Abstract
Effects of pure glyphosate and a glyphosate-based product were evaluated comparatively using two embryonic development stages of Xenopus laevis as model system. When pure glyphosate was applied in pH adjusted media, lethal or developmental effects were not observed at concentrations up to 500 mg L−1. The 96 h LC50 values for the commercial herbicide, in contrast, were 32.1 and 35.1 mg active ingredient L−1 for embryos and tadpoles, respectively. Since pure glyphosate has no effect on the selected biomarkers, it is thought that developmental toxic effects caused by glyphosate-based products are increased mainly due to formulation additives.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Glyphosate (GLY) is an extensively used herbicide around the world and is application increased more than 12 times to 826 million kg between 1995 and 2014 (Benbrook 2016). The main target of GLY is to inhibit 5-enolpyruvylshikimate-3-phosphate phosphate synthase, the key enzyme in aromatic amino acid synthesis pathways in plants. The number of toxicity studies on GLY or products containing GLY also continues to increase due to differences in the composition of commercial formulations and variability in effects among test species (Lajmanovich et al. 2011; Iummato et al. 2018; Samanta et al. 2014). Moreover, GLY products are one suggested cause of amphibian population declines similar to some other pesticides (Relyea, 2011). At the same time, amphibians represent one of the most threatened and rapidly declining group of vertebrates worldwide. The International Union for Conservation of Nature (IUCN) database reports, for instance, that 32% of the 6771 amphibian species worldwide are considered endangered or vulnerable (IUCN 2019). Consequently, several studies evaluate effects of commercial herbicides such as glyphosate-based products (GBPs) on amphibian species (Bonfanti et al. 2018; Carvalho et al. 2019; Edginton et al. 2004; Güngördü 2013; Güngördü et al. 2016; Mann and Bidwell 1999; Moore et al. 2012; Relyea and Jones 2009; Smith 2001; Wagner et al. 2013). GLY is the active ingredient (AI) of more than 750 different GBPs formulated with various adjuvants (Guyton et al. 2015; Li et al. 2005). Among all GBP, Roundup® is the original GBP introduced by Monsanto in 1974. It is still one of the most widely used formulation (Monsanto 2019; Szekacs and Darvas 2018). Roundup® contains glyphosate isopropylamine salt in combination with the surfactant polyoxyethylene tallow amine (POEA). POEA is added to assist GLY penetration into plant surfaces and thereby improve its effectiveness (Williams et al. 2000). In animal species, it is believed that POEA disrupts cell membranes on respiratory surfaces (Brausch and Smith 2007).
Most studies on GBP toxicity to aquatic organisms, focused on lethal effects (Tush et al. 2013, 2018; Lajmanovich et al. 2011). In relatively few studies, pure GLY showed low toxicity to non-target species in the ecosystem (Benbrook 2019). The World Health Organization (WHO) database reports low aquatic toxicity of GLY. However, the toxicity of Roundup® is considered to be a consequence of the surfactant additive POEA (Hong et al. 2018). Therefore, studies have reported that various additives may cause different levels of toxicity of GBPs (Edge et al. 2014; Mann and Bidwell 1999). Moreover, commercial GBPs disrupt various biochemical processes, including respiration, protein and nucleic acid synthesis, and may cause genotoxicity (Gill et al. 2018; Yanniccari et al. 2012). GBPs may affect various non-target aquatic organisms, including invertebrates, fish, and amphibians (Hansen and Roslev 2016; Lajmanovich et al. 2013; Pereira et al. 2018; Silveira et al. 2019; Wagner et al. 2013). Many studies reported GLY to be responsible in the adverse effects on non-target organisms caused by GBPs. However, comparative assessments of effects caused by GLY and its different formulations is limited (Babalola et al. 2019; Bonfanti et al. 2018; Lanzarin et al. 2019). For example, while discussing the lethal effects of GLY and commercial GBP on fish and amphibians, evaluation of its effects on biochemical markers have not been adequately assessed. Perez et al. (2011) report that LC50 values of pure GLY, Roundup®, and POEA were 140, 8.3, and 2.0 L−1 for Oncorhynchus mykiss, respectively. Moreover, GBPs cause serious deterioration of the health of aquatic organisms with significant changes in biomarker (AChE, catalase, and GST) (Samanta et al. 2014). However, there is still uncertainty about the extent GLY contributes to GBH toxicity.
