Abstract
Objective
To report five patients on hemodialysis via an indwelling central venous catheter who developed a thrombus located exclusively on the right atrial wall opposing the emptying site of the superior vena cava and to determine the possible cause of this location.
Design
Transthoracic echocardiography was performed in four of the patients as work-up for suspected endocarditis or pulmonary embolism and in one patient for syncope evaluation. The right atrial clots were confirmed in all the patients by transesophageal echocardiography.
Design and setting
General intensive care unit of a university hospital, tertiary referral center.
Patients
Five patients with end-stage chronic renal failure on hemodialysis via subclavian or internal jugular vein catheter.
Interventions
Three of these patients underwent surgical thrombectomy, and two others were medically treated.
Measurements and results
The clots were 2–4 cm in length and three of them were infected. Two of the three surgically treated patients and one of the two medically treated patients died. All the patients had the catheter tip in the right atrium, in two of them the bent catheter rubbed the atrial endocardium, and in all the cases the clot was located on the atrial free wall facing the superior vena cava emptying.
Conclusions
We postulate that the mechanism of thrombus formation at this location is related to friction of the catheter on the atrial endocardium, and therefore positioning the distal segment of the central venous catheters in the right atrium should be avoided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thrombosis is a relatively common complication of central venous indwelling catheters (CVC), with an incidence between 1.9% and 42% depending on the site of catheter insertion for comparable duration of catheterization and patients characteristics [1, 2]. Although right atrial (RA) thrombi are thought to be uncommon, a recent report showed that within 6–8 weeks of catheter insertion 12.5% of patients had thrombus at the tip of the catheter [3]. In that study all the thrombi were visualized in those patients in whom the catheter tip was in the RA and none in those with catheter tip at junction of the superior vena cava (SVC) and RA or within the SVC itself. The common mechanisms of clot formation appear to be related to intraluminal clot elongation [4] or endothelial RA damage secondary to the continuous friction of the catheter on the RA free wall [5].
We report five patients with end-stage renal failure on hemodialysis (Table 1) with indwelling RA catheters and in whom a clot was found by transthoracic echocardiography (TTE) and confirmed by transesophageal echocardiography (TEE) to be in the RA attached to its free wall opposite to the SVC emptying site.
Case reports
Patient 1
A 23-year-old woman with a history of type 1 diabetes, hypertension, and end-stage renal disease (ESRD) was admitted with a 4-day history of high fever and chills 6 weeks after placement of a right internal jugular catheter for initiation of dialysis. Examination showed her to have a temperature of 40.5°C and a 3/6 holosytolic murmur at the left lower sternal border. Blood and catheter tip cultures grew methicillin-sensitive Staphylococcus aureus. TTE revealed moderate tricuspid regurgitation and a possible a RA mass. Confirmed by TEE, the mass was attached to the RA free wall opposite site to SVC emptying. This mass was surgically excised, and on pathology an infected 3.5×3.0×0.8 cm clot was found. Following surgery the patient was transferred to a long-term care facility.
Patient 2
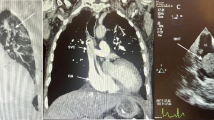
A 57-year-old man with history of hypertension, diabetes mellitus, and ESRD on hemodialysis via a subclavian vein inserted Quinton catheter for the past 6 months was admitted for recurrent syncope. Examination revealed increased jugular venous pressure, S3, and mild peripheral edema. TTE showed an enlarged, hypocontractile left ventricle and a RA mass of 3.1×2.5 cm prolapsing in diastole into the right ventricle (Figs. 1, 2) confirmed by TEE to be located on the atrial wall opposite to SVC emptying. At surgery an organized thrombus was found, but the patient developed postoperative worsening of congestive heart failure and died 8 days later.
Patient 3
A 55-year-old woman with 23-year history of diabetes mellitus, hypertension, and ESRD on hemodialysis via subclavian catheter for the past 4 months was admitted with 2 days history of fever and chest pain. On examination she had a temperature of 38.3°C and a 2/6 systolic murmur at the apex. Blood cultures grew Enterococcus and methicillin-resistant S. aureus. TTE showed moderate mitral regurgitation and a RA mass attached to the dialysis catheter which was confirmed by TEE. The 3.7×3.0 cm mass had the same location as those in the previous two patients. Anticoagulation and antibiotics were initiated, but the patient developed pulmonary embolism, myocardial infarction, and pulseless electrical activity and died.
