Abstract
The effects of copper (Cu) exposure on growth and physiological characteristics of three genotypes (CN-12, Cim-Sanjeevani and Cim-Arogya) of Artemisia annua L. were elucidated. The plants were grown under naturally illuminated greenhouse conditions and were harvested after physiological maturity (120 days after sowing). Results suggest that 10 mg kg− 1 Cu significantly enhanced the growth and physiological parameters like enzyme activities, photosynthesis. At higher concentrations, Cu inhibited the growth, biomass, photosynthetic parameters; while increased lipid peroxidation in all the genotypes. The activities of antioxidant enzymes viz. catalase, peroxidase and superoxide dismutase were upregulated by the Cu stress. The highest applied concentration of Cu (60 mg kg− 1) proved most toxic for plants. Moreover, artemisinin content was increased upto 10 mg kg− 1 of Cu treatment, compared with control, however, the artemisinin accumulation decreased at higher doses of Cu in all the genotypes. On the basis of studied parameters, Cim-Arogya was found to be most tolerant among all for Cu toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Soil, a quintessential resource for human survival, has been severely contaminated by anthropogenic activities and this gradually pollute soil with heavy metals. Anthropogenic origins of metal are more soluble in soil than naturally occurring (Ahsan et al. 2007). Copper (Cu) is an essential, redox-active microelement associated with normal plant growth and development. It is a component of regulatory proteins, a cofactor of phenol oxidase and it also embroils in plant biological processes such as photosynthesis, respiratory electron transport reactions and oxygen superoxide scavenging (Habiba et al. 2015; Lange et al. 2017). The rate of uptake and the concentration of the metal in a plant depends on environmental, physiological and biochemical factors (Hasanuzzaman et al. 2013). Excess concentration of essential metals in soil cause adverse effects on plant physiology (Feigl et al. 2015). Presence of high level of Cu in the soils reduce biomass, inhibit root growth; bronzing, necrosis and chlorosis are usually reported symptoms due to increased production of reactive oxygen species at cellular level (Lange et al. 2017). It can be strongly phytotoxic, deleterious in superfluous quantity, disrupts membrane permeability, proteins structures and inactivates enzymes and may activate senescence (Chen et al. 2015). Copper is an efficient generator of toxic oxygen species such as O2−, H2O2 and HO−, thus, an important factor of Cu toxicity is generation of oxidative stress (Ali et al. 2016; Saleem et al. 2019).
Malaria is a global health problem; in 2016 alone, there were an estimated 216 million new cases of malaria, 445,000 deaths, and nearly 1 billion people living in areas with a high risk of the disease in year 2016 (World Health Organization, WHO 2017). The artemisinin, which is naturally synthesised in the leaves and flowers of Artemisia annua L., is an antimalarial agent, which is effective against and can kill the multidrug resistant strains of malarial parasite i.e. Plasmodium falciparum (Aftab et al. 2010a, 2011, 2012, 2016). Now, WHO recommends the artemisinin-based combination therapy (ACT) for treatment of malaria, thus, considered one of the novel discoveries in the recent medicinal plant research (Ferreira et al. 2010). Exposure of A. annua to abiotic stress-factors have been reported to enhance ROS generation, which increase production of artemisinin by facilitating rapid conversion of dihydroartemisinic acid to artemisinin (Pu et al. 2009; Aftab et al. 2010a, b, 2011, 2012; Naeem et al. 2019) during biosynthetic pathway. The present research is the outcome of the hypothesis, that the artemisinin must be produced cheaply and if this important medicinal plant can be cultivated on contaminated lands. To test this, experiments were conducted on three different genotypes of A. annua to establish optimum and toxic levels of Cu in soil.
Materials and Methods
The pot experiments were conducted on three genotypes of A. annua (CN-12, CIM-Sanjeevani and CIM-Arogya, procured from Ipca Laboratories, MP, India and CIMAP, India) in net house conditions using 5 concentrations of Cu viz. Control, 5, 10, 20, 40 and 60 mg kg− 1 soil. Copper was applied as copper sulphate (CuSO4·5H2O) in the soil. Physicochemical characteristics of the soil were: texture-sandy loam, pH (1:2) 8.0, EC (1:2) 0.48 m mhos cm− 1 and available N, P and K 97.46, 10.21 and 147.0 mg kg− 1 soil, respectively. Soil Cu concentration was 0.01 mM, which is much below the required limit for plants. Seeds of A. annua were initially surface sterilized with 90% ethyl alcohol for 5 min and then washed thoroughly with double-distilled water before sowing. Each experimental pot (25 cm diameter × 25 cm height) was filled with 5.0 kg of a homogeneous mixture of soil and farmyard manure (4:1) prior to sowing seeds. Copper was applied when plants attained three to four true leaves. The plants were harvested after physiological maturity (120 days after sowing). There were four replicates of each treatment having five plants per replicate.