To address this gap amphibians were selected as test species, as they go through clear and distinct stages (Walsh et al. 2008) allowing to assess for age dependent effects. The latter is particularly relevant as different developmental stages of Xenopus laevis show different sensitivity to pollutants (Fort et al. 2004). Along this line, the aim of this study was to evaluate potential adverse effects of pure GLY and one of the widely used GBP on X. laevis from different developmental stages. Consequently, mortality, growth inhibition, and biomarker activity (metabolic, detoxification, and oxidative stress) were used as endpoints.
Materials and Methods
The commercial GBP (Roundup® Star, Belgium) was purchased from a local agricultural retailer. According to the information in the safety data sheet, 35.5% of its content is the potassium salt of GLY, 6% is ether alkylamine ethoxylate, and the remaining 58.5% are water and minor formulating ingredients. The GLY (N-(phosphonomethyl) glycine) concentration in the tested GBP is declared at 441 g L−1. The density of tested GBP is 1.25 kg L−1 at 20 °C. GLY as pure active ingredient was purchased from Sigma-Aldrich (PESTANAL®, analytic grade, 45521).
The embryos and tadpoles used in the tests were obtained from male and female frog pairs from an adult X. laevis colony in our laboratory. X. laevis breeding and acquisition of embryos were performed according to ASTM-E1439-98 (ASTM 2003). All amphibian eggs, tadpoles, and adults were handled and cared for following animal use protocols reviewed and approved by Inonu University Research Animals Ethics Committee (Research Protocol No. 2013/A-44, 22 May 2013). Embryos and tadpoles were maintained in a standard Frog Embryo Teratogenesis Assay Xenopus (FETAX) test medium (ASTM 2003). Composition of the FETAX test medium was: 625 mg NaCl, 96 mg NaHCO3, 75 mg MgSO4, 60 mg CaSO4·2H2O, 30 mg KCl, and 15 mg CaCl2 per liter of distilled water.
Both exposure solutions of GLY and GBP were prepared fresh daily in the standard FETAX test medium (ASTM 2003). In order to perform the comparative experiments, the pH of GLY and GBP solutions were adjusted to 7.9, as recommended for FETAX tests, using NaOH (ASTM 2003). Embryos and tadpoles were exposed to test solutions under semi-static test conditions with a 12:12 h light:dark photoperiod at 23 °C (± 1 °C).
Before starting the FETAX test, glyphosate levels in the test medium were measured using high-performance liquid chromatography (HPLC) (1100 system, Agilent Technologies, Santa Clara, CA, USA) equipped with a diode array detector and an auto-sampler. A reversed phase C18 column (5 µm, 4.6 mm internal diameter, and 250 mm length) was used. Ten microliters of the sample was injected. The column was eluted with a mobile phase of 10% acetonitrile (v/v) and 90% water (containing 0.1% TEA), with pH adjusted to 2.3 with phosphoric acid, and a flow rate of 1 mL min−1. GLY was determined at 196 nm and was quantified using a standard curve (12.5–500 mg L−1, R2 = 0.998). The measured glyphosate concentrations in both GLY and GBH exposure media were determined to be at least 92% of the nominal concentrations (Table 1).
For the FETAX test, stage8 embryos were exposed to different GLY and GBP concentrations for 96 h. Four embryos were randomly selected, and placed with 2 mL of test medium into each well of 24-well plates, serving as one replicate per treatment. All concentrations were tested with eight replicates and thus a total of 32 embryos. In the FETAX test, seven concentrations of GLY (282–500 mg L−1) and six concentrations of GBP (31–50 mg AI L−1) were tested plus controls. The test medium was changed every 24 h. Dead tadpoles were removed and incidences were recorded. The median lethal concentrations (LC50) were determined after 24, 48, 72, and 96 h of exposure. At termination of the bioassays, surviving embryos were euthanized with 200 mg L−1 of tricaine methanesulfonate (MS222, suitably buffered with sodium bicarbonate) and fixed in 3% formalin for observation of developmental anomalies. Malformed embryos and malformation types were determined using a dissection microscope as suggested by Bantle (1995). Body length was measured using Euromex ImageFocus 4.0 software.
