Abstract
Selenium is a trace element necessary for the growth of organisms. Moreover, selenium supplementation can improve the immunity and fertility of the body, as well as its ability to resist oxidation, tumors, heavy metals, and pathogenic microorganisms. However, owing to the duality of selenium, excessive selenium supplementation can cause certain toxic effects on the growth and development of the body and may even result in death in severe cases. At present, increasing attention is being paid to the development and utilization of selenium as a micronutrient, but its potential toxicity tends to be neglected. This study systematically reviews recent research on the toxicological effects of selenium, aiming to provide theoretical references for selenium toxicology-related research and theoretical support for the development of selenium-containing drugs, selenium-enriched dietary supplements, and selenium-enriched foods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selenium is a non-metallic element first discovered in the lead chamber mud of a sulfuric acid plant by Swedish chemist Jöns Jacob Berzelius in 1817. Schwarz et al. (1957) proved that selenium prevented dietary liver necrosis in rats and exudative diathesis in chickens. Rotruck et al. (1973) further confirmed its role as the active center of glutathione peroxidase (GSH-Px) in rats. In 1973, WHO declared it to be a trace element indispensable to human and animal life. Later, Awasthi et al. (1975) proved that selenium was a component of GSH-Px in human red blood cells. Since then, selenium has been deemed to be a trace element essential for human physiology, with selenium research entering a new era (Hybsier et al. 2017; Ullah et al. 2018). Biological effects of selenium have attracted considerable attention from experts in the medical and nutrition fields. Extensive reports have shown that selenium deficiency is related to a variety of diseases such as liver disease (Schwarz and Foltz 1957), Keshan disease (Fan and Kizer 1990), Kaschin-Beck disease (Peng and Yang 1991), kidney disease (Iglesias et al. 2013), cancer, cardio-cerebrovascular disease, acquired immunodeficiency syndrome (AIDS), and malignant malnutrition (Gladyshev et al. 1999; Xia et al. 2005), indicating that selenium plays an important role in human physiology. Selenium is known to have a protective effect as research has shown that it has several biological functions; it enhances immunity (Broome et al. 2004; Carlson et al. 2010), improves fertility (Ahsan et al. 2014), promotes the growth of livestock and poultry (Benton and Cook 1990; Marin-Guzman et al. 2000; Navarro-Alarcón et al. 2002; Kommisrud et al. 2005; Lubos et al. 2010; Shi et al. 2010; Roy et al. 2011; Hughes et al. 2012; Liu et al. 2014; Habibian et al. 2015; Aghwan et al. 2016; Talukdar et al. 2016), protects the liver, prevents some diseases (cancer, diabetes, AIDS, and cardio-cerebrovascular disease), delays aging, improves moods, regulates thyroid function (Rowntree et al. 2004; Lin et al. 2014), is resistant to heavy metals (Rusetskaya and Borodulin 2015), is resistant to pathogenic microorganisms (fungi, bacteria, viruses, and parasites) (Beck et al. 1994; Huang and Chen 1996; Qiao and Zhao 1997; Yu et al. 1997; Shisler et al. 1998; Wei et al. 2005; Huang 2006; Jaspers et al. 2007; Verma et al. 2008; Stone et al. 2010; Yu et al. 2011; Wang et al. 2012; Beheshti et al. 2013; Dagmar et al. 2014; De Miranda et al. 2014; Huang et al. 2014; Mahmoudvand et al. 2014; Cihalova et al. 2015; Liu et al. 2015; Mojtaba et al. 2015; Cheng et al. 2016; Dkhil et al. 2016; Guisbiers et al. 2016; Taylor et al. 2016; Guisbiers et al. 2017; Lv et al. 2018), and has an antioxidant activity (Wolonciej et al. 2016; Zhang et al. 2016b). However, selenium cannot be synthesized in the body and stored for a long time. Instead, it must be taken up by the body from external sources to maintain its dynamic balance (Rayman et al. 2008). The beneficial dose range of selenium is considerably narrow, and thus, a slight overdose above the necessary nutrient level will result in toxicity. Therefore, excessive selenium supplementation is harmful to the organism and may cause selenosis or induce mutations in severe cases (Han et al. 2016; Long et al. 2020). In 1990, the Joint FAO/IAEA/WHO Expert Committee on Trace Elements in Human Nutrition explicitly listed selenium as an “essential trace element in the human body” (Wang 2012). Moreover, in 2005, the US Food and Nutrition Board recommended that adults should not consume more than 400-µg selenium per day (Lee et al. 2015). In recent years, with the increase in cases of selenosis in humans as well as animals, such as horses, cattle, and sheep, due to long-term occupational exposure to excessive selenium compounds or due to excessive selenium in soils and foods in some areas, selenium toxicity research has been drawing increased attention across the globe (Wang and Niu 2010; Kohshahi et al. 2019). In the following sections, the progress in research on the toxic mechanism of selenium has been briefly reviewed to provide a theoretical basis for research on the development and utilization of selenium resources, their ecological risk assessment, and toxicological analysis.
Physical and Chemical Properties of Selenium
Physical Properties of Selenium
In nature, selenium is mainly distributed in rocks, soils, lakes, oceans, atmosphere, water, and the biosphere (Ren and Guo 2004), but the distribution is not uniform among many countries, with many areas naturally lacking selenium. In organisms, selenium mainly exists as selenomethionine obtained through diet and selenocysteine in selenoproteins; the former cannot be synthesized in vivo and serves as an unregulated storage form of selenium, so that when the dietary supply of selenium is interrupted, selenomethionine can provide selenium to the body, and can be substituted for protein methionine; the latter is a biologically active compound and also an important component of selenoproteins. Selenoproteins are the main functional form of selenium in the body, and there are more than 30 types of known selenoproteins so far, including nearly 15 that can be obtained through in vitro purification, which has provided a new basis for studying the functions of selenoproteins and their relationship with diseases (Kryukov et al. 2003; Qing and Zhang 2012).
Generally, selenium exists either as an element or as compounds, with the latter being the prevalent form. Studies have shown that the chemical species and quantity of selenium are closely related to its biological function, with the absorption efficiency, biological effect, and toxicity of selenium varying among different chemical species (Mu et al. 2004; Yu et al. 2006). The selenium element exists as solid powder in red or gray, with six solid allotropes, of which three are crystalline and the other three are amorphous. Nano-selenium (Nano-Se) is an elemental form of selenium with a valence of 0 in red and has a nanoscale size (generally < 60 nm in diameter) and possesses excellent nano-properties. Owing to the advantages of structural uniformity, small size, abundant unsaturated surface bonds, large surface area, high surface activity, wide range of safe concentrations, high bioavailability, and excellent bioactivity, Nano-Se has been widely used as a new selenium additive in livestock and poultry feed (Jia et al. 2005; Yoon et al. 2007; Zhang et al. 2008a, b; Zhou et al. 2009; Li et al. 2011, 2020; Hu et al. 2012; Forootanfar et al. 2014; Yang et al. 2016). Selenium compounds come in inorganic selenium compounds, such as selenate and selenite, and organic selenium compounds, such as selenium polysaccharides, selenoproteins, selenium-containing nucleic acids, alkylated selenium, and selenoamino acids and their derivatives. The use of inorganic selenium compounds have been rigorously forbidden owing to their high toxicity, low toxic thresholds close to the necessary nutrient level, and low utilization efficiency, with the US, Japan, and other countries having banned the addition of inorganic selenium compounds such as sodium selenite to foods (Kucukbay et al. 2010; Wang et al. 2011). In contrast, most organic selenium compounds are good sources of selenium and may serve as a biologically active form of selenium. Organic selenium compounds have the advantages of low toxicity, fewer side effects, environmental friendliness, high biological activity, and easy reuse by the body. Selenium polysaccharides, for example, have dual physiological functions of providing energy for the body and supplementing the body with selenium at the same time, and are thus the best selenium supplement (Zhang et al. 2016a).
