Abstract
Objective
To study left ventricular performance and hemodynamic abnormalities during different stages of dengue hemorrhagic fever (DHF).
Design and setting
Observational study in a tertiary medical school hospital.
Patients
Twenty-four patients with serologically confirmed diagnosis of dengue virus infection and DHF according to the WHO criteria.
Methods
Echocardiography was performed during toxic, convalescent stages and at least 2 weeks after discharge (recovery). Left ventricular ejection fraction, rate-corrected velocity of circumferential fiber shortening adjusted for end-systolic meridional wall stress (VCFC/ESS) Z score, end-diastolic volume Z score, cardiac index, heart rate, mean arterial pressure, and total systemic vascular resistance (SVR) were compared between different stages of DHF.
Results
Ejection fraction and VCFC/ESS were significantly lower during the toxic stage than after recovery. End-diastolic volume was low during toxic stage and returned to normal during convalescence and recovery. Cardiac index was low during the toxic stage due to decreased preload (low end-diastolic volume) and depressed left ventricular ejection fraction. Cardiac index remained subnormal during convalescence due to sinus bradycardia. Wide variation in heart rate during toxic stage resulted in a small, nonsignificant increase compared to recovery. With treatment, heightened SVR resulted in relatively normal mean arterial pressure throughout the course of the illness.
Conclusions
The mechanism of decreased cardiac output during toxic stage of DHF is complex. Decreased preload is accompanied by decreased left ventricular performance, and possibly a subnormal heart rate response in some patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dengue virus infection is one of the most important emerging infectious diseases affecting children in tropical countries [1]. Dengue hemorrhagic fever (DHF) occurs in some patients infected with the virus. Low cardiac output and shock are one of the most serious complications in DHF and may lead to death. Shock in DHF has been attributed largely to decreased intravascular volume from capillary leakage of plasma into interstitial space [2]. However, a few recent studies report a possible impairment of cardiac function in patients with DHF [3, 4]. The purpose of this study was to evaluate the left ventricular performance and hemodynamic abnormalities during toxic stage of DHF using echocardiography. Rate-corrected velocity of circumferential fiber shortening adjusted to the left ventricular wall stress (VCFC/ESS), a load-independent index of left ventricular contractility [5], was used as well as the ejection fraction as the indices for left ventricular performance.
Materials and methods
Study patients
The study was carried out in 24 children admitted with the diagnosis of DHF to King Chulalongkorn Memorial Hospital, Bangkok, Thailand, during April 2000–October 2001. Their demographic and clinical data are presented in Table 1. Serological studies (hemagglutination inhibition method) of acute and convalescent serum confirmed acute dengue viral infection in all subjects. DHF was diagnosed by the World Health Organization case definition [6] which includes (a) fever of 2–7 days, (b) hemorrhagic tendencies, (c) thrombocytopenia (<100,000/mm3), and (d) evidence of plasma leakage by rising of hematocrit more than 20% or presence of pleural effusion and/or ascites. No patient had prior history of heart disease or received intravenous inotropic support. The research protocol was approved by the Institutional Ethics Review Committee of the Faculty of Medicine, Chulalongkorn University. Informed consent was obtained from an appropriate guardian of each patient prior to enrollment.