For the tadpole-toxicity bioassays, stage 46 tadpoles were exposed to different GLY and GBP concentrations for 96 h. Five randomly selected tadpoles were placed in each well of 12-well plates containing 3 mL of test solution. All concentrations were tested with 6 replicates resulting in a total of 30 tadpoles per concentration. In the tadpole-toxicity tests, seven concentrations of GLY (250–403 mg L−1) and six concentrations of GBP (31–50 mg AI L−1) were tested excluding control groups. For all other procedures the embryotoxicity assays procedures were followed.
For biochemical assays, stage 46 tadpoles were exposed to three GLY (3.5–17.6 mg L−1) and GBP (50–250 mg AI L−1) concentrations for an exposure period of 96 h. Fifteen randomly selected tadpoles and 10 mL of test solution were placed into 25 mL polycarbonate containers. All concentrations were tested with five replicates resulting in a total of 75 tadpoles per treatment. At the end of the exposure period, surviving tadpoles were euthanized by MS222 and placed in microcentrifuge tubes as soon as possible. Each vial was cooled on ice and tadpoles were stored at − 80 °C until enzyme activity assays.
Frozen tadpoles were thawed on ice and weighed before biochemical studies. All homogenization, centrifugation, and biochemical measurements followed protocols reviewed by Güngördü et al. (2016). The tadpoles were homogenized in ice-cold buffer [0.1 M K-phosphate buffer (pH 7.4) with 0.15 M KCl, 1 mM EDTA, and 1 mM DTT]. The homogenate was centrifuged at 16,000×g for 20 min at 4 °C. The supernatant was transferred to a clean microcentrifuge tube. The activities of enzymes were measured as soon as possible after collecting postmitochondrial supernatant without freezing. The samples were maintained on ice during analyses. Enzyme activity was determined spectrophotometrically using a microplate reader at appropriate wavelengths (VersaMax, Molecular Devices Corp., USA). The enzyme activities were then normalized to the organisms protein content.
Glutathione S-transferase (GST) activity was measured in the supernatant according to Habig et al. (1974). Glutathione reductase (GR) activity was measured following Stephensen et al. (2000). Carboxylesterase (CaE) activity was assayed using the procedure of Santhoshkumar and Shivanandappa (1999). Acetylcholinesterase (AChE) activity was measured following Ellman et al. (1961). All procedures were modified for a microplate reader system. Superoxide dismutase (SOD) activity was measured using a SOD Assay Kit (Sigma-Aldrich 19160, St. Louis, MO, USA) following the manufacturer’s guidelines. Total protein concentration in the supernatant was measured using the Bradford method with bovine serum albumin (BSA) as standard (Bradford 1976). The protein content values were used to calculate the specific activities of each tested enzyme.
Graphpad Prism software (Version 5, USA) was used to calculate the average lethal concentration (LC50) and 95% confidence intervals (CI) and for other statistical analyses. A log(dose)-normalized response curve (Y = 100/(1 + 10(logEC50−x) × hillslope) to fit mortality data. For statistical analysis of biomarkers, data were tested initially for homogeneity of variances and normality distributions by the Bartlett and Kolmogorov–Smirnov tests, respectively. Nonparametric data were analyzed using Kruskal–Wallis test followed by pairwise comparisons of groups using Mann–Whitney U tests. Parametric data were analyzed using the One-way Analysis of Variance (ANOVA) followed by the unpaired t test. A Bonferroni correction was applied (0.05/3 = 0.016). In order to determine growth inhibition, the head-to-tail lengths were measured and the lengths were compared using ANOVA (Dunnett’s post hoc test, p < 0.05).