The plants from each treatment were carefully uprooted and shoot and root heights were recorded. Plants were washed with tap water to remove adhering foreign particles. Plant roots were removed and the individual shoot fresh weight was recorded. The shoots were dried at 80°C for 48 h and their dry weights were then recorded.
For determining Cu contents in plant tissues (Ouzounidou et al. 1997), root and shoot samples (0.5 g) were collected in a flask and then 15 mL of concentrated HNO3 was added. After mixing, the flasks were placed on a hot plate and temperature was gradually increased up to 275°C. Dense yellow fumes appeared from the flask and the hydrogen peroxide was continuously added until dense yellow fumes disappeared. When the samples became colourless, the flasks were removed from a hot plate, and volume was made up to 25 mL using distilled water. Copper content was determined using atomic absorption spectrometer (Perkin Elmer, AAnalyst 600, USA).
Net photosynthetic rate (PN) was measured on sunny days at 11:00 a.m. using fully expanded leaves with the help of an IRGA (infrared gas analyser, LI-COR 6400 Portable Photosynthesis System, LI-COR, Lincoln, NE, USA). Before carrying out the measurements, the IRGA was calibrated to zero was adjusted approximately every 30 min during the measurement period. Each leaf was enclosed in a gas exchange chamber for 60 s. Total chlorophyll content in the fresh leaves was estimated using the method of Lichtenthaler and Buschmann (2001). For chlorophyll extraction, the fresh tissue from interveinal leaf area (5 mg) was ground using a mortar and pestle containing 80% acetone. The absorbance of the solution (chlorophyll extract) was recorded at 662 nm and 645 nm using a spectrophotometer (Shimadzu UV-1700, Tokyo, Japan).
Nitrate reductase (NR) activity in the leaf was determined by the intact tissue assay method of Jaworski (1971). Chopped leaf pieces (200 mg) were incubated for 2 h at 30°C in a 5.5 mL reaction mixture, which contained 2.5 mL of 0.1 M phosphate buffer, 0.5 mL of 0.2 M potassium nitrate, and 2.5 mL of 5% isopropanol. The nitrite that formed subsequently was calorimetrically determined at 540 nm after azocoupling with sulphanilamide and naphthalene diamine dihydrochloride. NR activity was expressed as nM NO2 g− 1 FW h− 1. Carbonic anhydrase (CA) activity was measured in fresh leaves using the Dwivedi and Randhawa (1974) method. 200 mg of fresh leaf pieces were weighed and transferred to Petri dishes. The leaf pieces were dipped in 10 mL of 0.2 M cysteine hydrochloride solution for 20 min at 4°C. To each Petri dish, 4 mL of 0.2 M sodium bicarbonate solution and 0.2 mL of 0.022% bromothymol blue were added. The reaction mixture was titrated against 0.05 N HCl using methyl red as indicator. The enzyme activity was expressed as µM CO2 kg− 1 leaf FW s− 1.
The proline content was estimated using the method of Bates et al. (1973). The leaf material was homogenized in 3% aqueous sulfosalicylic acid and the homogenate was centrifuged at 10,000 × g rpm. The supernatant was used to estimate the proline content. The reaction mixture, consisting of 2 mL of acid ninhydrin and 2 mL of glacial acetic acid, was boiled at 100°C for 1 h. After terminating the reaction in an ice bath, the proline was extracted with 4 mL of toluene and its absorbance was recorded at 520 nm.
Lipid peroxidation in leaves was determined by the content of total 2 thiobarbituric acid reactive substances (TBARS) as described by the method of Cakmak and Horst (1991). The TBARS were extracted from 500 mg freshly chopped leaves, ground with 5 mL of 0.1% (w/v) trichloroacetic acid (TCA). The mixture is then centrifuged at 12,000 × g for 5 min, an aliquot of 1 mL of the supernatant was added to 4 mL of 0.5% (w/v) TBA in 20% (w/v) TCA. Samples were incubated at 90°C for 30 min. Thereafter, the reaction was stopped using an ice bath. The mixture was centrifuged at 10,000 × g for 5 min, and the absorbance of the supernatant was recorded at 532 nm with the help of a spectrophotometer and the values were corrected for non-specific turbidity by subtracting the absorbance at 600 nm. The TBARS content was expressed as nanomoles per gram FW.