Chemical Properties of Selenium
Selenium is a non-metallic chemical element in Group VIA and period 4 of the periodic table, with an atomic number of 34, a proton number of 34, a neutron number of 45, a molar mass of 79, and a relative atomic mass of 78.96 (Berzelius et al. 1817). Its valence electron arrangement is [Ar]3d104s24p4, with six valence electrons, including two unpaired electrons with the ability to form two covalent bonds, and a lone pair of p electrons not involved in bonding (Bjørklund et al. 2017). Selenium is a multivalent element in the chalcogen group, also known as the oxygen family, and has physical and chemical properties similar to those of sulfur and tellurium, especially with high similarity to tellurium in terms of atomic size, bonding energy, ionization energy, and electron affinity. Selenium has valences of − 2, 0, + 1, + 2, + 4, and + 6. Selenium with a valence of − 2 mainly exists as organic selenides, H2Se, and some metal selenides. Selenium with a valence of 0 is mostly elemental selenium, in black, gray, or red. Selenium with a valence of + 4 mainly exists as sodium selenite and selenium dioxide. Selenium with a valence of + 6 mainly exists as selenate.
Toxicology of Selenium
Toxicity of Selenium
Selenium has been considered a highly toxic element for more than 100 years since its discovery. For example, Franke (1934a, b) confirmed that selenium acts as a harmful toxic substance in mammal diets. In the same year, the US Department of Agriculture discovered that, in the agricultural experiment stations of Wyoming and South Dakota, animals consuming plants with high selenium content were subject to alkalosis and blind staggers. Currently, increasing importance has been attached to the safety assessment of drugs and chemicals, and selenosis is also divided into the classes of general toxicity versus specific toxicity. According to the time and dose of selenium intake, the general toxicity of selenium is mainly characterized as acute selenosis and chronic selenosis. Acute selenosis refers to acute toxicity caused by a large dose of selenium intake in a short period of time, which is mainly manifested as respiratory distress, ataxia, diarrhea, vomiting, abdominal pain, and even death. Chronic selenosis refers to cumulative toxicity caused by long-term low-dose intake of selenium, which is mainly manifested as fatigue, depression, garlic odor in breath, anemia, feed intake reduction, hair loss, onychomadesis, hoof rot, growth retardation, and liver cirrhosis (Ekermans and Schneider 1982; Ju et al. 2018). In addition, excessive selenium can induce specific toxicity, including genotoxicity (Biswas et al. 2000), embryotoxicity (Goldhaber 2003), reproductive toxicity (He et al. 2002), immunotoxicity (Brown and Arthur 2001), and cytotoxicity (Markus et al. 2004). Recent studies have shown that the toxicity of selenium depends on its chemical species, and the intensity of the toxic effects of selenium also varies with animal species, nutritional statuses, and drug administration routes (Mu et al. 2004; Chang et al. 2019). When the chemical species of selenium are concerned, inorganic selenium compounds, in general, are more toxic than organic selenium compounds, and organic selenium compounds are usually more toxic than Nano-Se (Mcadam and Orville 1987; Gao et al. 2003). Inorganic selenium compounds show almost the same toxicity, while selenium toxicity varies greatly among organic selenium compounds. For example, the toxicity of selenocysteine is greater than that of selenomethionine. Moreover, the same organic selenium compound may show different toxicity at different conformations, as is the case with l-selenomethionine having greater toxicity than d-selenomethionine (Wilber 1980; Brahser and Ogle 1993; Choy et al. 1993).
Mechanism of Selenium Phytotoxicity
Absorption and Accumulation of Selenium in Plants
The absorption of selenium by plants is affected by exogenous selenium species, sulfur elements, plant species and their tissues and organs, as well as growth stages (Garousi 2017). For example, different plant species have different selenium accumulation capabilities, with the highest selenium concentration usually existing in root and tuber crops as well as bulb crops, followed by field crops (including cereals, beans, and cruciferous plants) with the second highest concentration, and thereafter, leafy vegetables, seed vegetables, vegetable fruits, and tree fruits, in order of decreasing concentration (Mikkelsen et al. 1989; Hamilton and Beath 2002). Even for the same plant species, its absorption of selenium will vary with the chemical species of selenium (Arvy 1993; Zhang et al. 2003). For example, plants can absorb selenite faster than selenate. Plants absorb selenite through efficient sulfur transfer proteins and accumulate it mostly in the roots, while selenate is absorbed by plants, possibly through passive transport, and mainly accumulated in the tender shoots (Shrift and Ulrich 1969; Adel and Lytle 1998; Terry et al. 2000; White et al. 2004; Sors et al. 2005).
Mechanism of Selenium Phytotoxicity
At normal or low selenium levels, the selenium metabolite selenocysteine is transported from the chloroplast to the cytoplasm to continue the metabolic reaction. Conversely, at abnormally high or excessive selenium levels, selenium can have a negative effect on most selenium-sensitive plants, such as non-selenium-accumulating plants. In such plants, excessive selenocysteine will replace cysteine to participate in protein synthesis, which weakens the resistance of plants and damages their chlorophyll and anti-oxidative enzyme systems, thereby causing toxic effects (De Souza et al. 2000; Tai and Li 2002; Laure et al. 2007; Yi and Si 2007; Xu et al. 2011; Yang and Yang 2014; Li et al. 2016; Hou et al. 2018). Under normal circumstances, plants have the ability to adjust and adapt to adverse external environment, for which abscisic acid (ABA) is a very important indicator. Tai and Li (2002) have shown that excessive selenium can reduce the content of ABA in plants, suggesting that excessive selenium can inhibit the plants’ ability to resist external adverse factors by decreasing their self-regulatory function, which will eventually lead to symptoms of phytotoxicity. Lin et al. (2005) showed that a high concentration of selenium significantly inhibits the chlorophyll a content and the chlorophyll a/b ratio in rice, and produces a stress effect on the anti-oxidative enzyme system, which significantly increases GSH-Px and malondialdehyde (MDA), while significantly decreasing the activity of superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD). Li et al. (2003) showed that the effect of selenium on the antioxidant activity of plants is consistent with the effect of selenium on the growth of plants, suggesting that excessive selenium can decrease the content of reducing substances in plants by damaging the endogenous antioxidant system in plants, which further weakens the ability of plants to scavenge reactive oxygen species (ROS), thereby inhibiting plant growth. Sharma et al. (2014) observed increased levels of oxidative stress markers proline, H2O2, and MDA in rice leaves during the tillering and flowering periods, grown in high-selenium soils in India, confirming once again that selenium can inhibit plant growth by interfering with the antioxidant defense system of plants, and the affected plants show decreased dry matter weight.
Mechanism of Selenium Toxicity in Animals
Absorption, Distribution, Metabolism and Excretion of Selenium in Animals
Absorption of selenium varies among animal species, and is affected by physiological characteristics, functional status, amount of intestinal contents, chemical species of selenium, residence time of selenium in the intestine, and selenium administration routes (Sun et al. 2017; Allarabi et al. 2019; Lv and Wang 2019; Wei et al. 2019). Under normal circumstances, selenium enters the animal’s body mainly through the digestive tract, respiratory tract, skins, veins, or subcutis, after which it is absorbed in the stomach and intestine to enter the blood, where it combines with plasma proteins to become biologically active selenoproteins, which are then transported to various tissues and organs for utilization (Kang 2009; Cao et al. 2017). Although all cells and tissues in animals’ bodies contain selenium, the selenium content varies among tissues and is dependent on the dietary selenium level. For example, selenium will be preferentially distributed in body parts with sufficient blood supply, after which it is selectively redistributed according to the affinity of organs. As a result, selenium is mostly distributed in the liver, kidney, heart, and pancreas, followed by muscles, bones, and blood with the second highest abundance, and fat tissues with the lowest abundance (Hu et al. 2004). There are multiple pathways for selenium metabolism in animals. Inorganic selenium and organic selenium are involved in different metabolic pathways because they are different species of selenium. For example, sodium selenite, an inorganic selenium compound, is usually involved in only one metabolic pathway, where, under certain conditions it is first reduced to hydrogen selenide and then transported to the liver to become part of the selenium pool, where it is incorporated into selenoproteins as selenocysteine, or forms methylated metabolites to be finally excreted. In contrast, the organic selenium compound selenomethionine is mainly involved in two metabolic pathways, namely the methionine pathway and the selenium pathway. Generally, selenomethionine is absorbed by the body through the Na+-dependent neutral amino acid transport system in the small intestine, and then exists in a bound form in the liver, kidney, skeletal muscles, gastrointestinal mucosa, and pancreas to participate in protein synthesis (Eskil and Sten-Olof 1966; Vendeland et al. 1994; Shi et al. 2003). Under normal circumstances, selenium metabolized by animals is mainly excreted through urine and feces, but in the event of high selenium intake, exhalation also serves as one of the important routes for excreting selenium. In addition, selenium can be excreted through hair and sweat (Shi et al. 2003).