Results and Discussion
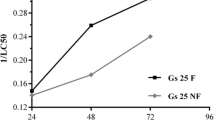
The LC50s of different GBPs for frog larvae of 37 species were reported in the range from 0.2 to 494 mg AI L−1 (Wagner et al. 2013). These differences in toxicity may be attributed to the type of adjuvants (surfactants, solvents, and antifoaming compounds) present in different commercial formulations. This may be assumed as adjuvants may actually be more toxic than the active ingredient GLY. It has, moreover, been suggested that the toxicity by GBPs may be the result of synergistic effects between glyphosate and adjuvants (Cattani et al. 2014; Jacques et al. 2019). However, the contribution of GLY to the toxicity of GBPs has not yet been assessed comprehensively, likely due to a large number of different surfactants (Edge et al. 2014). We tested Roundup® containing alkylamine ethoxylate and POEA as a surfactant (Brausch and Smith 2007). POEA increases membrane permeability of cells and therefore allows increased absorption of the GLY (Giesy et al. 2000). Perkins et al. (2000) reported POEA being more toxic than Roundup® for X. laevis embryos (9.3 mg AI L−1, LC50). Our results also showed that the 96 h LC50 values of the GBP were 32.1 and 35.1 mg AI L−1 for embryos and stage 46 tadpoles, respectively (Table 2). Please note that one of the developmental stages tested in this study is the early embryonic stage including gastrulation, neurulation and organogenesis, and the second was the premetamorphic tadpole stage. Consequently, the fact that similar LC50 values among developmental stages were reported here in combination with the absence of statistically significant malformations suggests that the toxicity is due to more general mechanisms such as oxidative stress rather than teratogenic effects. On the other hand, the calculated LC50 values in this study were relatively high compared to literature, even compared with a previous study that we conducted with the same GBP and X. laevis (the 96 h LC50 was 15 mg AI L−1, Güngördü 2013). This may be related to the variability of the active substances and adjuvants present in commercial pesticide formulations. Nonetheless, even the highest GLY concentrations did not cause a lethality higher than 17% for both X. laevis embryos and tadpoles in this study suggesting a significant role of surfactants for GBP toxicity.
Moreover, the highest GLY concentrations (403 and 500 mg L−1) caused no growth inhibition in embryos or tadpoles. However, nearly 15-fold lower concentrations of GBP resulted in significant inhibition of embryonic growth (Table 3). In contrast, another study reported both GLY (3–100 mg L−1) and GBP (0.37–5.25 mg AI L−1, Roundup Ultramax®) causing growth inhibition in Leptodactylus latrans embryos after pH adjustment to 7.7 (Bach et al. 2018). At the same time, Howe et al. (2004) found, similar to our study, that technical grade GLY had no acute or subchronic effect on tadpoles (96 h LC50 > 17.9 mg L−1). However, different formulations of Roundup (Roundup Original) caused higher lethality and developmental abnormalities in tadpoles that resulted in 96 h LC50 levels for four frog species between 2.2 and 8 mg AI L−1 (Howe et al. 2004). These insights support the importance surfactant(s) for toxicity of GBPs (Howe et al. 2004). In the light of the high number of GBP, with likely a different set of surfactants, being registered (SERA 2011) calls for further studies to understand the underly mechanisms as a generalization seems difficult.
In addition to the effects on development and survival, GBPs can cause histopathological, teratogenic, and behavioral changes. Furthermore, GBP may affect the metamorphic success and sexual differentiation of tadpoles (Bach et al. 2018; Bonfanti et al. 2018; Miko et al. 2017; Navarro-Martin et al. 2014). The selected biochemical markers in embryos and tadpoles exposed to GLY did not show any statistically significant change in this study. Following exposure to GBP, however, GR, CaE, AChE, and SOD activities were inhibited in a dose-dependent manner (Table 4). Similar results were reported for CaE and AChE (also glutathione S-transferases) in Rhinella arenarum tadpoles after exposure to four GBPs (Lajmanovich et al. 2011). Since pure GLY does not cause statistically significant inhibitions in enzyme activities even at much higher concentrations suggests that GR, CaE, AChE and SOD inhibitions are associated with another factor such as adjuvants. In contrast to the present study, another GBP caused an increase of catalase and AChE activities and lipid peroxidation levels in fish (Samanta et al. 2014). In combination with the results above, the role of adjuvants and other additives in formations for the toxicity the AI is causing requires further investigations.