Fresh leaf tissue was homogenized with an extraction buffer containing 0.5% (v/v), Triton-X-100 and 1% (w/v) polyvinyl pyrrolidone in potassium-phosphate buffer (100 mM, pH 7.8) using chilled mortar and pestle. The homogenate was centrifuged at 15,000 × g for 5 min at 4°C. The supernatant obtained after centrifugation was used for the assay of catalase, peroxidase and superoxide dismutase enzymes.
Catalase (CAT) activity was measured according the method given by Chandlee and Scandalios (1984) with a small modification. The assay mixture contained 2.6 mL of 50 mM potassium phosphate buffer (pH 7.0), 0.4 mL of 15 mM H2O2 and 0.04 mL of enzyme extract. The decomposition of H2O2 was followed by the decline in absorbance at 240 nm. The enzyme activity was expressed in units (U) mg− 1 protein (1 U = 1 mM of H2O2 reduction min− 1 mg− 1 protein).
Peroxidase (POX) activity was assayed by the method of Kumar and Khan (1982). The assay mixture of POX contained 2 mL of 0.1 M phosphate buffer (pH 6.8), 1 mL of 0.01 M pyrogallol, 1 mL of 0.005 M H2O2 and 0.5 mL of enzyme extract. The solution was incubated for 5 min at 25°C after which the reaction was terminated by adding 1 mL of 2.5 N H2SO4. The amount of purpurogallin formed was determined by measuring the absorbance at 420 nm against a reagent blank prepared by adding the extract after the addition of 2.5 N H2SO4 at time zero. The activity was expressed in U mg− 1 protein. One unit of the enzyme activity corresponded to an amount of enzyme that caused an increase in the absorbance of 0.1 min− 1 mg− 1 protein.
Superoxide dismutase (SOD) activity was assayed as described by Beauchamp and Fridovich (1971). The reaction mixture contained 1.17 × 10− 6 M riboflavin, 0.1 M methionine, 2 × 10− 5 M KCN and 5.6 × 10− 5 M nitroblue tetrazolium salt (NBT) dissolved in 3 mL of 0.05 M sodium phosphate buffer (pH 7.8) and 3 mL of the reaction medium was added to 1 mL of enzyme extract. The mixtures were illuminated in glass test tubes by two sets of Philips 40W fluorescent tubes in a single row. Illumination initiated the reaction at 30°C for 1 h. Identical solutions that were kept in the dark served as blanks. The absorbance was read at 560 nm in a spectrophotometer against a blank. SOD activity was expressed as U mg− 1 protein (1 U = the amount of change in absorbance by 0.1 h− 1 mg− 1 protein).
Dry leaf material (1 g) was used to estimate artemisinin modified to a compound Q260 and quantified using HPLC (Zhao and Zeng 1986). A standard curve was prepared using 1 mg of standard artemisinin dissolved in 1 mL of HPLC-grade methanol to make the stock solution. It was extracted with 20 mL petroleum ether in a shaker at 70 rpm for 24 h. After 24 h, solvent was decanted and pooled and 20 mL of petroleum ether added again; this step was repeated three times. Petroleum ether fractions were pooled and concentrated under reduced pressure and residues defatted with CH3CN (10 mL × 3). Precipitated fat was filtered out and the filtrate was concentrated under reduced pressure. Residues were dissolved in 1 mL of methanol. To a 100-µL aliquot of each sample of each treatment, 4 mL of 0.3% NaOH was added. The samples were incubated in a shaking water bath at 50°C for 30 min, thereafter cooled and neutralized with glacial acetic acid (0.1 M in 20% MeOH). The pH of the solution was maintained at 6.8. Derivatized artemisinin was analyzed and quantified through a reverse phase column (C18; 5 µm, 4.6 mm, 250 mm) using a premix of methanol and 10 mM K-phosphate buffer (pH 6.5) at a ratio of 60:40 as the mobile phase at a constant flow rate of 1 mL min− 1, with the detector set at 260 nm. Artemisinin was quantified against the standard curve of artemisinin (Sigma-Aldrich).
Descriptive statistics were presented as mean ± standard error of mean. One-way ANOVA was used to test differences among the treatments. Duncan’s multiple range test (DMRT ≤ 0.05%) was also used to identify different treatment followed by ANOVA. Statistical significance level was considered as 5% and SPSS (ver: 22) statistical program was used for all statistical computations.