Echocardiography
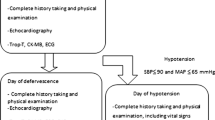
Echocardiographic studies were performed by one of the investigators (A.K. or M.S.) within 12 h of diagnosis of DHF, using Acuson 128XP/10 (Acuson, Mountain View, Calif., USA) or Aloka Prosound SSD5500 (Aloka, Tokyo, Japan) imaging system. An M-mode scan of the left ventricle obtained from a standard parasternal long-axis view, at the level of mitral valve tip was recorded on tape simultaneously with the electrocardiography and phonocardiography. Blood pressure was measured by oscillometric method (Dinamap 1486SX, Critikon, Tampa, USA) at the time of the echocardiography and the averaged value from three measurements was calculated. The study tapes were later reviewed randomly and measurements were carried out using the software provided with each echocardiographic machines, by two investigators (S.M. and A.K.), and the averaged values were used for further analysis. End-diastole was defined as the time at the onset of QRS complex and end-systole as the time at the first high frequency component of the second heart sound (S2) on phonocardiography. The following echocardiographic variables were collected: end-diastolic dimension (EDD, cm), end-systolic dimension (ESD, cm), posterior wall thickness at end-systole (ESPW, cm), ejection time (ET, s), and RR interval (s). Ejection time was measured from the earliest anterior motion of the left ventricular posterior wall endocardium to end-systole. All measurements were performed according to the American Society of Echocardiography guideline [7] in three consecutive heartbeats, and the averaged values were used for further calculation. Mean arterial pressure (MAP, torr) was used in lieu of end-systolic blood pressure for the calculation of end-systolic wall stress [8]. Echocardiography was repeated at follow-up at least 2 weeks after admission (at recovery). Seventeen patients (of 24) also had echocardiography on the last day of hospitalization, and the data were used to represent convalescent stage. These 17 patients recovered from DHF, had stable vital signs, and required no intravenous fluid at the time of echocardiography. The same echocardiographic machine was used for the same patient throughout the study. The following formulas were used for calculation of left ventricular performance index [5, 7, 9]:
-
Fraction shortening (FS)=(EDD-ESD)/EDD
-
End-diastolic volume (EDV) (Teichholz's method)=[7.0/(2.4+EDD)]*EDD3
-
End-systolic volume (ESV) (Teichholz's method)=[7.0/(2.4+ESD)]*ESD3
-
Ejection fraction (EF)=(EDV−ESV)/EDV
-
Velocity of circumferential fiber shortening (VCFC)=FS/ET
-
Rate-corrected VCFC=FS/(ET/square root of RR interval)
-
Meridional end-systolic wall stress (ESS)=[(1.35)(MAP)(ESD)]/{[(4)(ESPW)][1+(ESPW/ESD)]}
-
Heart rate (HR)=60/RR
-
Cardiac index (CI)=(EDV-ESV)(HR)/body surface area (BSA, m2)
-
Total systemic vascular resistance (SVR)=mean arterial pressure (MAP)/CI
Z scores of VCFC/ESS and EDV were derived based on the number of standard deviations from the normal population mean [5, 9].
Treatment of DHF
DHF was treated by house staff and attending staff of our department according to WHO [6] guidelines. Echocardiographic data were not provided to the clinician unless specifically asked for. All patients in this study received intravenous fluid as dictated by their clinical severity prior to echocardiography.
Statistics
Paired t test and analysis of variance with repeated measures were used to compare hemodynamic variables between stages of DHF. Data are expressed as mean ±SD. Statistical significance is defined as p<0.05.
Results
The first echocardiograms (toxic stage) were performed 4.6±3.0 h after the diagnosis of DHF. The echocardiograms representing convalescent and recovery stages of DHF were performed at 55.8±16.5 h and 29±23.6 days after the first echocardiogram, respectively. Echocardiographic variables during toxic stage compared with after recovery are presented in Table 2. The indexes of left ventricular systolic function (both EF and VCFC/ESS Z score) and index of preload (EDV Z score) were significantly lower during toxic stage. Systemic vascular resistance was significantly elevated during the toxic stage, resulting in similar mean arterial pressure despite decreased cardiac output caused by both decrease in preload and EF. The heart rate showed a small but nonsignificant increase during toxic stage compared to after recovery. While end-diastolic volume was uniformly low during toxic stage in all patients, there was a high degree of individual variation in ejection fraction, VCFC/ESS Z score and heart rate during toxic stage. Figure 1 demonstrates the changes in EF, VCFC/ESS Z score, EDV Z score, SVR, CI, HR, and MAP during toxic, convalescent and recovery stages of DHF.
Changes in left ventricular ejection fraction, rate-corrected velocity of left ventricular circumferential fiber shortening/end-systolic wall stress (VCFC/ESS) Z score, end-diastolic volume (EDV) Z score, total systemic vascular resistance (SVR), cardiac index, heart rate, and mean arterial blood pressure (MAP) during toxic stage, convalescent stage and after recovery of DHF. 1 Toxic stage; 2 convalescent stage; 3 recovery
Discussion
Low cardiac output and shock are one of the most serious consequences of dengue virus infection. Rising hematocrit, evidence of plasma leakage into abdominal and pleural cavity, and relatively normal central venous pressure [10] suggest intravascular hypovolemia as the cause of shock in DHF. Cardiac function was not previously thought to be significantly affected.