Finally, the data generated from this study provides important information for assessing the toxic effects GBPs in non-target aquatic organisms by relating the effects to pure GLY. It was highlighted that GBP cause higher toxicity than pure glyphosate in X. laevis tadpoles. Consequently, understanding the mechanisms behind this presumably synergizing effect of surfactants and other ingredients in GBP, or other pesticide formulations, seems of high relevance from a fundamental and applied perspective.
References
ASTM (2003) American Society for Testing and Materials, Standard guide for conducting the Frog Embryo Teragonesis Assay-Xenopus (FETAX), E1439-98. In: ASTM standards on biological effects and environmental fate, Vol. 11.05. Philadelphia, PA, pp 447–457
Babalola OO, Truter JC, van Wyk JH (2019) Mortality, teratogenicity and growth inhibition of three glyphosate formulations using frog embryo teratogenesis Assay-Xenopus. J Appl Toxicol. https://doi.org/10.1002/jat.3811
Bach NC, Marino DJG, Natale GS, Somoza GM (2018) Effects of glyphosate and its commercial formulation, Roundup((R)) Ultramax, on liver histology of tadpoles of the neotropical frog, Leptodactylus latrans (amphibia: Anura). Chemosphere 202:289–297
Bantle JA (1995) FETAX-A developmental toxicity assay using frog embryos. In: Rand GM (ed) Fundamental aquatic toxicology. Taylor and Francis, Washington, DC, pp 207–230
Benbrook CM (2016) Trends in glyphosate herbicide use in the United States and globally. Environ Sci Eur 28:3. https://doi.org/10.1186/s12302-016-0070-0
Benbrook CM (2019) How did the US EPA and IARC reach diametrically opposed conclusions on the genotoxicity of glyphosate-based herbicides? Environ Sci Eur 31:2. https://doi.org/10.1186/s12302-018-0184-7
Bonfanti P, Saibene M, Bacchetta R, Mantecca P, Colombo A (2018) A glyphosate micro-emulsion formulation displays teratogenicity in Xenopus laevis. Aquat Toxicol 195:103–113
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brausch JM, Smith PN (2007) Toxicity of three polyethoxylated tallowamine surfactant formulations to laboratory and field collected fairy shrimp, Thamnocephalus platyurus. Arch Environ Contam Toxicol 52:217–221
Cattani D, de Liz Oliveira Cavalli VL, Heinz Rieg CE, Domingues JT, Dal-Cim T, Tasca CI, Mena Barreto Silva FR, Zamoner A, (2014) Mechanisms underlying the neurotoxicity induced by glyphosate-based herbicide in immature rat hippocampus: involvement of glutamate excitotoxicity. Toxicology 320:34–45
Carvalho WF, Ruiz de Arcaute C, Pérez-Iglesias JM, Laborde MRR, Soloneski S, Larramendy ML (2019) DNA damage exerted by mixtures of commercial formulations of glyphosate and imazethapyr herbicides in Rhinella arenarum (Anura, Bufonidae) tadpoles. Ecotoxicology 28(3):367–377
Edge C, Gahl M, Thompson D, Hao C, Houlahan J (2014) Variation in amphibian response to two formulations of glyphosate-based herbicides. Environ Toxicol Chem 33:2628–2632
Edginton AN, Sheridan PM, Stephenson GR, Thompson DG, Boermans HJ (2004) Comparative effects of pH and Vision (R) herbicide on two life stages of four anuran amphibian species. Environ Toxicol Chem 23:815–822
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Fort DJ, Guiney PD, Weeks JA, Thomas JH, Rogers RL, Noll AM, Spaulding CD (2004) Effect of methoxychlor on various life stages of Xenopus laevis. Toxicol Sci 81:413–454
Giesy JP, Dobson S, Solomon KR (2000) Ecotoxicological risk assessment for Roundup (R) Herbicide. Rev Environ Contam Toxicol 167(167):35–120
Gill JPK, Sethi N, Mohan A, Datta S, Girdhar M (2018) Glyphosate toxicity for animals. Environ Chem Lett 16:401–426
Güngördü A (2013) Comparative toxicity of methidathion and glyphosate on early life stages of three amphibian species: Pelophylax ridibundus, Pseudepidalea viridis, and Xenopus laevis. Aquat Toxicol 140–141:220–228