Mechanism of Selenium Toxicity in Animals
Studies have shown that selenium as a micronutrient works within a narrow beneficial range for animals, with the relationship between the biological effects and the concentrations of selenium following the well-known Weinberg’s principle (Scott 1973; Chen and Hou 2015). Selenosis is divided into acute selenosis and chronic selenosis. The main cause of the disease is that, after high concentrations of selenium enter the body, there is a competitive relationship between selenium and sulfur owing to the similarity of selenium to sulfur, with selenium being prone to act as a substitute for sulfur in sulfur-containing amino acids, to become selenomethionine and selenocysteine, which disturbs protein expression and deactivates SH-enzymes (such as succinate dehydrogenase and δ-aminolevulinic acid dehydratase), thereby hindering the biochemical reaction in the body and causing damage to tissues and organs. As a result, animals exhibit abnormal physiological functions such as growth inhibition, fertility decline, and immunosuppression (Spallholz et al. 1973; Kajander et al. 1991; Daniels 1996; Gu et al. 1998; Lemly 1998; Vickerman et al. 2002; Popham et al. 2005; Wang et al. 2007; Han and Hou 2012; Han et al. 2016). In addition, some studies have shown that selenosis in animals is mainly attributed to the fact that selenium methylation, the main detoxification pathway of selenium in the body, is inhibited. For example, Hasegawa et al. found that high doses of selenocysteine administered to mice can inhibit selenium methylation by deactivating methionine adenosyltransferase (an enzyme responsible for the synthesis of S-adenosyl methionine), which leads to the accumulation of hepatotoxic selenides, especially large quantities of hydrogen selenide, and eventually liver damage in animal bodies (Tatsuya et al. 1996).
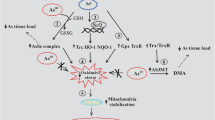
Moreover, there is a controversial mechanism underlying selenosis in animal bodies, namely the oxidative stress mechanism. Studies have shown that many selenium compounds (such as selenites, selenium dioxide, and diselenides) that are prone to form Se2− or SeH− anions can react with oxygen in the presence of thiols (such as glutathione and cysteine) to produce superoxide or ROS, which leads to redox cycling, cell cycle arrest, endoplasmic reticulum (ER) stress and apoptosis in animal bodies (Per et al. 1988; Seko et al. 1988; Yan and Spallholz 1993; Lanfear et al. 1994; Nakamuro et al. 1997; Wang et al. 2002; Mu et al. 2004; Spallholz et al. 2004; Wycherly et al. 2004; Rayman et al. 2008; Pacini et al. 2013). For example, Yao et al. (2015) indicated that selenium induced oxidative stress in chicken liver and the occurrence of oxidative stress can induce continuous ER stress through interfering with the oxidation of the internal environment of the ER, which process may be involved in the PERK, ATF6 and IRE1 pathways. Kim et al. (2004) illustrated that the oxidative-ER stress pathway participates in selenite-induced apoptosis in Chang liver cells. Hwang et al. (2007) showed that selenium treatment led to the activation of the ER stress signal through the phosphorylation of JNK and eIF2 protein and Zachariah et al. (2015, 2016) verified that high selenium induced endothelial dysfunction via endoplasmic reticulum stress. In contrast, some studies have revealed that selenodiglutathione (SDG), the primary metabolite of selenites, cannot induce oxidative stress similar to that induced by typical oxidants such as H2O2 (Harrison et al. 1997). However, as pointed out by Seko and Imura (1997), experimental designs adopted in earlier studies on proving that the production of ROS might be a mechanism of selenium toxicity are subject to a few shortcomings. In addition, some scholars believe that excessive selenium can damage cellular functions by affecting the level of vitamin A in the body (Chen et al. 1997; Mu et al. 2004), whereas others believe that excessive selenium can oxidize sulfhydryl groups to inhibit or damage some enzymes or functional proteins in the mitochondria, resulting in the inhibition of mitochondrial energy metabolism and blockage of the function (Deagen et al. 1987; Mu et al. 2004). Furthermore, few other scholars believe that excessive selenium can cause a large amount of intracellular Ca2+ to accumulate by disturbing the Ca2+ signaling pathway in the body, which in turn activates Ca2+-dependent intracellular enzymes (such as endonucleases, transglutaminase, and calpain) and causes poisoning phenomena such as DNA breakage in the host cell chromatin, cytoplasmic protein cross-linking, and cytoskeleton destruction (Carson and Ribeiro 1993; Xu et al. 2002; Zhivotovsky and Orrenius 2011).
Mechanism of Selenium Toxicity in Microorganisms
Metabolism of Selenium in Microorganisms
Microorganisms play an important role in the geochemical cycling of selenium. Studies have shown that microorganisms can metabolize selenium through various pathways of transport, reduction, oxidation, assimilation, and methylation, to achieve selenium species transformation and self-detoxification (Nancharaiah and Lens 2015; Xu et al. 2017).
Mechanism of Selenium Toxicity in Microorganisms
Currently, increasing evidence shows that in addition to toxic effects on the growth and related physiological functions of animals and plants, selenium also has certain negative effects on microbial growth. It has been reported that Nano-Se has a significant antibacterial effect on Staphylococcus aureus, and it is speculated that Nano-Se may affect the growth of bacteria by inhibiting the formation of cell membranes (Tran and Webster 2011; Dagmar et al. 2014; Khiralla and El-Deeb 2015; Wang et al. 2015; Guisbiers et al. 2016). It is also reported that selenium can inhibit pathogenic bacteria such as Salmonella spp. and E. coli, but the specific mechanism remains unclear (Wang et al. 2015; Li et al. 2017). For viruses, numerous studies have shown that the replication of many viruses is affected by selenium, including DNA viruses, RNA viruses, and subviruses (Lv et al. 2018). For mouse mammary tumor virus and porcine parvovirus, studies have shown that methylselenic acid can inhibit the replication of mouse mammary tumor virus by regulating epigenetic markers (De Miranda et al. 2014), whereas sodium selenite, selenomethionine, and seaweed selenium polysaccharides inhibit the replication of porcine parvovirus by scavenging oxygen free radicals (Wei et al. 2005). For fungi, many studies have shown that selenium can antagonize the infection of fungi such as Aspergillus flavus, Magnaporthe oryzae, A. fumigatus, and Candida albicans (Mojtaba et al. 2015; Yao et al. 2016; Guisbiers et al. 2017; Hosnedlova et al. 2017; Zhou 2018). For example, Guisbiers et al. (2017) have shown that Nano-Se can destroy the cell structure in C. albicans by replacing sulfur on the biofilm, thereby inhibiting the formation of biofilm, eventually inhibiting the fungal growth.
Summary and Prospects
This article reviewed the progress in research on the selenosis mechanism in plants, animals, and microorganisms (summarized in Table 1). Although selenosis may involve many different mechanisms, the mechanisms of selenium toxicity are far from being clear. Generally, the mechanisms of selenium toxicity may involve three main aspects: (i) oxidative stress, (ii) inhibition of biofilm formation, and (iii) disturbance of enzyme activity. The ever-growing understanding of selenium toxicity, especially in-depth research on the nutrition and toxicity mechanisms of various newly developed selenium-enriched foods, selenium-containing drugs, and selenium-enriched dietary supplements in different organisms, will provide theoretical support for the rational utilization of selenium resources, assessment of ecological risks, and appropriate use of selenium products.