Results and Discussion
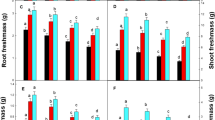
Copper is an essential micronutrient, however, it works as a beneficial element for plants at a specific concentration. Maximum increase in growth parameters was reported when 10 mg kg− 1 Cu was applied to the plants, and growth was negatively affected as the concentration of Cu increased further (Table 1). Maximum shoot length was recorded at the application of 10 mg kg− 1 Cu in all the genotypes while maximum inhibition of shoot length was found when 60 mg kg− 1 Cu was applied, compared with control. Root length also decreased significantly at 60 mg kg− 1 Cu and fresh weight reduced by 22.8%, 17.5% and 20.5% in CN-12, CIM-Sanjeevani and CIM-Arogya of A. annua respectively, compared with control. At highest concentration of Cu, reduction in dry weight was maximum while at 10 mg kg− 1 Cu significant increase in dry weight was noted in all tested genotypes, compared with control (Table 1). The effects of Cu have been previously reported in case of rice (Ahsan et al. 2007), barley (Gupta and Kalra 2006), wheat (Azooz et al. 2012) and Phaseolus vulgaris L. (Khalil et al. 2017). In this study, growth and biochemical parameters increased with increasing concentration of Cu upto 10 mg kg− 1, but Cu had negative impact on plant growth beyond this concentration (10 mg kg− 1). Fageria (2001) reported the reduction in root growth, total chlorophyll content in the leaves and increase in copper uptake. However, application of 50 kg ha− 1 Cu had no effect on yield in wheat and barley, however, yield was reduced at higher levels of Cu (Gupta and Kalra 2006). Additionally, the toxic levels of Cu in plants reduces plant growth and yield due to rapid alterations in photosynthesis, enzyme activities, mineral nutrients, and decrease in chlorophyll biosynthesis (Adrees et al. 2015).
Copper concentrations significantly increased in root and shoot with increasing Cu levels in the soil, compared with control (Table 2). The highest Cu contents were found in root tissues. Among genotypes, the accumulation of Cu was highest in CN-12 while lowest in Cim-Arogya. Significant elevations of Cu concentration were noted in Cu-stressed A. annua genotypes. Greater Cu accumulation in the roots and less transport to the shoots further strengthen the findings of Minnich et al. (1987), who suggested that higher Cu contents in growth media were more detrimental to root growth of plant species. Despite of relatively higher Cu concentration in root tissues, efficient translocation of Cu was noticed to leaves which damaged chlorophyll pigment and consequently caused biomass reduction.
Total chlorophyll content and chlorophyll fluorescence decreased maximally at 60 mg kg− 1 Cu, while the parameters were reported to be increased significantly at 10 mg kg− 1 Cu application, compared with control in all genotypes (Table 3). A significant reduction was also noted for chlorophyll fluorescence at highest applied concentration of Cu over the control. The reduction in total chlorophyll content and chlorophyll fluorescence under heavy metal stress was noted in previous studies in several crops such as spinach (Baszynski et al. 1988), wheat (Lanaras et al. 1993), lettuce (Doncheva and Stoyanova 1997) and coontail (Devi and Prasad 1998). The interference of heavy metals in synthesis of chlorophyll either inhibit direct enzymatic steps or influence the deficiency of an essential nutrients (Meers et al. 2010) and the formation of free radicals due to cellular injury was also reported by Cu treatment (Gupta et al. 2006).
Maximum inhibition in NR and CA activities were recorded at 60 mg kg− 1 of Cu application in case of all three tested genotypes, compared with control (Table 3). Nitrate reductase catalyses the assimilation of nitrogen. Transcriptional, post transcriptional and post translational levels are highly regulated in nitrogen assimilation in which inputs are supplied by photosynthesis, oxygen availability, light and nutrient status (Von Wiren et al. 2000). Besides, carbonic anhydrase enzyme plays a pivotal role in the physiological processes like ion-exchange, acid-base balance, carboxylation/decarboxylation reactions and inorganic carbon diffusion with in the cell and between the cell and its environment (Georgios et al. 2004). The activities of NR and CA have been reported to be suppressed under stress conditions in A. annua L. (Aftab et al. 2010a, 2011).
