Abstract
Objective
To assess the predictive ability of preillness and illness variables, impact of care, and discharge variables on the post-intensive care mortality.
Setting and patients
5,805 patients treated with high intensity of care in 89 ICUs in 12 European countries (EURICUS-I study) surviving ICU stay.
Methods
Case-mix was split in training sample (logistic regression model for post-ICU mortality: discrimination assessed by area under ROC curve) and in testing sample. Time to death was studied by Cox regression model validated with bootstrap sampling on the unsplit case-mix.
Results
There were 5,805 high-intensity patients discharged to ward and 423 who died in hospital. Significant odds ratios were observed for source of admission, medical/surgical unscheduled admission, each year age, each SAPSII point, each consecutive day in high-intensity treatment, and each NEMS point on the last ICU day. Time to death in ward was significantly shortened by different source of admission; age over 78 years, medical/unscheduled surgical admission; SAPSII score without age, comorbidity and type of admission over 16 points; more than 2 days in high-intensity treatment; all days spent in high treatment; respiratory, cardiovascular, and renal support at discharge; and last ICU day NEMS higher than 27 points
Conclusions
Worse outcome is associated with the physiological reserve before admission in the ICU, type of illness, intensity of care required, and the clinical stability and/or the grade of nursing dependence at discharge.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Age, chronic health status, and severity of illness are known to affect mortality after discharge from an intensive care unit (ICU) [1, 2, 3, 4, 5, 6, 7, 8, 9]. A significant role is also often played by premature ICU discharge [2, 4, 5, 7, 9], often caused by the overwhelming demand on ICU resources [6] and generally recognized by the instability of vital function [9] or by the need of high nursing support [3, 8]. The relevant question behind these professional issues concerns the appropriateness of availability/use of ICU resources in the hospital [10].
This study analyzed the relationship between the clinical variables considered to be related to the need of care in the ICU and post-ICU mortality. In addition to variables commonly used in such studies, we also used a new classification identifying the intensity of treatment on a daily basis at patient level [11, 12].
Materials and methods
The study used data on all consecutive admissions to 89 ICUs in 12 European countries between October 1994 and February 1995 [13] collected by the Foundation for Research on Intensive Care in Europe. The ICUs included 74 general, 9 medical, and 6 surgical; pediatric, cardiac, and neurosurgical units were excluded
Data base
The data base included the following variables.
Case-mix
These variables included: source of admission (operating theater, recovery room, emergency room, ward, other ICU, other hospital, other), age, diagnosis [14]; type of admission (medical, surgical scheduled and surgical emergency), and severity of illness (Simplified Acute Physiological Score, SAPSII) [15]. The APS II (acute physiology score of SAPSII) was used to evaluate the effect of physiological variables independently of age, comorbidity, and type of admission.
Nursing workload
Data on the Nine Equivalents Nursing Manpower Score (NEMS [16]) were collected daily, and the cumulative score per patient was calculated. The NEMS of the last day was divided into three levels, or classes [18, 17], and the degree of respiratory, cardiovascular, and renal support was recorded.
Intensity of care
To classify intensity of daily medical treatment in the ICU we used the six NEMS items relating to organ failure: monitoring, mechanical ventilation support/continuous positive airway pressure, multiple vasoactive medication, supplementary ventilatory care, single vasoactive medication, dialysis [11]. Intensity of care was classified as highly intensive/complex in the case of monitoring coupled with invasive/active support of respiration and/or with multiple vasoactive drugs or with a less invasive support of at least two organs/systems (respiratory, circulatory and renal). All other combinations of interventions were classified as less intensive treatment.
Use of resource
We used length of stay (LOS) and intensity of care in the ICU: overall and consecutive days in high treatment, i.e. "critical" length of stay, trends in clinical courses [12], and the length of low-intensity treatment before discharge.
Outcome
ICU and in-hospital death/survival was recorded. The protocol did not include do-not-resuscitate orders or their equivalent, or institutional ethical attitude.
Patients
Exclusion criteria were: patients with missing data and discharged before registration of the first daily NEMS; patients who did not receive intensive/complex treatment; and patients discharged to other ICUs and to other hospitals.
The data set included 13,472 patients without missing data and with at least one NEMS record. Of these, 12,615 were discharged to ward, and 7,191 of these receiving high-intensity treatment were analyzed; 1,386 died in the ICU (19.3%) and 5,805 were discharged to ward. After ICU discharge 423 patients (7.3%) died in hospital in 15.4±20.5 days (median 9), and the others left hospital in 14.5±15.8 days (median 10). Patients receiving intensive treatment and discharged to intermediate care units were separately handled (n=609). The latter patients had 2.7% hospital mortality (p=0.000 vs. mortality of patients discharged to ward). Most of them were surgical scheduled patients [78.2 vs. 37.1%, p=0.000, with 28.8.±11.7 SAPSII points (median 27) vs. 33.5±14.2 (median 31), p=0.000].