Güngördü A, Uckun M, Yologlu E (2016) Integrated assessment of biochemical markers in premetamorphic tadpoles of three amphibian species exposed to glyphosate- and methidathion-based pesticides in single and combination forms. Chemosphere 144:2024–2035
Guyton KZ, Loomis D, Grosse Y, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Scoccianti C, Mattock H, Straif K (2015) Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol 16:490–491
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hansen LR, Roslev P (2016) Behavioral responses of juvenile Daphnia magna after exposure to glyphosate and glyphosate-copper complexes. Aquat Toxicol 179:36–43
Hong YH, Yang XZ, Huang Y, Yan GW, Cheng YX (2018) Assessment of the oxidative and genotoxic effects of the glyphosate-based herbicide roundup on the freshwater shrimp, Macrobrachium nipponensis. Chemosphere 210:896–906
Howe CM, Berrill M, Pauli BD, Helbing CC, Werry K, Veldhoen N (2004) Toxicity of glyphosate-based pesticides to four North American frog species. Environ Toxicol Chem 23:1928–1938
IUCN (2019) www.iucnredlist.org. Accessed Nov 4, 2019
Iummato MM, Sabatini SE, Cacciatore LC, Cochón AC, Cataldo D, de Molina MDCR, Juárez ÁB (2018) Biochemical responses of the golden mussel Limnoperna fortunei under dietary glyphosate exposure. Ecotoxicol Environ Saf 15:69–75
Jacques MT, Bornhorst J, Soares MV, Schwerdtle T, Garcia S, Avila DS (2019) Reprotoxicity of glyphosate-based formulation in Caenorhabditis elegans is not due to the active ingredient only. Environ Pollut 252(Pt B):1854–1862
Lanzarin GAB, Felix LM, Santos D, Venancio CAS, Monteiro SM (2019) Dose-dependent effects of a glyphosate commercial formulation - Roundup® UltraMax - on the early zebrafish embryogenesis. Chemosphere 223:514–522
Lajmanovich RC, Attademo AM, Peltzer PM, Junges CM, Cabagna MC (2011) Toxicity of Four Herbicide Formulations with Glyphosate on Rhinella arenarum (Anura: Bufonidae) Tadpoles: B-esterases and Glutathione S-transferase Inhibitors. Arch Environ Contam Toxicol 60:681–689
Lajmanovich RC, Junges CM, Attademo AM, Peltzer PM, Cabagna-Zenklusen MC, Basso A (2013) Individual and Mixture Toxicity of Commercial Formulations Containing Glyphosate, Metsulfuron-Methyl, Bispyribac-Sodium, and Picloram on Rhinella arenarum Tadpoles. Water Air Soil Pollut 224:1404
Li J, Smeda RJ, Sellers BA, Johnson WG (2005) Influence of formulation and glyphosate salt on absorption and translocation in three annual weeds. Weed Sci 53:153–159
Mann RM, Bidwell JR (1999) The toxicity of glyphosate and several glyphosate formulations to four species of southwestern Australian frogs. Arch Environ Contam Toxicol 36:193–199
Miko Z, Ujszegi J, Gal Z, Hettyey A (2017) Effects of a glyphosate-based herbicide and predation threat on the behaviour of agile frog tadpoles. Ecotoxicol Environ Saf 140:96–102
Monsanto (2019) Monsanto product safety assistance website. https://www.sdslibrary.monsanto.com/Pages/Default.aspx. Accessed Dec 4, 2019
Moore LJ, Fuentes L, Rodgers JH Jr, Bowerman WW, Yarrow GK, Chao WY, Bridges WC Jr (2012) Relative toxicity of the components of the original formulation of Roundup to five North American anurans. Ecotoxicol Environ Saf 78:128–133
Navarro-Martin L, Lanctot C, Jackman P, Park BJ, Doe K, Pauli BD, Trudeau VL (2014) Effects of glyphosate-based herbicides on survival, development, growth and sex ratios of wood frogs (Lithobates sylvaticus) tadpoles. I: Chronic laboratory exposures to VisionMax (R). Aquat Toxicol 154:278–290
Pereira AG et al (2018) Low-concentration exposure to glyphosate-based herbicide modulates the complexes of the mitochondrial respiratory chain and induces mitochondrial hyperpolarization in the Danio rerio brain. Chemosphere 209:353–362
Perez GL, Vera MS, Miranda L (2011) Effects of herbicide glyphosate and glyphosate-based formulations on aquatic ecosystems. In: Kortekamp A (ed) Herbicides and environment. IntechOpen, Rijeka.