Copper at higher concentrations decreased photosynthesis significantly while increased at lower concentration (Table 4). Stomatal conductance decreased at highest concentration of Cu by 28.0%, 24.0% and 22.5% in CN-12, CIM-Sanjeevani and CIM-Arogya genotypes respectively, compared with control. Internal CO2 and net photosynthetic rate also increased significantly when Cu application was applied at 10 mg kg− 1 of Cu. Net photosynthetic rate was found maximum at 10 mg kg− 1 of Cu and further decreased significantly in all the tested genotypes of A. annua, over their respective controls (Table 4). The PS II of photosynthetic electron transport chain system is more sensitive site to copper toxicity than PS I (Ouzounidou et al. 1997; Yruela 2005). The damage caused in the photosynthetic machinery of plants under abiotic stress is due to the generation of reactive oxygen species (ROS). Photosynthetic apparatus particularly PS II could be damaged by the ROS followed by the inhibition of photosynthesis as a result of variation in the photosynthetic redox signalling pathways and retardation of PS II repair (Gururani et al. 2015). A least photosynthetic activity was found at highest applied concentration of Cu in Cucumis sativus L. (Stepien and Klbus 2006). It was noticed that Cu at 80 µM concentration resulted in the disintegration of cytoplasm in cortical or stellar cells in Thlaspi ochroleucum (Ouzounidou et al. 1992), while in Zea mays, the toxicity of Cu resulted in the accumulation of higher amount of it in roots than the above ground parts (Ouzounidou et al. 1995).
The accumulation trend of proline increased under stress conditions. The content of proline was recorded maximum at 60 mg kg− 1 Cu which was 71.0%, 76.1% and 66.73% higher in CN-12, CIM-Sanjeevani and CIM-Arogya respectively, compared with untreated plants (Table 4). Proline functions as osmoprotectant and helps in antioxidant defence (Hasanuzzaman et al. 2014). Osmotic adjustment, protection of membrane integrity and detoxification of ROS is thought to be the contribution of proline. The proline content of radish leaves decreased at lower concentration and significantly increase as the concentration of Cu increased in the soil (Christhuuthayam et al. 2018).
Lipid peroxidation of membrane which results in the formation of MDA content, increased under stress conditions and the highest TBARS content was observed at 60 mg kg− 1 Cu in all three genotypes of A. annua as compared to control (Table 5). This is in agreement with the effect of high Cu concentration observed on total lipid content of plasma membrane and/or reduction in cell division and damage to root epidermal cells (Ouzounidou et al. 1995; Keller et al. 2015). One of the most harmful impressions induced by heavy metal in plants is lipid peroxidation; membrane disfiguration instantly caused by lipid peroxidation. It mainly maintain the oxidative stress and formed in cellular metabolic processes like photosynthesis and respiration and in different process like photosynthetic electron transport chain system (Younis et al. 2018). Lipid peroxidation used as a marker for oxidative stress under heavy metal stress in case of tomato (Mazhoudi et al. 1997), rice (Verma and Dubey 2003), pea (Metwally et al. 2005), rice (Ahsan et al. 2007), and wheat (Azooz et al. 2012).
The generation of ROS under high concentrations of Cu in the soil is enhanced by cuprous and cupric Cu ions, which induce oxidative damage in plant cells/tissues (Saleem et al. 2019). Increased activities of antioxidant enzymes were recorded after Cu treatments in all three genotypes (Table 4). Plant responses to oxidative stress depend on the plant species and variety. Activity of CAT showed progressive and significantly increasing trend under Cu application in all three genotypes of A. annua, over their respective controls. Highest POX activity was observed at the highest concentration of applied Cu, which was 55.1%, 66.1% and 70.9% increase in CN-12, CIM-Sanjeevani and CIM-Arogya respectively, compared with untreated plants (Table 4). Also, there was a significant increase in the activity of SOD under Cu treatments. It showed maximum increase at highest concentration of applied Cu in all three genotypes of A. annua, compared with control (Table 5). The highest increase in the activity of antioxidant enzymes was noticed in CIM-Arogya, which might be genotype specific and provide tolerance towards oxidative stress to this genotype. Overall, antioxidant enzymes play an important role in the antioxidative defence mechanism. The enzymatic and non-enzymatic antioxidants protect the cells from oxidative stress and control the cascades of unlimited oxidation by scavenging reactive oxygen species (Gill and Tuteja 2010). In the present study, Cu exhibited a significant increase in the activities of CAT, POX and SOD in a dose dependent manner, as compared with control. The SOD, POD, GR activities increased with increase in the concentration of salt in ‘Pora’, a variety of cotton (Meloni et al. 2003). It is reported that Cu toxicity could promote and induce the production the ROS which leads to increase the activities of antioxidant enzymes as defence mechanism (Mittler et al. 2004; Azooz et al. 2012). Furthermore, the Cu toxicity causes redox cycling, deplete glutathione and protein bounded sulfhydryl group leads to the production of ROS as superoxide ion, hydrogen peroxide and hydroxyl radical (Stohs and Bagchi 1995).