The following variables were considered. At admission: source, age, type, diagnostic categories, high-intensity treatment, severity score; at ICU stay: LOS, total NEMS score, number of consecutive and total days spent in high-treatment, number of days in low-treatment, entire ICU stay in high treatment or any other combination between high and low treatment [12], number of low-intensity days upon discharge (0, 1, 2, >2); on discharge day: NEMS points, single (renal) or minor (respiratory or cardiovascular) organ support, discharge at night (22.00–06.59 or 00.00–04.59 hours).
Statistical analysis
Data are reported as mean ±standard deviation, median, and interquartile range (25th–75th percentiles, IQR). Quantitative variables were compared with unpaired Student's t test; the χ2 test was used for categorical variables. Using the random numbers supplied by statistical program, the dataset of patients discharged to ward was split in two samples, one training set and one testing set of equal size. On the training set we developed a logistic regression model for post-ICU mortality; we tested all available variables and selected the independent variables with a stepwise procedure. The odds ratio (OR) and corresponding 95% confidence limit (CI) for each variable were computed. The ability of the model to predict the outcome of patients (dead or alive) was assessed by the area under the receiver operating characteristic (ROC) curve. Calibration ability for the model was measured in the testing set as the capability in matching estimated to observed mortality and was evaluated by Hosmer-Lemeshow goodness-of-fit statistic.
Association of ICU-related variables with time to death in hospital after ICU discharge was studied by Cox regression model on the unsplit case-mix. The proportional hazard assumption was tested by Schoenfeld residuals, and we introduced only the variables satisfying the criterion into the model. Independent variables predicting early hospital death were selected with a stepwise procedure. Bootstrap sampling was used to validate the model, and bootstrap hazard ratios (HR) and corresponding 95% CI for each variable were computed. To assess the impact on post-ICU mortality of the discharge to intermediate units we performed a sensitivity analysis by comparing the hospital outcome of the enrolled patients discharged to ward with that of patients discharged to Intermediate Units. The level of significance was set at p<0.05 for all analyses. Statistical analysis was performed with Intercooled Stata 7.0 statistical package (Stata, Tex., USA).
Results
Survivors and nonsurvivors to hospital discharge differed regarding the following: age; source and type of admission; SAPSII/APS II score; ICU LOS/'critical LOS' (number of consecutive days with high-intensity of care); cumulative NEMS score, NEMS score on the last ICU day among classes; and degree of organ support inferred by intensity/complexity of care during the last ICU day (Table 1).
Multivariate analysis showed post-ICU mortality to be significantly related to: medical admission (OR 3.59, 95% CI 1.71–7.55); admission from recovery room (OR 3.34, 95% CI 1.17–9.58); admission from other ICU (OR 3.15, 95% CI 1.47–6.72); unscheduled surgical admission (OR 2.59, 95% CI 1.57–4.27); admission from ward (OR 2.19, 95% CI 1.49–3.23); admission from operating theater (OR 2.05, 95% CI 1.02–4.13); every consecutive day in high-intensity treatment (OR 1.05, 95% CI 1.02–1.07); each SAPSII point (OR 1.03, 95% CI 1.02–1.04); each year of age (OR 1.03, 95% CI 1.02–1.04); and each NEMS point on the last ICU day (OR 1.02, 95% CI 1.00–1.05).
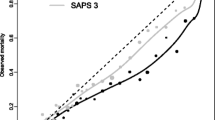
The area under the ROC curve was 0.77 either for training and testing sample. The model calibration was assessed in the testing sample (Hosmer-Lemeshow χ2 test p=0.17; Fig. 1). A Cox regression model validated by bootstrap technique was applied on 5,805 patients. Post-ICU time to death (Fig. 2) was independently shortened by: medical admission (HR 4.05, 95% CI 2.50–6.57); unscheduled surgical admission (HR 2.57, 95% CI 1.82–3.62); age over 78 years (90th percentile; HR 2.41, 95% CI 1.87–3.09); admission from recovery room (HR 2.28, 95% CI 1.16–4.48); all ICU stay spent in high treatment (HR 1.98, 95% CI 1.40–2.81); APS II higher than 16 points (median value; HR 1.78, 95% CI 1.42–2.23); last ICU day with only dialysis or the minimal support of respiration or circulation (HR 1.68, 95% CI 1.23–2.31); admission from operating theater (HR 1.60, 95% CI 1.03–2.47); last ICU day NEMS higher than 27 points (75th percentile; HR 1.51, 95% CI 1.11–2.05); admission from ward (HR 1.44, 95% CI 1.12–1.86); and more than 2 days on high-intensity treatment during ICU stay (median value; HR 1.28, 95% CI 1.03–1.59.