Patient 4
A 55-year-old woman with history of diabetes mellitus, below-knee amputation, and 6-year history of ESRD on hemodialysis for the past 8 months via subclavian CVC was admitted with fever and diarrhea. On examination we found a 3/6 systolic murmur at left lower sternal border. Blood cultures grew S. aureus and yeast (non-Candida albicans). TEE revealed a 3.9×1.4 cm RA mass attached to its free wall and severe tricuspid regurgitation. In spite of anticoagulation therapy and antibiotics the patient continued to be febrile and developed septic pulmonary embolism and endophthalmitis. Atriotomy was performed, and a 4×2.1 cm infected thrombus was found attached to the RA endocardium facing the SVC. The postoperative course was complicated by pulmonary embolism and cardiogenic shock, and the patient died the following day.
Patient 5
A 23-year old woman with diabetes mellitus and ESRD on dialysis via subclavian Quinton catheter underwent TTE for evaluation of pericardial effusion. This revealed a moderately sized pericardial effusion, moderate tricuspid regurgitation, and a RA mass. On TEE a 2.5×2.1×1.3 cm RA mass attached to its free wall just was identified. The tip of the Quinton catheter was in the RA moving back and forth being embedded into the thrombus. The catheter was removed, and anticoagulation therapy was initiated. After 6 months of coumadin treatment the thrombus resolved completely, and the clinical course remained uneventful.
Discussion
The incidence of thrombus formation in SVC or RA associated with an indwelling CVC varies from 2% to 17%, with a systemic infection rate of 6.7% [1, 2, 3, 4, 5]. A number of case reports have been published regarding CVC-associated RA thrombi, and the postulated mechanisms include intraluminal clot elongation [3], fluid dynamics of the RA with the catheter tip being located in the region of relative separation or stagnation of blood flow [2], and mechanical irritation of the RA free wall [4].
The five patients presented here had thrombosis associated with CVC placed for hemodialysis; three of them had signs of systemic infection, and all had the catheter tip extending into the RA. The thrombi were located on the RA wall opposite to the entrance of the SVC. Trauma to the free wall by the catheter tip or, less likely, by the jet of infused medication might be the underlying mechanism of endothelial damage and clot formation, as previously proposed [4]. In our series only one patient had an uneventful course. In this case the clot was an incidental finding on TTE, and there were no symptoms related to the presence of the blood clot. However, three patients presented with systemic infection and one with syncope possibly due to transient obstruction of right ventricle inflow. It is clear from the surgical specimen obtained from these patients that with long-term indwelling catheters the thrombus can organize and infiltrate the RA musculature and, secondarily, become infected, with dire consequences.
In a recent prospective study [2] in which 55 patients were evaluated by TEE 46% of the catheters placed in the RA developed thrombus within 1 week, in contrast to patients in whom the catheter was at the RA-SVC junction or within the SVC in whom no thrombus was found at 1 or 6–8 weeks. All the patients who developed thrombus in that study were asymptomatic. There have been cases reported in the literature with infected thrombus in the RA in the presence of a CVC [3, 5, 6, 7, 8, 9, 10, 11]. Although not very common, an infected atrial thrombus can be potentially lethal if not treated, and in many cases surgical removal is required [10].
In cases of a RA thrombus associated with CVC, if the thrombus is not adherent to the atrial wall or SVC, it has been proposed that removal of the CVC might result in massive pulmonary emboli and possible death [8]. Therefore the use of TEE even before the CVC is removed can be advocated. Apart from removing the CVC antibiotic use is of utmost importance. Most common organism in these cases appears to be Staphylococcus species [11]. Anticoagulation to prevent further propagation of the thrombus and emboli has been suggested, but occasionally this is not effective [12]. Thrombolytic therapy or IIb/IIIa receptor antagonist has also been used in some cases [13, 14]. In the case of a freely moving thrombus or prolapsing thrombus across the tricuspid valve the risk of massive pulmonary embolism is great, and thrombolytics should probably not be used [15]. Although a meta-analysis of such cases showed no difference in outcome after treatment with heparin, thrombolytics, or surgery, the reviewed patients were not infected. Therefore it has been suggested that when there is an infected RA thrombus, surgery should be considered [10].
