, 4, 5, 6, 7, 16] studies have supported this hypothesis. In a recent experimental study, the magnitude of the respiratory changes in aortic velocity time integral (recorded by transthoracic echocardiography at the level of the aortic annulus), was shown to be highly sensitive to provoked hemorrhage and blood restitution in rabbits receiving mechanical ventilation. Moreover, this dynamic parameter predicted fluid responsiveness more reliably than static markers of cardiac preload measured by echocardiography.
The superiority of dynamic parameters over static ventricular preload parameters in predicting fluid responsiveness in critically ill patients has been recently emphasized [17]. In this way, Feissel et al. [7], using transesophageal echocardiography, demonstrated that the measurement of respiratory variation of aortic blood velocity (at the level of the aortic annulus) was better than measurement of left ventricular dimensions for predicting the hemodynamic effects of volume expansion in septic shock patients under mechanical ventilation. Moreover, the respiratory changes in arterial pulse pressure that have been shown to reflect the respiratory changes in left ventricular stroke volume [18] were also demonstrated accurately to predict fluid responsiveness in mechanically ventilated patients with septic shock. Analysis of the respiratory changes in arterial systolic pressure has also been proposed to assess fluid responsiveness in mechanically ventilated patients [2, 4, 5].
The results of the present work are in agreement with these findings since the magnitude of the respiratory changes of PP and SBP correlated with the amount of blood withdrawn, and returned to baseline values after blood restitution. Interestingly, in comparison with respiratory changes in arterial pressure, the magnitude of ΔV appeared as the best index of blood volume losses, as indicated by ΔV changes of 6.7% compared with changes of 5.6% after 1 ml of blood withdrawal (normalized slope) and a correlation coefficient of 0.96 compared with 0.75. This is probably explained by the fact that aortic blood velocity and its respiratory changes are physiologically a more direct reflection of stroke volume and its respiratory changes than arterial pressure-derived parameters and their respiratory changes are.
The purpose of this paper is to test a new index using a non-invasive monitoring technique. Even if dynamic indices have previously been described using conventional echocardiography, this technique cannot be used for monitoring. In addition, in this study we tested the reliability of this technique both in detecting volume changes and in terms of responsiveness. To our knowledge no study using this technique and this kind of protocol has previously been reported. Further clinical studies are required to confirm these findings.
The present study may suffer from some limitations. First, although measurements of aortic blood velocity and flow by esophageal Doppler have been well validated in patients [9, 14], no data are available in small animals. Second, the animals studied received anesthetic agents which might have depressed sympathetic activity, as reflected by the absence of tachycardia during the progressive hemorrhage. Moreover, we did not modify sympathetic tone during the experiments, therefore our data cannot be applied readily to less anesthetized or awake animals. Heart failure animals were not studied, which limits our conclusions to normal heart animals. Because this study was performed using a model of volume static changes, the results cannot be extrapolated to any other cause of hypovolemia and further experiments are needed to validate this concept in other conditions, such as in septic shock for example. Lastly, an effect of right ventricular afterload changes during mechanical ventilation may be play a role in the respiratory changes of aortic blood flow velocity [19], even if this effect seems to be trivial [20].
In conclusion, in this mechanically ventilated rabbit model, a stepwise blood withdrawal period resulted in decreases in stroke volume, cardiac output, arterial pressures and velocity of descending aorta blood, recorded using esophageal Doppler, while the magnitude of the respiratory changes in descending aorta blood velocity increased. Each of these variables returned to baseline values after blood restitution. Among all the parameters studied, respiratory variation in the descending aorta blood velocity was found to be the most accurate to hemorrhage and the best correlated to the amount of blood withdrawal. Therefore, esophageal Doppler seems to be a suitable technique for monitoring patients in ICUs, to detect hypovolemia and for a follow-up during a fluid challenge.