Variables selected by logistic regression model to predict post-ICU mortality. OT Operating theater; RR recovery room; adm admission; M medical; SU unscheduled surgical; SAPS II Simplified Acute Physiological Score; critical LOS each consecutive day spent in high treatment; l-NEMS Nine Equivalents Nursing Manpower Score of the last ICU day
Variables shortening post-ICU time to death according to Cox regression model validated by bootstrap technique. OT Operating theater; RR recovery room; adm admission; M medical; SU unscheduled surgical; APS II Simplified Acute Physiological Score controlling for age, comorbidity, and type of admission; HT ICU day with high-intensity level of care; HT trend all ICU days spent in high treatment; l-LT O supply last ICU day with low-intensity level of care and minor organ supply; l-NEMS Nine Equivalents Nursing Manpower Score of the last ICU day
Discussion
Post-ICU mortality can be explained by poor physiological patient reserves before illness, severity of illness, intensity of the process of care, degree of organ functions support, and nursing dependence at discharge [1, 2, 3, 4, 5, 6, 7, 8, 9]. Several proxy measures of these causes have been used, and their reported incidence is higher in patients not surviving after ICU discharge: age [1, 2, 3, 4, 5, 6, 7, 8, 9], chronic health status (e.g., malignancy [1, 2, 6]), underlying pathology (sepsis [1], respiratory and abdominal disorders [1, 2], organ failure [8, 9]), severity at ICU admission [1, 2, 3, 4, 5, 6, 7, 8, 9] and discharge [9], and nursing need at discharge [3, 5, 8]. Most of the authors [2, 3, 4, 5, 6, 7, 8, 9] indicate the shortage of ICU/high-dependency beds to be the reason for early discharge of patients to the ward (with insufficient normalization of physiology). These findings challenge the current availability of ICU resources in Europe [10].
We investigated the weight of all the variables independently associated with post-ICU mortality in an adult international case-mix (with the exclusion of neuro-/cardiosurgical patients). The strength of this study is based on three factors. Firstly, the large international database encompasses different types of patients and different ways of working. Secondly, only severely ill patients undergoing high level of care [11] were enrolled in the study. Thirdly, the use of markers of process of care in ICU [12] allowed the integration of physiological derangement and markers of treatment in the ICU, as recommended elsewhere [4]. The markers of process of care were LOS [8], number of days (consecutive or not) spent in high-intensity of care, trends of treatment (high and low intensity) in the courses of illness [12] and cumulative NEMS points [8]. These can be considered a proxy for physiological reserve consumption during the critical illness. In addition to, the number of low-treatment days before discharge is also considered an indicator of the external demand of ICU beds and physiological stability [9]. On the last day the intensity of respiratory, cardiovascular or renal support [16], the dependence from nursing care (NEMS and class distribution [17]), and discharge at night [5, 7] were used to define the patient stability at the discharge and indirectly to assess the pressure on ICU.
Our multivariate analysis demonstrates more extensively than other reports [3, 7, 8, 9, 17] that at least one variable from each group (preillness, illness, process of care, discharge status) has an independently effect on post-ICU mortality of intensively treated patients (Fig. 1), i.e., location before ICU admission (other ICU, ward, operating theater, recovery room [4, 8, 18]), medical and unscheduled surgical status. Moreover, in-hospital mortality increases with severity score evaluating comorbidity and physiological derangement at admission [3, 9, 17], the number of consecutive days spent in high-intensity care (acuity/intensity of treatment), and the nursing workload on the last day [3, 8] as marker of the appropriateness of discharge. This study found that the number of days in high-intensity care, rather than the total number of ICUs days (as found by Daly et al. [9]), and the cumulative NEMS score (weak statistical weigh also in the Moreno et al. study [8]) can be taken as a proxy of process of care responsible of the consumption of physiological reserve.
Each group of causes is also independently associated with shortened in-hospital survival time (Fig. 2). These include admission from recovery room, operating theater, and ward, advanced age (as noted by Wallis et al. [2]), medical and unscheduled surgical admission, and severity of illness. High-intensity care is important when present more than 2 days, even if not consecutive days. Clinical instability at (or "inappropriateness" of) discharge was associated with a NEMS greater than 27 points in the last day (one nurse per 1.5 patients), consistent with other reports [3, 5]. Cox analysis also highlighted low-intensity respiratory, cardiovascular, and renal organ supply on the last ICU day as a sign of residual instability at discharge [8]. The number of days spent in low treatment/observation monitoring before ICU discharge [9] and night discharge [5, 7] did not affect outcome, in contrast to that which was found by a study carried out in a single country [5, 7, 9]. Differences in organization of intensive medicine across Europe may have reduced the importance of this scenario.