The artemisinin content was higher when 5 and 10 mg kg− 1 Cu was applied, as compared with untreated plants, however a decrease in artemisinin content was noted when further high doses of Cu were applied (Fig. 1). A considerable augment in the artemisinin content due to Cu application could be attributed to a high conversion rate of dihydroartemisinic acid into artemisinin. Presumably, applied Cu enhanced the synthesis of artemisinin by encouraging the level of H2O2, which converts intermediate precursor (dihydroartemisinic acid) to a final product of artemisinin biosynthesis. Regarding the escalation of artemisinin, the present results are in accordance with the earlier studies of Wallaart et al. (2000), Ferreira (2007), Pu et al. (2009) and Aftab et al. (2011) suggested a direct relationship between the ROS and artemisinin content, that consecutively enhance the conversion rate of the dihydroartemisinic acid to artemisinin.
In summary, this study revealed that the Cu supplementation at higher concentrations showed inhibitory effect on the growth and physiological functions of three selected genotypes of A. annua plants. Among all genotypes, CIM-Arogya showed tolerant behaviour and had maximum artemisinin content under moderate Cu stress. Copper stress stimulated the lipid peroxidation activity and production of antioxidant defence system in the leaf tissues. It was observed that the Cu showed biphasic activity i.e., at low concentration, Cu, acted as beneficial element for plants, but it became toxic on high concentration. The relevance of changes in both plant metabolisms, primary and secondary, can drive to responses that allow the survival of plants exposed to high Cu concentrations. The present study also suggest that at higher Cu concentrations, A. annua behaves as metal excluders which accumulate heavy metals from the substrate in their roots, but restrict their transport and entry into their aerial parts. Therefore, A. annua can be used for phytoremediation of high Cu concentrations.
References
Adrees M, Ali S, Rizwan M, Ibrahim M, Abbas F, Farid M, Zia-Ur-Rehman M, Irshad MK, Bharwana SA (2015) The effect of excess copper on growth and physiology of important food crops: a review. Environ Sci Pollut Res Int 22:8148–8162
Aftab T, Khan MMA, Idrees M, Naeem M, Ram M (2010) Boron induced oxidative stress, antioxidant defence response and changes in artemisinin content in Artemisia annua L. J Agron Crop Sci 196:423–430
Aftab T, Khan MMA, Idrees M, Naeem M, Hashmi N, Moinuddin (2010) Effect of salt stress on growth, membrane damage, antioxidant metabolism and artemisinin accumulation in Artemisia annua L. Plant Stress 4:36–43
Aftab T, Khan MMA, Teixeira da Silva JA, Idrees M, Naeem M, Moinuddin (2011) Role of salicylic acid in promoting salt stress tolerance and enhanced artemisinin production in Artemisia annua L. J Plant Growth Regul 30:425–435
Aftab T, Khan MMA, Naeem M, Idrees M, Moinuddin, Teixeira da Silva JA, Ram M (2012) Exogenous nitric oxide donor protects Artemisia annua from oxidative stress generated by boron and aluminium toxicity. Ecotoxicol Environ Saf 80:60–68
Aftab T, Naeem M, Idrees M, Khan MMA, Moinuddin, Varshney L (2016) Simultaneous use of irradiated sodium alginate and nitrogen and phosphorus fertilizers enhance growth, biomass and artemisinin biosynthesis in Artemisia annua L. J Appl Res Med Aromat Plant 3:186–194
Ahsan N, Lee DG, Lee SH, Kang KY, Lee JJ, Kim PJ, Yoon HS, Kim JS, Lee BH (2007) Excess copper induced physiological and proteomic changes in germinating rice seeds. Chemosphere 67(6):1182–1193
Ali S, Rizwan M, Ullah N, Bharwana SA, Waseem M, Farooq MA, Abbasi GH, Farid M (2016) Physiological and biochemical mechanisms of silicon-induced copper stress tolerance in cotton (Gossypium hirsutum L.). Acta Physiol Plant 38:262
Azooz MM, Abou-Elhamd MF, Al-Fredan MA (2012) Biphasic effect of copper on growth, proline, lipid peroxidation and antioxidant enzyme activities of wheat (Triticum aestivum cv. Hasaawi) at early growing stage. Aust J Crop Sci 6:688–694
Baszynski T, Tukendorf A, Ruszkowska M, Skorzynska E, Maksymieci W (1998) Characteristics of the photosynthetic apparatus of copper non-tolerant spinach exposed to excess copper. J Plant Physiol 132(6):708–713