Abbreviations
- ABA:
-
Abscisic acid
- AIDS:
-
Acquired immunodeficiency syndrome
- GSH-Px:
-
Glutathione peroxidase
- Nano-Se:
-
Nano-selenium
- MDA:
-
Malondialdehyde
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- POD:
-
Peroxidase
- ROS:
-
Reactive oxygen species
- SDG:
-
Selenodiglutathione
References
Adel Z, Lytle CM (1998) Accumulation and volatilization of different chemical species of selenium by plant. Planta 206(2):284–292. https://doi.org/10.1007/s004250050402
Aghwan ZA, Sazili AQ, Kadhim KK et al (2016) Effects of dietary supplementation of selenium and iodine on growth performance, carcass characteristics and histology of thyroid gland in goats. Anim Sci J 87(5):690–696. https://doi.org/10.1111/asj.12484
Ahsan U, Kamran Z, Raza I et al (2014) Role of selenium in male reproduction—a review. Anim Reprod Sci 146:55–62. https://doi.org/10.1016/j.anireprosci.2014.01.009
Allarabi H, Fadayifar A, Alimohamady R et al (2019) The effect of maternal supplementation of zinc, selenium, and cobalt as slow-release ruminal bolus in late pregnancy on some bloodmetabolites and performance of ewes and their lambs. Biol Trace Elem Res 187(2):403–410. https://doi.org/10.1007/s12011-018-1409-8
Arvy MP (1993) Selenate and selenite uptake and translocation in bean plant. J Exp Bot 44(6):1083–1087. https://doi.org/10.1093/jxb/44.6.1083
Awasthi YC, Beutler E, Srivastava SK (1975) Purification and properties of human erythrocyte glutathione peroxidase. J Biol Chem 250(13):5144–5149. https://doi.org/10.1007/BF01648966
Beck MA, Kolbeck PC, Shi Q et al (1994) Increased virulence of a human enterovirus (coxsackievirus B3) in selenium-deficient mice. J Infect Dis 170(2):351–357. https://doi.org/10.1093/infdis/170.2.351
Beheshti N, Soflaei S, Shakibaie M et al (2013) Efficacy of biogenic selenium nanoparticles against Leishmania major: in vitro and in vivo studies. J Trace Elem Med Biol 27(3):203–207. https://doi.org/10.1016/j.jtemb.2012.11.002
Benton D, Cook R (1990) Selenium supplementation improves mood in a double-blind crossover trial. Psychopharmacology 102(4):549–550. https://doi.org/10.1007/BF02247139
Berzelius JJ (1817) Lettre de M. Berzelius a M. Berthollet sur deux metaux nouveaux. Ann Chim Phys 7:199–207
Bjørklund G, Aaseth J, Ajsuvakova OP et al (2017) Molecular interaction between mercury and selenium in neurotoxicity. Coord Chem Rev 332:30–37. https://doi.org/10.1016/j.ccr.2016.10.009
Biswas S, Talukder G, Sharma A (2000) Chromosome damage induced by selenium salts in human peripheral. Toxicol In Vitro 14:405–408. https://doi.org/10.1016/s0887-2333(00)00040-0
Brahser AM, Ogle S (1993) Comparative toxicity of selenite and selenate to the amphipod Hyalella azteca. Arch Environ Contam Toxicol 24(2):182–186. https://doi.org/10.1007/bf01141346
Broome CS, Ardle FM, Kyle JAM et al (2004) An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am J Clin Nutr 80(1):154–162. https://doi.org/10.1093/ajcn/80.1.154
Brown KM, Arthur JR (2001) Selenium selenoproteins and human health: a review. Public Health Nutr 4(2B):593–599. https://doi.org/10.1079/PHN2001143
Cao DD, Meng TT, Shu XG et al (2017) Research progress on absorption and metabolism of selenium in animals. J Zhongkai Univ Agric Eng 30(4):66–70. https://doi.org/10.3969/j.issn.1674-5663.2017.04.012 (in Chinese with English Abstract)
Carlson BA, Yoo MH, Shrimali RK et al (2010) Role of selenium- containing proteins in T-cell and macrophage function. Proc Nutr Soc 69(3):300–310. https://doi.org/10.1017/S002966511000176X
Carson DA, Ribeiro JM (1993) Apoptosis and disease. Lancet 341(8855):1251–1254. https://doi.org/10.1016/0140-6736(93)91154-E
Chang C, Yin R, Zhang H et al (2019) Bioaccumulation and health risk assessment of heavy metals in the soil–rice system in a typical seleniferous area in central China. Environ Toxicol Chem 38:1577–1584. https://doi.org/10.1002/etc.4443
Chen S, Hou ZZ (2015) Effects of subacute selenium poisoning on testicular cell structure in mice. Anim Husb Vet Med 47(8):97–100 (in Chinese with English Abstract)
Chen C, Liu Y, Zhou J et al (1997) Microcalorimetric study of the toxic effect of selenium on the mitochondrial metabolism of Cyprinus carpio liver. Biol Trace Elem Res 60(1–2):115–122. https://doi.org/10.1007/BF02783314
Cheng ZK, Zhi XG, Sun G et al (2016) Sodium selenite suppresses hepatitis B virus transcription and replication in human hepatoma cell lines. J Med Virol 88(4):653–663. https://doi.org/10.1002/jmv.24366
Choy WN, Henika PR, Willhite CC et al (1993) Incorporation of a micronucleus study into a developmental toxicology and pharmacokinetic study of L-selenomethionine in nonhuman primates. Environ Mol Mutagen 21(1):73–80. https://doi.org/10.1002/em.2850210110
Cihalova K, Chudobova D, Michalek P et al (2015) Staphylococcus aureus and MRSA growth and biofilm formation after treatment with antibiotics and SeNPs. Int J Mol Sci 16(10):24656–24672. https://doi.org/10.3390/ijms161024656
Dagmar C, Kristyna C, Simona D et al (2014) Comparison of the effects of silver phosphate and selenium nanoparticles on Staphylococcus aureus growth reveals potential for selenium particles to prevent infection. FEMS Microbiol Lett 351(2):195–201. https://doi.org/10.1111/1574-6968.12353
Daniels RL (1996) Selenium metabolism and bioavailability. Biol Trace Elem Res 54(3):185–199. https://doi.org/10.1007/BF02784430
De Miranda JX, Andrade FO, de Conti A et al (2014) Effects of selenium compounds on proliferation and epigenetic marks of breast cancer cells. J Trace Elem Med Biol 28(4):486–491. https://doi.org/10.1016/j.jtemb.2014.06.017
De Souza MP, Lytle CM, Mulholland MM et al (2000) Selenium assimilation and volatilization from dimethyselenoniopropionate by Indian mustard. Plant Physiol 122(4):1281–1288. https://doi.org/10.1104/pp.122.4.1281
Deagen J, Butler JA, Beilstein MA et al (1987) Effects of dietary selenite, selenocystine and selenomethionine on selenocysteine lyase and glutathione peroxidase activities and on selenium levels in rat tissues. J Nutr 117(1):91–98. https://doi.org/10.1093/jn/117.1.91
Dkhil MA, Bauomy AA, Diab MSM et al (2016) Protective role of selenium nanoparticles against Schistosoma mansoni induced hepatic injury in mice. Biomed Res 27(1):214–219
Ekermans LG, Schneider JV (1982) Selenium in livestock production: a review. J S Afr Vet Assoc 53(4):223–228
Eskil H, Sten-Olof J (1966) Uptake of [75Se] selenomethionine in the tissues of the mouse studied by whole-body autoradiography. BBA Gen Subj 115(2):285–293. https://doi.org/10.1016/0304-4165(66)90427-2
Fan AM, Kizer KW (1990) Selenium: nutritional, toxicologic, and clinical aspects. West J Med 153(2):160–167. https://doi.org/10.1620/tjem.161.343
Forootanfar H, Adeli-Sardou M, Nikkhoo M et al (2014) Antioxidant and cytotoxic effect of biologically synthesized selenium nanoparticles in comparison to selenium dioxide. J Trace Elem Med Biol 28(1):75–79. https://doi.org/10.1016/j.jtemb.2013.07.005
Franke KW (1934a) A new toxicant occurring naturally in certain samples of plant foodstuffs: I. Results obtained in preliminary feeding trials: eight figures. J Nutr 8(5):597–608. https://doi.org/10.1093/jn/8.5.597
Franke KW (1934b) A new toxicant occurring naturally in certain samples of plant foodstuffs: II. The occurrence of the toxicant in the protein fraction: four figures. J Nutr 8(5):609–613. https://doi.org/10.1093/jn/8.5.609