While we agree that premature discharge means pressure on ICUs [2, 5, 7, 9], we have no information on another possible effect, i.e., decision to withdraw life-sustaining treatments [2, 6, 18]. Of note, the importance of diagnosis was discounted in all of our analyses. This finding, apparently illogical, seems to indicate the insufficiency of the use of the main diagnostic categories [14] that we made to reduce the huge numbers of diagnoses. The sensitivity analysis on hospital mortality of patients discharged to intermediate units shows a better outcome than that of patients discharged to the ward, as proposed [3, 4, 10] or demonstrated [5, 7]. In any case these were prevalently surgical scheduled patients, and their conditions were significantly less severe than those of patients discharged to ward, at odds with the report of Beck et al. [5]. This surprising finding could be explained by the small number (24%) of the participating ICUs with intermediate unit availability in a large European sample.
In conclusion, post-ICU mortality and time at which death occurs are chiefly and independently related to initial severity of illness, physiological stability, and, even in stable patients, physiological reserve at discharge as shown by the number of days with high-intensity care. These variables, with the single exception of stability at discharge, are not under the direct control of intensivists.
References
Ridley S, Purdie J (1992) Cause of death after critical illness. Anaesthesia 47:116–119
Wallis CB, Davies HT, Shearer AJ (1997) Why do patients die on general wards after discharge from intensive care units? Anaesthesia 52:9–14
Smith L, Orts CM, O'Neil I, Batchelor AM, Gascoigne AD, Baudouin SV (1999) TISS and mortality after discharge from intensive care. Intensive Care Med 25:1061–1065
Goldhill DR, Sumner A (1998) Outcome of intensive care patients in a group of British intensive care units. Crit Care Med 26:1337–1345
Beck DH, McQuillan P, Smith GH (2002) Waiting for the break of dawn? The effects of discharge time, discharge TISS scores and discharge facility on hospital mortality after intensive care. Intensive Care Med 28:1287–1293
Lawrence A, Havill JH (1999) An audit of deaths occurring in hospital after discharge from the intensive care unit. Anaesth Intensive Care 27:185–189
Goldfrad C, Rowan K (2000) Consequences of discharge from intensive care at night. Lancet 355:1138–1142
Moreno R, Miranda DR, Matos R, Fevereiro T (2001) Mortality after discharge from intensive care: the impact of organ system failure and nursing workload use at discharge. Intensive Care Med 27:999–1004
Daly K, Beale R, Chang SWR (2001) Reduction in mortality after inappropriate early discharge from intensive care unit: logistic regression triage model. BMJ 322:1–6
McPerson K (2001) Safer discharge from intensive care to hospital wards. BMJ 322:1261–1262
Iapichino G, Radrizzani D, Bertolini G, Ferla L, Pasetti G, Pezzi A, Porta F, Miranda DR (2001) Daily classification of the level of care. A method to describe clinical course of illness, use of resources and quality of intensive care assistance. Intensive Care Med 27:131–136
Iapichino G, Radrizzani D, Ferla L, Pezzi A, Porta F, Zanforlin G, Miranda DR (2002) Description of trends in the course of illness of critically ill patients. Markers of intensive care organization and performance. Intensive Care Med 28:985–989
Miranda DR, Ryan DW, Schaufeli WB, Fidler V (1998) Organisation and management of intensive care: a prospective study in 12 European countries. Springer, Berlin Heidelberg New York
Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy Dj, Lotring T, Damiano A (1991) The APACHE III prognostic system: risk prediction of hospital mortality for critically ill hospitalized adults. Chest 100:1619–1636
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPSII) based on a European-North American multicenter study. JAMA 270:2957–2963
Miranda DR, Moreno R, Iapichino G (1997) Nine Equivalents of nursing Manpower Score (NEMS). Intensive Care Med 23:760–765
Cullen DJ, Civetta JM, Briggs BA, Ferrara LC (1974) Therapeutic intervention scoring system: a method for quantitative comparison of patient care. Crit Care Med 2:57–60
Azoulay E, Adrie C, De Lassence A, Pochard F, Moreau D, Thiery G, Cheval C, Moine P, Garrouste-Orgeans M, Alberti C, Cohen Y, Timsit J-F (2003) Determinants of postintensive care unit mortality: a prospective multicenter study. Crit Care Med 31:428–432
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported in part, by the Foundation for Research on Intensive Care in Europe (FRICE) and by a grant from the Commission of the European communities (BMH1-CT93-1340)
Rights and permissions
About this article
Cite this article
Iapichino, G., Morabito, A., Mistraletti, G. et al. Determinants of post-intensive care mortality in high-level treated critically ill patients. Intensive Care Med 29, 1751–1756 (2003). https://doi.org/10.1007/s00134-003-1915-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-003-1915-8