References
Slama M, Masson H, Teboul JL, Arnout ML, Susic D, Frohlich E, Andrejak M (2002) Respiratory variations of aortic VTI: a new index of hypovolemia and fluid responsiveness. Am J Physiol Heart Circ Physiol 283:H1729–1733
Perel A (1998) Assessing fluid responsiveness by the systolic pressure variation in mechanically ventilated patients. Anesthesiology 89:1309–1310
Berkenstadt H, Margalit N, Hadani M, Friedman Z, Segal E, Villa Y, Perel A (2001) Stroke volume variation as a predictor of fluid responsiveness in patients undergoing brain surgery. Anesth Analg 92:984–989
Tavernier B, Makhotine O, Lebuffe G, Dupont J, Scherpereel P (1998) Systolic pressure variation as a guide to fluid therapy in patients with sepsis-induced hypotension. Anesthesiology 89:1313–1321
Coriat P, Vrillon M, Perel A, Baron JF, Le Bret F, Saada M, Viars P (1994) A comparison of systolic blood pressure variations and echocardiographic estimates of end-diastolic left ventricular size in patients after aortic surgery. Anesth Analg 78:46–53
Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, Richard C, Pinsky MR, Teboul JL (2000) Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med 162:134–138
Feissel M, Michard F, Mangin I, Ruyer O, Faller JP, Teboul JL (2001) Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest 119:867–873
Tousignant CP, Walsh F, Mazer CD (2000) The use of transesophageal echocardiography for preload assessment in critically ill patients. Anesth Analg 90:351–355
Cariou A, Monchi M, Joly LM, Bellenfant F, Claessens YE, Thebert D, Brunet F, Dhainaut JF (1998) Noninvasive cardiac output monitoring by aortic blood flow determination: evaluation of the Sometec Dynemo-3000 system. Crit Care Med 26:2066–2072
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1(8476): 307–310
Side CD, Gosling RG (1971) Non-surgical assessment of cardiac function. Nature 232:335–336
Lavandier B, Cathignol D, Muchada R, Xuan BB, Motin J (1985) Noninvasive aortic blood flow measurement using an intraesophageal probe. Ultrasound Med Biol 11:451–460
Singer M, Clarke J, Bennett ED (1989) Continuous hemodynamic monitoring by esophageal Doppler. Crit Care Med 17:447–452
Valtier B, Cholley BP, Belot JP, de la Coussaye JE, Mateo J, Payen DM (1998) Noninvasive monitoring of cardiac output in critically ill patients using transesophageal Doppler. Am J Respir Crit Care Med 158:77–83
Perel A, Pizov R, Cotev S (1987) Systolic blood pressure variation is a sensitive indicator of hypovolemia in ventilated dogs subjected to graded hemorrhage. Anesthesiology 67:498–502
Reuter DA, Felbinger TW, Schmidt C, Kilger E, Goedje O, Lamm P, Goetz AE (2002) Stroke volume variations for assessment of cardiac responsiveness to volume loading in mechanically ventilated patients after cardiac surgery. Intensive Care Med 28:392–398
Michard F, Teboul JL (2002) Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest 121:2000–2008
Jardin F, Farcot JC, Gueret P, Prost JF, Ozier Y, Bourdarias JP (1983) Cyclic changes in arterial pulse during respiratory support. Circulation 68:266–274
Vieillard-Baron A, Loubieres Y, Schmitt JM, Page B, Dubourg O, Jardin F (1999) Cyclic changes in right ventricular output impedance during mechanical ventilation. J Appl Physiol 87:1644–1650
Vieillard-Baron A, Augarde R, Prin S, Page B, Beauchet A, Jardin F (2001) Influence of superior vena caval zone condition on cyclic changes in right ventricular outflow during respiratory support. Anesthesiology 95:1083–1088
Acknowledgements
Very special thanks to Olivier des Horts and Sophie Mourier from Agilent technologies (Philips Medical Systems), who made it possible for us to carry out this study by lending us an echocardiographic machine, and to Olivier Fructus from Arrow who lent us the esophageal Doppler advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by Philips Medical System and Arrow.
Rights and permissions
About this article
Cite this article
Slama, M., Masson, H., Teboul, JL. et al. Monitoring of respiratory variations of aortic blood flow velocity using esophageal Doppler. Intensive Care Med 30, 1182–1187 (2004). https://doi.org/10.1007/s00134-004-2190-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-004-2190-z