Gao XY, Zhang JS, Zhang LD et al (2003) The role of nano red element selenium in inhibiting tumors and improving immune function. Chin J Public Health 19(3):309–310. https://doi.org/10.11847/zgggws2003-19-03-30 (in Chinese with English Abstract)
Garousi F (2017) The essentiality of selenium for humans, animals, and plants, and the role of selenium in plant metabolism and physiology. Acta Univ Sapientiae Alimentaria 10(1):75–90. https://doi.org/10.1515/ausal-2017-0005
Gladyshev VN, Martin-Romero FJ, Xu XM et al (1999) Molecular biology of selenium and its role in cancer, AIDS and other human diseases. Recent Res Dev Biochem 1:145–167
Goldhaber SB (2003) Trace element risk assessment: essentiality vs. toxicity. Regul Toxicol Pharmacol 38(2):232–242. https://doi.org/10.1016/s0273-2300(02)00020-x
Gu QP, Xia YM, Ha PC et al (1998) Distribution of selenium between plasma fractions in guinea pigs and humans with various intakes of dietary selenium. J Trace Elem Med Biol 12(1):8–15. https://doi.org/10.1016/S0946-672X(98)80015-1
Guisbiers G, Wang Q, Khachatryan E (2016) Inhibition of E. coli and S. aureus with selenium nanoparticles synthesized by pulsed laser ablation in deionized water. Int J Nanomed 11:3731–3736. https://doi.org/10.2147/IJN.S106289
Guisbiers G, Lara H, Mendoza-Cruz R et al (2017) Inhibition of Candida albicans biofilm by pure selenium nanoparticles synthesized by pulsed laser ablation in liquids. Nanomed Nanotechnol Biol Med 13(3):1095–1103. https://doi.org/10.1016/j.nano.2016.10.011
Habibian M, Sadeghi G, Ghazi S et al (2015) Selenium as a feed supplement for heat-stressed poultry: a review. Biol Trace Elem Res 165(2):183–193. https://doi.org/10.1007/s12011-015-0275-x
Hamilton JW, Beath OA (2002) Selenium in vegetables, amount and chemical form of selenium in vegetable plants. J Agric Food Chem 12(4):371–374. https://doi.org/10.1021/jf60134a018
Han Y, Hou ZZ (2012) Studies on toxic effects of Sodium selentite on female reproductive system. Chin J Vet Med 48(7):8–10. https://doi.org/10.3969/j.issn.0529-6005.2012.07.002 (in Chinese with English Abstract)
Han XX, Yao ZW, Wei HY (2016) Advances in the study of toxicity biology of selenium. Jiangsu Agric Sci 44(12):24–28. https://doi.org/10.15889/j.issn.1002-1302.2016.12.006 (in Chinese with English Abstract)
Harrison PR, Lanfear J, Wu L et al (1997) Chemopreventive and growth inhibitory effects of selenium. Biomed Environ Sci 10(2–3):235–245
He JP, Zhang JX, Hou YP (2002) Study on the effect of excess selenium on the structure of testicular tissue and cell of breeder rooster by using electron microscopic. J Chin Electron Microsc Soc 21(5):511–512. https://doi.org/10.3969/j.issn.1000-6281.2002.05.023 (in Chinese with English Abstract)
Hosnedlova B, Kepinska M, Skalickova S et al (2017) A summary of new findings on the biological effects of selenium in selected animal species—a critical review. Int J Mol Sci 18(10):2209–2255. https://doi.org/10.3390/ijms18102209
Hou D, Yang LF, Yu TL (2018) Selenium content of konjac in different soil environment. J Hubei Univ Nat Sci 34(2):202–207. https://doi.org/10.3969/j.issn.1000-2375.2012.02.020 (in Chinese with English Abstract)
Hu XR, Wang LX, Xu PY et al (2004) The toxic interaction of mercuric chloride and sodium selenite to rats. Chem Res Appl 16(3):317–319. https://doi.org/10.3969/j.issn.1004-1656.2004.03.006 (in Chinese with English Abstract)
Hu CH, Li YL, Xiong L et al (2012) Comparative effects of nano elemental selenium and sodium selenite on selenium retention in broiler chickens. Anim Feed Sci Technol 177(3–4):204–210. https://doi.org/10.1016/j.anifeedsci.2012.08.010
Huang S (2006) Biological activity of selenium and related diseases. B Biol 41(3):17–19 (in Chinese with English Abstract)
Huang KH, Chen WF (1996) Effect of selenium on chicken’s resistance to Marek’s disease and its mechanism. Chin J Anim Vet Sci 26(5):448–455 (in Chinese with English Abstract)
Huang FY, Zhang RL, Zhang QW et al (2014) Effect of selenomethionine and sodium selenite on proliferation of EV71 in vitro and in vivo. Chin Trop Med 14(7):792–794, 868. https://doi.org/10.13604/j.cnki.46-1064/r.2014.07.053 (in Chinese with English Abstract)
Hughes CM, Woodside JV, Gartland CM et al (2012) Nutritional intake and oxidative stress in chronic heart failure. Nutr Metab Cardiovasc Dis 22(4):376–382. https://doi.org/10.1016/j.numecd.2010.08.006
Hwang DY, Seo SJ, Kim YK et al (2007) Selenium acts as an insulin-like molecule for the down-regulation of diabetic symptoms via endoplasmic reticulum stress and insulin signalling proteins in diabetes-induced non-obese diabetic mice. J Biosci 32(4):723–735. https://doi.org/10.1007/s12038-007-0072-6
Hybsier S, Schulz T, Wu Z et al (2017) Sex-specific and inter-individual differences in bio-markers of selenium status identified by a calibrated ELISA for selenoprotein P. Redox Biol 11:403–414. https://doi.org/10.1016/j.redox.2016.12.025
Iglesias P, Selgas R, Romero S et al (2013) Selenium and kidney disease. J Nephrol 26(2):266–272. https://doi.org/10.5301/jn.5000213
Jaspers I, Hang W, Brighton LE et al (2007) Selenium deficiency alters epithelial cell morphology and responses to influenza. Free Radic Biol Med 42(12):1826–1837. https://doi.org/10.1016/j.freeradbiomed.2007.03.017
Jia X, Li N, Chen J (2005) A subchronic toxicity study of elemental Nano-Se in Sprague-Dawley rats. Life Sci 76(17):1989–2003. https://doi.org/10.1016/j.lfs.2004.09.026
Ju W, Ji M, Li X et al (2018) Relationship between higher serum selenium level and adverse blood lipid profile. Clin Nutr 37(5):1512–1517. https://doi.org/10.1016/j.clnu.2017.08.025
Kajander EO, Harvima RJ, Eloranta TO et al (1991) Metabolism, cellular actions, and cytotoxicity of selenomethionine in cultured cells. Biol Trace Elem Res 28(1):57–68. https://doi.org/10.1007/bf02990463
Kang ST (2009) Nutritional research progress of trace element selenium. China Anim Husb Vet Med 36(1):34–37 (in Chinese with English Abstract)
Khiralla GM, El-Deeb BA (2015) Antimicrobial and antibiofilm effects of selenium nanoparticles on some foodborne pathogens. LWT Food Sci Technol 63(2):1001–1007. https://doi.org/10.1016/j.lwt.2015.03.086
Kim YS, Jhon DY, Lee KY (2004) Involvement of ROS and JNK1 in selenite-induced apoptosis in Chang liver cells. Exp Mol Med 36:157–164. https://doi.org/10.1038/emm.2004.22
Kohshahi AJ, Sourinejad I, Sarkheil M et al (2019) Dietary co-supplementation with curcumin and different selenium sources (nanaoparticulate, organic, and inorganic selenium): influence on growth performance, body composition, immune responses, and glutathione peroxidase activity of rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 45(2):793–804. https://doi.org/10.1007/s10695-018-0585-y
Kommisrud E, Østerås O, Vatn T (2005) Blood selenium associated with health and fertility in Norwegian dairy herds. Acta Vet Scand 46(4):229–240. https://doi.org/10.1186/1751-0147-46-4-229
Kryukov GV, Castellano S, Novoselov SV et al (2003) Characterization of mammalian selenoproteomes. Science 300(5624):1439–1443. https://doi.org/10.1126/science.1083516
Kucukbay FZ, Yazlak H, Karaca I et al (2010) The effects of dietary organic or inorganic selenium in rainbow trout (Oncorhynchus mykiss) under crowding conditions. Aquac Nutr 15(6):569–576. https://doi.org/10.1111/j.1365-2095.2008.00624.x