Bates LS, Walden RP, Teare ID (1973) Rapid determination of free proline for water stress studied. Plant Soil 39:205–207
Beauchamp CO, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Cakmak I, Horst J (1991) Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83:463–468
Chandlee JM, Scandalios JG (1984) Analysis of variants affecting the catalase development program in maize scutellum. Theor Appl Genet 69:71–77
Chen J, Shafi M, Li S, Wang Y, Wu J, Ye Z, Peng D, Yan W, Liu D (2015) Copper induced oxidative stresses, antioxidant responses and phytoremediation potential of Moso bamboo (Phyllostachys pubescens). Sci Rep 5:13554
Christhuuthayam M, Vijayarengan P, Natarajan A, Maheshwari M (2018) Changes in biochemical constitution of radish (Raphanus sativus L.) under copper toxicity. J Plant Stress Physiol 4:22–24
Devi SR, Prasad MNV (1998) Copper toxicity in Ceratophyllum demersum L. (Coontail), a free floating macrophyte: response of antioxidant enzymes and antioxidants. Plant Sci 138:157–165
Doncheva S, Stoyanova Z (1997) Plant response to copper and zinc hydroxidesulphate and hydroxidecarbonate used as an alternative copper and zinc sources in mineral nutrition. Rom Agric Res 7–8:15–20
Dwivedi RS, Randhawa NS (1974) Evaluation of rapid test for hidden hunger of zinc in plants. Plant Soil 40:445–451
Fageria VD (2001) Nutrient interactions in crop plants. J Plant Nutr 24(8):1269–1290
Feigl G, Kumar D, Lehotai N, Pető A, Molnár Á, Rácz É, Ördög A, Erdei L, Kolbert Z, Laskay G (2015) Comparing the effects of excess copper in the leaves of Brassica juncea (L. Czern) and Brassica napus (L.) seedlings: growth inhibition, oxidative stress and photosynthetic damage. Acta Biol Hung 66:205–221
Ferreira JFS (2007) Nutrient deficiency in the production of artemisinin, dihydroartemisinic acid, and artemisinic acid in Artemisia annua L. J Agric Food Chem 55:1686–1694
Ferreira JFS, Luthria DL, Sasaki T, Heyerick A (2010) Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules 15:3135–3170
Georgios A, Dimou M, Flemetakis E, Plati F, Katinakis P, Drossopoulos JB (2004) Immunolocalization of carbonic anhydrase and phosphoenolpyruvate carboxylase in developing seeds of Medicago sativa. Plant Physiol Biochem 42:181–186
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930
Gupta UC, Kalra YP (2006) Residual effect of copper and zinc from fertilizers on plant concentration, phytotoxicity and crop yield response. Commun Soil Sci Plant Anal 37(15–20):2505–2511
Gururani MA, Venkatesh J, Tran LSP (2015) Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol Plant 8(9):1304–1320
Habiba U, Ali S, Farid M, Shakoor MB, Rizwan M, Ibrahim M, Abbasi GH, Hayat T, Ali B (2015) EDTA enhanced plant growth, antioxidant defense system, and phytoextraction of copper by Brassica napus L. Environ Sci Pollut Res 22:1534–1544
Hasanuzzaman M, Nahar K, Alam MM, Roychowdhury R, Fujita M (2013) Physiological, biochemical and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14(5):9643–9684
Hasanuzzaman M, Alam MM, Rahman A, Hasanuzzaman M, Nahar K, Fujita M (2014) Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. BioMed Res Int.https://doi.org/10.1155/2014/757219
Jaworski EG (1971) Nitrate reductase assay in intact plant tissue. Biochem Biophys Res Commun 43:1274–1279
Keller C, Rizwan M, Davidian J-C, Pokrovsky OS, Bovet N, Chaurand P, Meunier J-D (2015) Effect of silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics and exposed to 0 to 30 µM Cu. Planta 241:847–860
Khalil RR, Moustafa AN, Bassuony FM, Haroun SA (2017) Kinetin and/or calcium affect growth of Phaseolus vulgaris L. plant grown under heavy metals stress. J Environ Sci 46(2):103–120
Kumar KB, Khan PA (1982) Peroxidase and polyphenol oxidase in excised ragi (Eleusine coracana cv. PR 202) leaves during senescence. Indian J Exp Bot 20:412–416
Lanaras M, Ssymeonidi SL, Diomantoglou S, Karatagli SS (1993) Plant metal content, growth responses and some photosynthetic measurements of field-cultivated wheat growing on ore bodies enriched in Cu. Physiol Plant 88:307–314
Lange B, Ent AVD, Baker AJM, Echevarria G, Mahy G, Malaisse F, Meerts P, Pourret O, Verbruggen N, Faucon MP (2017) Copper and cobalt accumulation in plants: a critical assessment of the current state of knowledge. N Phytol 213:537–551