Based on the results of this report and on the previous studies we believe that CVC should be advised to be positioned in such a manner to avoid its tip protruding into the RA and potentially contributing to a clot formation. Although routine echocardiographic surveillance of such patients is not considered indicated by the present guidelines, further studies are warranted to determine the cost-effectiveness of such a strategy for identifying the presence of an intra-atrial thrombus at an early stage of development when anticoagulation therapy might be more effective and the potential for infection is less threatening.
References
Merrer J, De Jonghe B, Golliot F, Lefrant JY, Raffy B, Barre E, Rigaud JP, Casciani D, Misset B, Bosquet C, Outin H, Brun-Buisson C, Nitenberg G, French Catheter Study Group in Intensive Care (2001) Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA 286:700–707
Timsit JF, Farkas JC, Boyer JM, Martin JB, Misset B, Renaud B, Carlet J (1988) Central vein catheter-related thrombosis in intensive care patients: incidence, risks factors, and relationship with catheter-related sepsis. Chest 114:207–213
Gilon D, Schechter D, Rein AJ, Gimmon Z, Or R, Rozenman Y, Slavin S, Gotsman MS, Nagler A (1998) Right atrial thrombi are related to indwelling central venous catheter position: insights into time course and possible mechanism of formation. Am Heart J 135:457–462
Murphy PT, Sivakumaran M, Ghosh K, Chapman CS, Wood JK (1994) Non fatal massive right atrial thrombosis associated with Hickman catheter in an adult receiving chemotherapy for acute leukemia. Postgrad Med J 70:520–521
Fuchs S, Pollak A, Gilon D (1999) Central venous catheter mechanical irritation of the right atrial free wall: a cause for thrombosis formation. Cardiology 91:169–172
Hollingsed MJ, Morales JM, Roughneen PT, Burch KD (1997) Surgical management of catheter tip thrombus: surgical therapy for right atrial thrombus and fungal endocarditis (Candida tropicalis) complicating paediatric sickle-cell disease. Perfusion 12:197–201
Kentos A, Dufaye P, Jacobs F, De Smet JM, Serruys E, Thys JP (1995) Candida albicans septic thrombosis of the right atrium is associated with a central venous catheter. Clin Infect Dis 21:440–442
Spotnitz WD, Dent JM, Mintz PD, Erickson NL, Fechner RE, Groh MA (1994) Removal of an infected right atrial mass in a patient with sickle cell disease. Ann Thorac Surg 58:1762–1764
Tamary H, Goshen J, Carmi D, Yaniv I, Kaplinsky C, Cohen IJ, Zaizov R (1994) Long-term intravenous deferoxamine treatment for noncompliant transfusion-dependent beta-thalassemia patients. Isr J Med Sci 30:658–664
Horner SM, Bell JA, Swanton RH (1993) Infected right atrial thrombus-an important but rare complication of central venous lines. Eur Heart J 14:138–140
Korzets A, Katz S, Chagnac A, Katz M, Gafter U, Zevin D, Levi J (1994) An infected right atrial thrombus—a new complication of haemodialysis associated subclavian vein catheterisation. Nephrol Dial Transplant 9:1652–1654
Kinney EL, Allen RP, Weidner WA, Pierce WS, Leaman DM, Zelis RF (1979) Recurrent pulmonary emboli secondary to right atrial thrombus around a permanent pacing catheter: a case report and review of the literature. Pacing Clin Electrophysiol 2:196–202
Jolly N, Kaul UA, Khalilullah M (1991) Right atrial thrombus over eustachian valve-successful lysis with streptokinase. Int J Cardiol 30:354–356
Joshi P, Bullingham A, Soni N (1991) Septic atrial thrombus. A complication of central venous catheterisation. Anaesthesia 46:1030–1032
Borges AC, Reibis RK, Claus M, Baumann G (2001) Right atrial thrombus treated successfully with abciximab and heparin. J Thromb Thrombolysis 12:283–287
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghani, M.K., Boccalandro, F., Denktas, A.E. et al. Right atrial thrombus formation associated with central venous catheters utilization in hemodialysis patients. Intensive Care Med 29, 1829–1832 (2003). https://doi.org/10.1007/s00134-003-1907-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-003-1907-8