Lanfear J, Fleming J, Wu L et al (1994) The selenium metabolite selenodiglutathione induces p53 and apoptosis: relevance to the chemopreventive effects of selenium? Carcinogenesis 15(7):1387–1392. https://doi.org/10.1093/carcin/15.7.1387
Laure G, Rodolphe G, Olivier S et al (2007) Effect of selenate on growth and photosynthesis of Chlamydomonas reinhardtii. Aquat Toxicol 83(2):149–158. https://doi.org/10.1016/j.aquatox.2007.04.001
Lee J, Finley JW, Harnly JM (2015) Effect of selenium fertilizer on free amino acid composition of broccoli (Brassica oleracea Cv. Majestic) determined by gas chromatography with flame ionization and mass selective detection. J Agric Food Chem 53(23):9105–9111. https://doi.org/10.1021/jf051221x
Lemly AD (1998) Pathology of selenium poisoning in fish. In: Frankenberger WT Jr, Engberg RA (eds) Environmental chemistry of selenium. Marcel Dekker, New York, pp 281–296
Li DC, Zhu ZJ, Han QM et al (2003) Effect of selenium on growth and antioxidative system in spinach and pakchoi. J Shanghai Jiaotong Univ Agric Sci 21(1):5–8. https://doi.org/10.3969/j.issn.1671-9964.2003.01.002 (in Chinese with English Abstract)
Li BC, Zhou JX, Chen HL et al (2011) Study on effect of nano-selenium on growth performance and peptic development of egg chickens. J Chin Cereals Oils Assoc 26(11):65–69 (in Chinese with English Abstract)
Li YY, Wang L, Peng WX et al (2016) Study on the suitable application of selenium in tobacco-planting soils. Chin J Tob Sci 37(4):1–5. https://doi.org/10.13496/j.issn.1007-5119.2016.04.001 (in Chinese with English Abstract)
Li MZ, Liao XW, Qu WH et al (2017) Rapid isolation and identification of Bacillus licheniformis with bacteriostatic ability and its antibacterial activity against four resistant pathogens. J Nanjing Agric Univ 40(2):325–331. https://doi.org/10.7685/jnau.201602005 (in Chinese with English Abstract)
Li YH, Zhang FF, Huang LJ et al (2020) Influence of dietary nano-selenium on growth, muscle composition, serum biochemical and antioxidant indices of Schizothorax prenanti. J Fish Sci China 27(6):682–691. https://doi.org/10.3724/SP.J.1118.2020.19307 (in Chinese with English Abstract)
Lin KF, Xu XQ, Jin X et al (2005) Eco-toxicological effects of selenium and its critical value on Oryza sativa. Chin J Appl Ecol 16(4):678–682 (in Chinese with English Abstract)
Lin SL, Wang CW, Tan SR et al (2014) Selenium deficiency inhibits the conversion of thyroidal thyroxine (T4) to triiodothyronine (T3) in chicken thyroids. Biol Trace Elem Res 161(3):263–271. https://doi.org/10.1007/s12011-014-0083-8
Liu HM, Li XM, Qin F et al (2014) Selenium suppresses oxidative-stress-enhanced vascular smooth muscle cell calcification by inhibiting the activation of the PI3K/AKT and ERK signaling pathways and endoplasmic reticulum stress. J Biol Inorg Chem 19(3):375–388. https://doi.org/10.1007/s00775-013-1078-1
Liu G, Yang G, Guan GP et al (2015) Effect of dietary selenium yeast supplementation on porcine circovirus type 2 (PCV2) infections in mice. PLoS One 10(2):e0115833. https://doi.org/10.1371/journal.pone.0115833
Long ZD, Xiang JQ, Song JP et al (2020) Soil selenium concentration and residents daily dietary intake in a selenosis area: a preliminary study in Yutangba Village, Enshi City, China. Bull Environ Contam Toxicol. https://doi.org/10.1007/s00128-020-02983-x
Lubos E, Sinning CR, Schnabel RB et al (2010) Serum selenium andprognosis in cardiovascular disease: results from the AtheroGene study. Atherosclerosis 209(1):271–277. https://doi.org/10.1016/j.atherosclerosis.2009.09.008
Lv QZ, Wang DB (2019) Study on the antiviral effect of selenium in animals. Northeast Normal University Press, Changchun (in Chinese)
Lv QZ, Yan Q, Chen Y et al (2018) Research progress of antiviral effects of microelement selenium in animal body. Soils 50(6):1113–1118. https://doi.org/10.13758/j.cnki.tr.2018.06.008 (in Chinese with English Abstract)
Mahmoudvand H, Harandi MF, Shakibaie M et al (2014) Scolicidal effects of biogenic selenium nanoparticles against protoscolices of hydatid cysts. Int J Surg 12(5):399–403. https://doi.org/10.1016/j.ijsu.2014.03.017
Markus W, Markus L, Matthias K et al (2004) Toxicity of nutritionally available selenium compounds in primary and transformed hepatocytes. Toxicology 201(1):21–30. https://doi.org/10.1016/j.tox.2004.03.026
Marin-Guzman J, Mahan DC, Pate JL (2000) Effect of dietary selenium and vitamin E on spermatogenic development in boars. J Anim Sci 78(6):1537–1543. https://doi.org/10.2527/2000.7861537x
Mcadam PA, Orville AL (1987) Chronic toxicity and retention of dietary selenium fed to rats and D-or L-selenomethionine selenite or selenite. Nutr Res 7(6):601–610. https://doi.org/10.1016/S0271-5317(87)80053-2
Mikkelsen RL, Page AL, Bingham FT (1989) Factors affecting selenium accumulation by agricultural crops. Selenium Agric Environ 23:65–94. https://doi.org/10.2136/sssaspecpub23.c4
Mojtaba S, Naser SM, Amin AMS (2015) Antifungal activity of selenium nanoparticles synthesized by bacillus species msh-1 against Aspergillus fumigatus and Candida albicans. Jundishapur J Microbiol 8(9):e26381. https://doi.org/10.5812/jjm.26381
Mu WP, Tian Y, Piao JH et al (2004) Studies on comparing the toxicity between sodium selenite and selenomethionine in rats. J Hyg Res 33(6):700–703. https://doi.org/10.3969/j.issn.1000-8020.2004.06.015 (in Chinese with English Abstract)
Nakamuro K, Nakanishi K, Okuno T et al (1997) Comparison of methylated selenium metabolites in rats after oral administration of various selenium compounds. Jpn J Toxicol Environ Health 43(1):P13. https://doi.org/10.1248/jhs1956.43.P13
Nancharaiah YV, Lens PNL (2015) Ecology and biotechnology of selenium-respiring bacteria. Microbiol Mol Biol Rev 79(1):61–80. https://doi.org/10.1128/MMBR.00037-14
Navarro-Alarcón M, de la Serrana HLG, Pérez-Valero V et al (2002) Selenium concentrations in serum of individuals with liver diseases (cirrhosis or hepatitis): relationship with some nutritional and biochemical markers. Sci Total Environ 291:135–141. https://doi.org/10.1016/s0048-9697(01)01088-9
Pacini N, Elia AC, Abete MC et al (2013) Antioxidant response versus selenium accumulation in the liver and kidney of the Siberian sturgeon (Acipenser baeri). Chemosphere 93(10):2405–2412. https://doi.org/10.1016/j.chemosphere.2013.08.042
Peng A, Yang C (1991) Examination of the roles of selenium in the Kaschin-Beck disease. Biol Trace Elem Res 28(1):1–9. https://doi.org/10.1007/BF02990457
Per G, Annika S, Margareta W et al (1988) Studies of the role of DNA fragmentation in selenium toxicity. Biochem Pharmacol 37(18):3401–3406. https://doi.org/10.1016/0006-2952(88)90688-0
Popham HJ, Shelby KS, Popham TW (2005) Effect of dietary selenium supplementation on resistance to baculovirus infection. Biol Control 32(3):419–426. https://doi.org/10.1016/j.biocontrol.2004.12.011
Qiao J, Zhao LH (1997) Effects of free radical scavengers on pathologic process in infectious bursal disease. Chin J Anim Vet Sci 28(4):362–365 (in Chinese with English Abstract)
Qing Y, Zhang LS (2012) Study advances on toxicity of selenium. J Prev Med Inform 28(3):216–218 (in Chinese with English Abstract)