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV–Vis spectroscopy. Curr Protoc Food Anal Chem 1(1):F4–F3
Mazhoudi S, Chaoui A, Ghorbal MH, El Ferjani E (1997) Response of antioxidant enzymes to excess copper in tomato (Lycopersicon esculentum Mill). Plant Sci 127:129–137
Meers E, Van Slycken S, Adriaensen K, Ruttens A, Vangronsveld J, Du Laing G, Witters N, Thewys T, Tack FMG (2010) The use of bio-energy crops (Zea mays) for ‘phytoattenuation’of heavy metals on moderately contaminated soils: a field experiment. Chemosphere 78(1):35-41
Meloni DA, Oliva MA, Martinez CA, Cambraia J (2003) Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot 49(1):69–76
Metwally A, Safronova VI, Belimov AA, Dietz KJ (2005) Genotypic variation of the response to cadmium toxicity in Pisum sativum L. J Exp Bot 56:167–178
Minnich M, McBride M, Chaney R (1987) Copper activity in soil solution: II. Relation to copper accumulation in young snapbeans. Soil Sci Soc Am J 51:573–578
Mittler R, Vanderauwer S, Gollery M, Breusegem FV (2004) Abiotic stress series. Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Naeem M, Nabi A, Aftab T, Khan MMA (2019) Oligomers of carrageenan regulate functional activities and artemisinin production in Artemisia annua L. exposed to arsenic stress. Protoplasma.https://doi.org/10.1007/s00709-019-01475-y
Ouzounidou G, Eleftheriou EP, Karataglis S (1992) Ecophysical and ultrastructural effects of copper in Thlaspi ochroleucum (Cruciferae). Can J Bot 70(5):947–957
Ouzounidou G, Ciamporova M, Moustakas M, Karataglis S (1995) Responses of maize (Zea mays L.) plants to copper stress—I. Growth, mineral content and ultrastructure of roots. Environ Exp Bot 35(2):167–176
Ouzounidou G, Moustakas M, Strasser RJ (1997) Sites of action of copper in the photosynthetic apparatus of maize leaves: kinetic analysis of chlorophyll fluorescence, oxygen evolution, absorption changes and thermal dissipation as monitored by photoacoustic signals. Aust J Plant Physiol 24:81–90
Pu GB, Ma DM, Chen JL, Ma LQ, Wang H, Li GF, Ye HC, Liu BY (2009) Salicylic acid activates artemisinin biosynthesis in Artemisia annua L. Plant cell Rep 28(7):1127–1135
Saleem MH, Ali S, Seleiman MF, Rizwan M, Rehman M, Akram NA, Liu L, Alotaibi M, Al-Ashkar I, Mubushar M (2019) Accessing the correlation between different traits in copper-sensitive and copper-resistant varieties of jute (Corchorus capsularis L.). Plants 8:1–12
Stepien P, Klbus G (2006) Water relations and photosynthesis in Cucumis sativus L. leaves under salt stress. Biol Plant 50:610
Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med 18(2):321–336
Verma S, Dubey RS (2003) Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci 164:645–655
Von Wiren N, Gazzarini S, Gojon A, Frommer WB (2000) The molecular physiology of ammonium uptake and retrieval. Curr Opin Plant Biol 3:254–261
Wallaart TE, Pras N, Beekman AC, Quax WJ (2000) Seasonal variation of artemisinin and its biosynthetic precursors in plants of Artemisia annua of different geographical origin: proof for the existence of chemotypes. Planta Medica 66(01):57–62
World Health Organization (2017) Artemisinin and artemisinin-based combination therapy resistance: status report. World Health Organization. https://apps.who.int/iris/handle/10665/255213
Younis ME, Tourky SMN, Elsharkawy SEA (2018) Symptomatic parameters of oxidative stress and antioxidant defense system in Phaseolus vulgaris L. in response to copper or cadmium stress. S Afr J Bot 117:207–214
Yruela I (2005) Copper in plants. Braz J Plant Physiol 17(1):145–156
Zhao SS, Zeng MY (1986) Determination of qinghaosu in Artemisia annua L. by high performance liquid chromatography. Chin J Pharm Anal 6:3–5
Acknowledgements
Ms. Andleeb Zehra and Ms. Sadaf Choudhary would like to thank University Grants Commission (UGC), New Delhi, India for providing financial support in the form of Non-NET Fellowship to conduct this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zehra, A., Choudhary, S., Mukarram, M. et al. Impact of Long-Term Copper Exposure on Growth, Photosynthesis, Antioxidant Defence System and Artemisinin Biosynthesis in Soil-Grown Artemisia annua Genotypes. Bull Environ Contam Toxicol 104, 609–618 (2020). https://doi.org/10.1007/s00128-020-02812-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-020-02812-1