Perkins PJ, Boermans HJ, Stephenson GR (2000) Toxicity of glyphosate and triclopyr using the frog embryo teratogenesis assay–Xenopus. Environ Toxicol Chem 19:940–945
Relyea RA, Jones DK (2009) The toxicity of Roundup Original Max to 13 species of larval amphibians. Environ Toxicol Chem 28:2004–2008
Relyea RA (2011) Amphibians Are Not Ready for Roundup®. In: Elliott JE, Bishop CA, Morrissey CA (eds) Wildlife ecotoxicology: forensic approaches. Springer, New York, pp 267–300
Samanta P, Pal S, Mukherjee AK, Ghosh AR (2014) Biochemical effects of glyphosate based herbicide, Excel Mera 71 on enzyme activities of acetylcholinesterase (AChE), lipid peroxidation (LPO), catalase (CAT), glutathione-S-transferase (GST) and protein content on teleostean fishes. Ecotoxicol Environ Saf 107:120–125
SERA (2011) Glyphosate—human health and ecological risk assessment—final Report. USDA, Forest Service, Forest Health Protection. SERA TR-052-22-03b. Atlanta, Georgia. https://www.fs.fed.us/foresthealth/pesticide/pdfs/Glyphosate_SERA_TR-052-22-03b.pdf
Silveira T et al (2019) Roundup (R) Herbicide decreases quality parameters of spermatozoa of silversides odontesthes humensis. Bull Environ Contam Toxicol 102:1–6
Smith GR (2001) Effects of acute exposure to a commercial formulation of glyphosate on the tadpoles of two species of anurans. Bull Environ Contam Toxicol 67:483–488
Stephensen E, Svavarsson J, Sturve J, Ericson G, Adolfsson-Erici M, Forlin L (2000) Biochemical indicators of pollution exposure in shorthorn sculpin (Myoxocephalus scorpius), caught in four harbours on the southwest coast of Iceland. Aquat Toxicol 48:431–442
Szekacs A, Darvas B (2018) Re-registration challenges of glyphosate in the European Union. Front Environ Sci 6:1–35
Tush D, Loftin KA, Meyer MT (2013) Characterization of polyoxyethylene tallowamine surfactants in technical mixtures and glyphosate formulations using ultra-high performance liquid chromatography and triple quadrupole mass spectrometryle. J Chromatogr A 1319:80–87
Tush D, Maksimowicz MM, Meyer MT (2018) Dissipation of polyoxyethylene tallowamine (POEA) and glyphosate in an agricultural field and their co-occurrence on streambed sediments. Sci Total Environ 636:212–219
Walsh PT, Downie JR, Monaghan P (2008) Plasticity of the duration of metamorphosis in the African clawed toad. J Zool 274:143–149
Wagner N, Reichenbecher W, Teichmann H, Tappeser B, Lotters S (2013) Questions concerning the potential impact of glyphosate-based herbicides on amphibians. Environ Toxicol Chem 32:1688–1700
Williams GM, Kroes R, Munro IC (2000) Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans. Regul Toxicol Pharmacol 31:117–165
Yanniccari M, Istilart C, Gimenez DO, Castro AM (2012) Effects of glyphosate on the movement of assimilates of two Lolium perenne L. populations with differential herbicide sensitivity. Environ Exp Bot 82:14–19
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Turhan, D.Ö., Güngördü, A. & Ozmen, M. Developmental and lethal effects of glyphosate and a glyphosate-based product on Xenopus laevis embryos and tadpoles. Bull Environ Contam Toxicol 104, 173–179 (2020). https://doi.org/10.1007/s00128-019-02774-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-019-02774-z