Rayman MP, Infante HG, Sargent M (2008) Food-chain selenium and human health: spotlight on speciation. Br J Nutr 100(2):254–268. https://doi.org/10.1017/S0007114508922522
Ren FL, Guo X (2004) Characteristics, toxicity and present situation of selenium in some countries. Foreign Med Sci Sect Medgeogr 25(3):117–119. https://doi.org/10.3969/j.issn.1001-8883.2004.03.007 (in Chinese with English Abstract)
Rotruck JT, Pope AL, Ganther HE et al (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179(4073):588–590. https://doi.org/10.1126/science.179.4073.588
Rowntree JE, Hill GM, Hawkins DR et al (2004) Effect of Se on selenoprotein activity and thyroid hormone metabolism in beef and dairy cows and calves. J Anim Sci 82:2995–3005. https://doi.org/10.2527/2004.82102995x
Roy S, Dontamalla SK, Mondru AK et al (2011) Downregulation of apoptosis and modulation of TGF-β1 by sodium selenate prevents streptozotocin-induced diabetic rat renal impairment. Biol Trace Elem Res 139(1):55–71. https://doi.org/10.1007/s12011-010-8635-z
Rusetskaya NY, Borodulin VB (2015) Biological activity of selenorganic compounds at heavy metal salts intoxication. Biomed Khim 61(4):449–461. https://doi.org/10.18097/PBMC20156104449
Schwarz K, Foltz CM (1957) Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. J Am Chem Soc 79:3292–3293. https://doi.org/10.1111/j.1753-4887.1978.tb03701.x
Schwarz K, Bieri JG, Briggs GM et al (1957) Prevention of exudative diathesis in chicks by factor 3 and selenium. Proc Soc Exp Biol Med 95(4):621–625. https://doi.org/10.3181/00379727-95-23308
Scott ML (1973) The selenium dilemma. J Nutr 103(6):803–810. https://doi.org/10.1093/jn/103.6.803
Seko Y, Imura N (1997) Active oxygen generation as a possible mechanism of selenium toxicity. Biomed Environ Sci 10(2–3):333–339
Seko Y, Saito Y, Kitahara J et al (1988) Active oxygen generation by the reaction of selenite with reduced glutathione in vitro. In: Wendel A (ed) Selenium in biology and medicine. Springer, New York, pp 70–73
Sharma S, Goyal R, Sadana US (2014) Selenium accumulation and antioxidant status of rice plants grown on seleniferous soil from northwestern India. Rice Sci 21(6):327–334. https://doi.org/10.1016/S1672-6308(14)60270-5
Shi J, Xu JX, Li XL (2003) Research progress on the physiological function of trace element selenium. Feed Rev 1:4–7 (in Chinese with English Abstract)
Shi L, Yue WB, Zhang CX et al (2010) Effects of maternal and dietary selenium (Se-enriched yeast) on oxidative status in testis and apoptosis of germ cells during spermatogenesis of their offspring in goats. Anim Reprod Sci 119(3–4):212–218. https://doi.org/10.1016/j.anireprosci.2010.02.012
Shisler JL, Senkevich TG, Berry MJ et al (1998) Ultraviolet-induced cell death blocked by a selenoprotein from a human dermatotropic poxvirus. Science 279(5347):102–105. https://doi.org/10.1126/science.279.5347.102
Shrift A, Ulrich JM (1969) Transport of selenate and selenite into astragalus roots. Plant Physiol 44(6):893–896. https://doi.org/10.1104/pp.44.6.893
Sors TG, Ellis DR, Salt DE (2005) Selenium uptake, translocation, assimilation and metabolic fate in plant. Photosynth Res 86:373–389. https://doi.org/10.1007/s11120-005-5222-9
Spallholz JE, Martin JL, Gerlach ML et al (1973) Enhanced immunoglobulin M and immunoglobulin G antibody titers in mice fed selenium. Infect Immun 8(5):841–842
Spallholz JE, Palace VP, Reid TW (2004) Methioninase and selenomethionine but not Se-methylselenocysteine generate methylselenol and superoxide in an in vitro chemiluminescent assay: implications for the nutritional carcinostatic activity of selenoamino acids. Biochem Pharmacol 67(3):547–554. https://doi.org/10.1016/j.bcp.2003.09.004
Stone CA, Kawai K, Kupka R et al (2010) Role of selenium in HIV infection. Nutr Rev 68(11):671–681. https://doi.org/10.1111/j.1753-4887.2010.00337.x
Sun P, Wang J, Liu W et al (2017) Hydroxy -selenomethionine: a novel organic selenium source that improves antioxidant status and selenium concentrations in milk and plasma of mid-lactation dairy cows. J Dairy Sci 100(12):9602–9610. https://doi.org/10.3168/jds.2017-12610
Tai PD, Li PJ (2002) Phyto-toxicity of selenium on plant. J Agro Environ Sci 6:496–498. https://doi.org/10.3321/j.issn:1672-2043.2002.06.005 (in Chinese with English Abstract)
Talukdar DJ, Talukdar P, Ahmed K (2016) Minerals and its impact on fertility of livestock: a review. Agric Rev 37(4):333–337. https://doi.org/10.18805/ag.v37i4.6464
Tatsuya T, Makoto M, Katsuhiko N et al (1996) Mechanisms of selenium methylation and toxicity in mice treated with selenocystine. Arch Toxicol 71(1–2):31–38. https://doi.org/10.1007/s002040050355
Taylor EW, Ruzicka JA, Premadasa L et al (2016) Cellular selenoprotein mRNA tethering via antisense interactions with Ebola and HIV-1 mRNAs may impact host selenium biochemistry. Curr Top Med Chem 16(13):1530–1535. https://doi.org/10.2174/1568026615666150915121633
Terry N, de Souza MP, Tarun AS et al (2000) Selenium in higher plants. Annu Rev Plant Physiol Mol Biol 51:401–432. https://doi.org/10.1146/annurev.arplant.51.1.401
Tran PA, Webster TJ (2011) Selenium nanoparticles inhibit Staphylococcus aureus growth. Int J Nanomed 6:1553–1558. https://doi.org/10.2147/IJN.S21729
Ullah H, Liu G, Yousaf B et al (2018) Developmental selenium exposure and health risk in daily foodstuffs: a systematic review and meta-analysis. Ecotoxicol Environ Saf 149:291–306. https://doi.org/10.1016/j.ecoenv.2017.11.056
Vendeland SC, Deagen JT, Butler JA et al (1994) Uptake of selenite, selenomethionine and selenate by brush border membrane vesicles isolated from rat small intestine. Biochem Med 7(4):305–312. https://doi.org/10.1007/BF00144126
Verma S, Molina Y, Lo YY et al (2008) In vitro effects of selenium deficiency on West Nile virus replication and cytopathogenicity. Virol J 5(1):66. https://doi.org/10.1186/1743-422X-5-66
Vickerman DB, Young JK, Trumble JT (2002) Effect of selenium-treated alfalfa on development, survival, feeding, and oviposition preferences of Spodoptera exigua (Lepidoptera: Noctuidae). Environ Entomol 31(6):953–959. https://doi.org/10.1603/0046-225X-31.6.953
Wang FD (2012) Trace element research in China: present and future. Chin Bull Life Sci 24(8):713–730. https://doi.org/10.13376/j.cbls/2012.08.020 (in Chinese with English Abstract)
Wang GZ, Niu ZX (2010) Research progress on the toxicity of trace element selenium. Northwest Pharm J 25(3):237–238. https://doi.org/10.3969/j.issn.1004-2407.2010.03.044in (Chinese with English Abstract)
Wang Z, Jiang C, Lv JX (2002) Induction of caspase-mediated apoptosis and cell-cycle G1 arrest by selenium metabolite methylselenol. Mol Carcinog 34(3):113–120. https://doi.org/10.1002/mc.10056
Wang HL, Zhang JS, Yu HQ (2007) Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice. Free Radic Biol Med 42(10):1524–1533. https://doi.org/10.1016/j.freeradbiomed.2007.02.013
Wang BT, Xie LQ, Lin YK et al (2011) Determination of selenium species in food by high performance liquid chromatography with inductively coupled plasma mass spectrometry. Chin J Chromatogr 29(3):223–227 (in Chinese with English Abstract)
Wang AP, Yu KH, Zou WW et al (2012) Anti-herpes simplex virus type 1 activity of trace element selenium in vitro. J Nanchang Univ Med Sci 52(9):1–4 (in Chinese with English Abstract)