In 1998 Wali et al. [3] reported significantly lower in left ventricular EF in adult patients during toxic stage of DHF than after recovery. In the same year Kabra et al. [4] similarly reported decreased ejection fraction (<50%) in 16.7% of children with DHF. While ejection fraction is widely used as an index of left ventricular contractility, its value is sensitive to changes in preload and afterload of the left ventricle. Since diminished preload commonly occurs in a patient with DHF, a question still remains whether the left ventricular contractility is depressed in DHF [3].
Velocity of the left ventricular circumferential fiber shortening adjusted for end-systolic wall stress is an index of left ventricular contractility that is insensitive to change in preload and incorporates afterload [5]. It is one of the most accepted methods to evaluate myocardial contractility noninvasively. In this study we found that the VCFC/ESS Z score was low and significantly depressed compared to the value taken after the patients recovered from DHF. This finding indicates that myocardial contractility is impaired during toxic stage of DHF. The degree of depressed left ventricular contractility was mild (ejection fraction >45%) in most patients. The degree of cardiac dysfunction also varied markedly with some patient actually showed an increase in contractility during the toxic stage. In most patients with this degree of myocardial dysfunction, inotropic support would not be required to maintain hemodynamic stability and cannot replace early and effective replacement of plasma lost as the mainstay of treatment for DHF.
Two other important responses to maintain cardiac output and blood pressure during shock are increased heart rate and systemic vascular resistance. While systemic vascular tone is well maintained during DHF, the degree of heart rate response varies. Heart rate is significantly lower during the convalescent stage, a well recognized phenomenon in DHF [6]. One of the usual beliefs is that an increase in preload and cardiac output due to reabsorption of fluid during convalescent stage is the cause of this sinus bradycardia. Our findings disagree with this hypothesis because neither supernormal preload nor high cardiac output was seen during convalescent stage of DHF. In fact, the cardiac output during convalescent stage was still lower than normal primarily because of sinus bradycardia.
References
Jelinek T (2000) Dengue fever in international travelers. Clin Infect Dis 31:144–147
Rigau-Perez JG, Clak GG, Gubler DJ, Reiter P, Sanders EJ, Vorndam AV (1998) Dengue and dengue hemorrhagic fever. Lancet 352:971–977
Wali JP, Biswas A, Chandra S, Malhotra A, Aggarwal P, Handa R, Wig N, Bahl VK (1998) Cardiac involvement in dengue hemorrhagic fever. International J Cardiol 64:31–36
Kabra SK, Juneja R, Madhulika, Jain Y, Singhal T, Dar L, Kothari SS, Broor S (1998) Myocardial dysfunction in children with dengue hemorrhagic fever. Natl Med J India 11:59–61
Colan SD, Borow KM, Newmann A (1984) Left ventricular end-systolic wall stress-velocity of fiber shortening relation: a load independent index of myocardial contractility. J Am Coll Cardiol 4:715–724
World Health Organization (1997) Dengue hemorrhagic fever: diagnosis, treatments and control, 2nd edn. World Health Organization, Geneva (http://www.who.int/emc/diseases/ebola/Denguepublication/)
Shah DJ, DeMaria A, Kisslo J, Wayman A (1978) Recommendation regarding quantitative in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 58:1072–1083
Rowlan DG, Gutgesell HP (1994) Use of arterial pressure for noninvasive determination of left ventricular end-systolic wall stress in infants and children. Am J Cardiol 74:98–99
Colan SD, Parness IA, Spevak PJ, Sanders SP (1992) Developmental modulation of myocardial mechanics: age- and growth-related alterations in afterload and contractility. J Am Coll Cardiol 19:619–629
Pongpanich B (1983) Hemodynamic changes in shock associated with dengue hemorrhagic fever. Southeast Asian J Trop Med Public Health 18:326–330
Acknowledgements
The authors thank Profs. Chule Thisyakorn and Usa Thisyakorn for their advice and encouragement in this study. The study was supported by a Rachadapiseksompotch research grant from the Faculty of Medicine, Chulalongkorn University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khongphatthanayothin, A., Suesaowalak, M., Muangmingsook, S. et al. Hemodynamic profiles of patients with dengue hemorrhagic fever during toxic stage: an echocardiographic study. Intensive Care Med 29, 570–574 (2003). https://doi.org/10.1007/s00134-003-1671-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-003-1671-9