Wang Q, Larese-Casanova P, Webster TJ (2015) Inhibition of various gram-positive and gram-negative bacteria growth on selenium nanoparticle coated paper towels. Int J Nanomed 10:2885–2894. https://doi.org/10.2147/IJN.S78466
Wei ZY, Cui BA, Huang KH et al (2005) Inhibitory effects of different selenium sources on porcine parvovirus infection in vitro. Virol Sin 20(6):613–617
Wei JY, Wang J, Liu W et al (2019) Short communication: effects of different selenium supplements on rumen fermentation and apparent nutrient and selenium digestibility of mid-lactation dairy cows. J Dairy Sci 102(4):3131–3135. https://doi.org/10.3168/jds.2018-15455
White PJ, Bowen HC, Parmaguru P et al (2004) Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. J Exp Bot 55:1927–1937. https://doi.org/10.1093/jxb/erh192
Wilber CG (1980) Toxicology of selenium: a review. Clin Toxicol 17(2):171–230. https://doi.org/10.3109/15563658008985076
Wolonciej M, Milewska E, Roszkowska-Jakimiec W (2016) Trace elements as an activator of antioxidant enzymes. Postepy Hig Med Dosw 70:1483–1498. https://doi.org/10.5604/17322693.1229074
Wycherly BJ, Moak MA, Christensen MJ (2004) High dietary intake of sodium selenite induces oxidative DNA damage in rat liver. Nutr Cancer 48(1):78–83. https://doi.org/10.1207/s15327914nc4801_11
Xia YM, Hill KE, Byrne DW et al (2005) Effectiveness of selenium supplements in a low-selenium area of China. Am J Clin Nutr 81(4):829–834. https://doi.org/10.1093/ajcn/81.4.829
Xu HB, Yang XL, Liu Q et al (2002) The signal transduction in ROS-mediated apoptosis induced by selenium compounds. Prog Chem 14(4):305–310 (in Chinese with English Abstract)
Xu ZC, Shao HF, Sun SG et al (2011) Effects of selenium added to soil on physiological indexes in flue-cured tobacco. Acta Ecol Sin 31(2):7179–7187 (in Chinese with English Abstract)
Xu QL, Wu WL, Zhao GS et al (2017) Selenium metabolism in microorganisms. Microbiol China 44(1):207–216. https://doi.org/10.13344/j.microbiol.china.160380 (in Chinese with English Abstract)
Yan L, Spallholz JE (1993) Generation of reactive oxygen species from the reaction of selenium compounds with thiols and mammary tumor cells. Biochem Pharmacol 45(2):429–437. https://doi.org/10.1016/0006-2952(93)90080-G
Yang YX, Yang LF (2014) Research progress of effects of soil selenium on growth of plant. Tillage Cultiv 2:50–52. https://doi.org/10.3969/j.issn.1008-2239.2014.02.027 (in Chinese with English Abstract)
Yang QL, Qu XY, Chen JF et al (2016) Application of nano-selenium in livestock and poultry production. China Poultry 38(1):47–52. https://doi.org/10.16372/j.issn.1004-6364.2016.01.010 (in Chinese with English Abstract)
Yao LL, Du Q, Yao HD et al (2015) Roles of oxidative stress and endoplasmic reticulum stress in selenium deficiency-induced apoptosis in chicken liver. Biometals 28:255–265. https://doi.org/10.1007/s10534-014-9819-3
Yao W, Zhao FH, Liu LN et al (2016) Study on the inhibition effect of different concentrations selenium content in selenium-rich lactobacillus on the growth and toxin-producing effect of Aspergillus. China Feed 12(19–21):26. https://doi.org/10.15906/j.cnki.cn11-2975/s.20161205 (in Chinese with English Abstract)
Yi HL, Si L (2007) Vicia root-mirconucleus and sister chromatid exchange assays on the genotoxicity of selenium compounds. Mutat Res 630(1–2):92–96. https://doi.org/10.1016/j.mrgentox.2007.03.003
Yoon I, Werner TM, Butler JM (2007) Effect of source and concentration of selenium on growth performance and selenium retention in broiler chickens. Poult Sci 86(4):727–730. https://doi.org/10.1093/ps/86.4.727
Yu SY, Zhu YJ, Li WG (1997) Protective role of selenium against hepatitis B virus and primary liver cancer in Qidong. Biol Trace Elem Res 56(1):117–124. https://doi.org/10.1007/bf02778987
Yu HW, Chen CY, Gao YX et al (2006) Chemical speciation analysis of selenium in biological samples by a hyphenated technique of high performance liquid chromatography-inductively coupled plasma mass spectrometry. Chin J Anal Chem 34(6):749–753 (in Chinese with English Abstract)
Yu L, Sun L, Nan Y et al (2011) Protection from H1N1 influenza virus infections in mice by supplementation with selenium: a comparison with selenium-deficient mice. Biol Trace Elem Res 141(1–3):254–261. https://doi.org/10.1007/s12011-010-8726-x
Zachariah M, Maamoun H, Rayman MP et al (2015) High selenium intake is associated with endothelial dysfunction: critical role for endoplasmic reticulum stress. Heart 100(4):A5. https://doi.org/10.1136/heartjnl-2014-306916.13
Zachariah M, Maamoun H, Milano L et al (2016) P31 endoplasmic reticulum stress drives high selenium induced endothelial dysfunction. Heart 102(8):A11–A12. https://doi.org/10.1136/heartjnl-2016-310696.35
Zhang YL, Pan GX, Chen J et al (2003) Uptake and transport of selenite and selenate by soybean seeding of two genotypes. Plant Soil 253(2):437–443. https://doi.org/10.1023/A:1024874529957
Zhang YS, Bian LQ, You SQ (2008a) Effects of three selenium sources on tissue selenium deposition and antioxidant capacity of growing-finishing pigs. Feed Ind 29(1):18–20 (in Chinese with English Abstract)
Zhang JS, Wang XF, Xu TW (2008b) Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: comparison with se-methylselenocysteine in mice. Toxicol Sci 101(1):22–31. https://doi.org/10.1093/toxsci/kfm221
Zhang P, Wan YX, Du XF et al (2016a) Research progress of the resistance of selenium in animals and plants. Appl Chem Ind 45(8):1579–1582. https://doi.org/10.16581/j.cnki.issn1671-3206.20160527.015 (in Chinese with English Abstract)
Zhang X, Liu C, Guo J et al (2016b) Selenium status and cardiovascular diseases: meta-analysis of prospective observational studies and randomized controlled trials. Eur J Clin Nutr 70(2):162–169. https://doi.org/10.1038/ejcn.2015.78
Zhivotovsky B, Orrenius S (2011) Calcium and cell death mechanisms: a perspective from the cell death community. Cell Calcium 50(3):211–221. https://doi.org/10.1016/j.ceca.2011.03.003
Zhou C (2018) Study on biosynthesis and antifungal activity of Nano-selenium. Dissertation, Anhui Agricultural University (in Chinese with English Abstract)
Zhou XX, Wang YB, Gu Q et al (2009) Effects of different dietary selenium sources (selenium nanoparticle and selenomethionine) on growth performance, muscle composition and glutathione peroxidase enzyme activity of crucian carp (Carassius auratus gibelio). Aquaculture 291(1):78–81. https://doi.org/10.1016/j.aquaculture.2009.03.007
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No. 31860708), the Natural Science Foundation of Guangxi Zhuang Autonomous Region (No. 2017GXNSFBA198025) and the Guangxi driving innovation major project (No. GK-AA17202037 and No. GK-AB16380164)
Author information
Authors and Affiliations
Contributions
QL had the idea for the article, XL, KN, ZG, TQ and XQ performed the literature search and data analysis, QL drafted the work, DW and YZ critically revised the work. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research Involving Human Participants and/or Animals
Not applicable.
Informed Consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lv, Q., Liang, X., Nong, K. et al. Advances in Research on the Toxicological Effects of Selenium. Bull Environ Contam Toxicol 106, 715–726 (2021). https://doi.org/10.1007/s00128-020-03094-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-020-03094-3