Abstract
A hydroponic experiment was carried out to study the accumulation characteristics of copper (Cu) and lead (Pb) combined pollution in three ornamental plants. The results showed that these tested ornamental plants had higher tolerance to Cu–Pb combined pollution and could effectively accumulate the heavy metals. The Cu and Pb concentrations were higher in the roots of the ornamental plants than that in the shoots. For Panax notoginseng (P. notoginseng), Chlorophytum comosum (C. comosum) and Calendula officinalis (C. officinalis), the average Cu and Pb concentration in the three ornamental plants were 1402.1 mg/kg, 829.5 mg/kg, and 1473.4 mg/kg for Cu and 2710.4 mg/kg, 4250.3 mg/kg, and 4303.6 mg/kg for Pb, respectively. The three ornamental plants accumulation and tolerance to Cu–Pb were demonstrated through the hydroponic-culture method in this study. Therefore, the three ornamental plants should have great potential to be used in remediation of soils contaminated by Cu and Pb and beautifying the environment simultaneously.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
With the rapid increase of urbanization and industrialization, environmental pollution and ecological destruction have become two important issues that are receiving more attention due to their threats to the health and survival of human beings. Among these threats, heavy metals cause the most serious pollution and damage to the soil environment (Doumett et al. 2010; Doumett et al. 2008; Yan et al. 2017). Pb and Cu are ubiquitous metal contaminants in urban soils, especially in larger urban areas (Datko-Williams et al. 2014) and around city roads (Zaidi et al. 2005). Recent studies had shown that varying levels of Pb and Cu pollution were found in many provinces and cities, such as Beijing, Nanjing, Guizhou, Fujian, Hebei, Guangxi, Jiangxi, Hainan, Chongqing, and Hong Kong (Sun et al. 2007a). The concentrations of Pb and Cu in urban soils range from 25.0 mg/kg to 28.6 mg/kg and 17.6 mg/kg to 111.3 mg/kg, respectively, which are higher than the background values of Pb and Cu for soils in China (26.0 mg/kg and 22.6 mg/kg, respectively) (Wei and Yang 2010). Moreover, heavy metal pollutants cannot be chemically or biologically degraded and have the characteristics of concealment, persistence, hysteresis, etc., and they endanger human health, life and safety through direct contact or food chain transmission (Li et al. 2014; Rajkumar et al. 2009). Therefore, the remediation of heavy metal-contaminated soil in urban areas represents a major problem that the environmental science community must resolve (Li et al. 2016).
Phytoremediation has become the preferred option for remediating heavy metal-contaminated soil (Liu et al. 2008) than conventional chemical and physical remediation methods (Pereira et al. 2010), which are generally costly and often produce other destructive effects (Mahar et al. 2016; Yan et al. 2017). Phytoextraction is the use of hyperaccumulators to extract heavy metals from contaminated soils or waters and transfer them to the relatively manageable aboveground environment (Dahmani-Muller et al. 2000). Thus, it has become important to screen out effective hyperaccumulators. Ornamental plants represent an important research direction that can simultaneously remedy and beautify contaminated environments. P. notoginseng, C. comosum and C. officinalis are herbaceous ornamental plants. Studies have shown that Chlorophytum comosum has a high tolerance to Pb when this element is not combined with other heavy metals (Youbao et al. 2011), and Wang found that Impatiens balsamina, Calendula officinalis and Althaea rosea had a higher tolerance and accumulation ability to cadmium (Cd) and lead (Pb) (Wang 2005). Therefore, the use of ornamental plants to remediate environmental pollution has important practical significance. In fact, the heavy metal elements cannot exist alone, and more are a mixture of two or more pollutants. (Zhao et al. 2013). However, few studies have focused on the combined pollution of Pb and Cu in the abovementioned ornamental plants. In urban areas, ornamental plants have been used for the remediation of metal-contaminated soils and thus have high practical and application value (Cameselle 2015).
The hyperaccumulation ability of three ornamental plants for heavy metals was studied using the method of hydroponic culture under the combination of Cu–Pb combined pollution in the paper so as to provide a scientific basis for the phytoremediation of heavy metal combined pollution in soils.
Materials and Methods
The hydroponic test method based on the work of Liu et al. (2008) was carried out on June 4th, 2016 in a green-house at Jilin Agricultural University. The three ornamentals seeds with full grain and uniform size are selected, and soaked and germinated at 25°C for sowing. Healthy and consistent seedlings of the three ornamentals were selected after one month of growth, and the roots were immersed in 0.1% KMnO4 solution for 10 min and then rinsed with deionized water. Then, seedlings of the three ornamentals were cultivated in 250 mL conical flasks. In this study, we used a 20-fold dilution Hoagland nutrient solution (pH 6.7), which was replaced in the hydroponic experiments when the seedlings of the three ornamentals were cultivated for 3 to 4 days. The hydroponic solution was mixed with Pb (CH3COOH)2·3H2O and CuSO4·5H2O to obtain the treatment concentrations. A total of eleven treatments (CK and T1–T10) were conducted, and the Cu and Pb concentrations in each treatment solution were 0 + 0 (CK), 5 + 50 (T1), 10 + 50 (T2), 20 + 50 (T3), 40 + 50 (T4), 80 + 50 (T5), 5 + 100 (T6), 10 + 100 (T7), 20 + 100 (T8), 40 + 100 (T9), 80 + 100 mg/L (T10). Three replications were performed for each treatment. One seedling was planted in each conical flask, and the solution was replaced every 5 days. All ornamental plants were harvested after 20 days of growth in the culture solution, rinsed repeatedly with deionized water and then divided into shoots and roots. The plant samples were dried at 105°C for 30 min and dried at 75°C to a constant weight and then ground to powder. The plant samples were digested with a HNO3/HClO4 (3:1, V/V) mixture (Yan et al. 2017). The content of Cu and Pb in plants were determined using a flame atomic absorption spectrophotometer (TAS-990, Beijing Purkinje General Instrument Co., Ltd., China).
Statistical analyses were performed using SAS statistical package (SAS Institute Inc., Cary, North Carolina, USA). Differences among treatment means were determined using a one-way analysis of variance (ANOVA) followed by Duncan’s new multiple range test at significant levels (p < 0.05).
Results and Discussion
The Cu concentrations in the shoots and roots of the three ornamental plants are shown in Table 1. An analysis of variance showed that there were significant differences in Cu accumulation among the three ornamental plants (p < 0.05). After growing in conical flasks for 15 days, the Cu concentration in the roots of the three ornamental plants was much higher than that in the shoots and increased with increasing Cu and Pb concentration in the solution. The Cu or Pb enrichment factor (BC) was the ratio of the Cu or Pb content in plants and that of the Cu or Pb concentration in hydroponic solution, which reflected the the Accumulate ability of plants for Cu or Pb, and the translocation factor (TF) was the ratio of the Cu or Pb content in shoots and that of the roots, which reflected the Cu or Pb transfer ability of plants shoots (Tanhan 2007). When the Pb concentration in the solution was 50 mg/L, the Cu accumulation in the shoots and roots of P. notoginseng, C. comosum and C. officinalis was 76.0 to 158.8 mg/kg, 98.4 to 160.9 mg/kg and 66.7 to 166.7 mg/kg, and 451.2 to 2603.6 mg/kg, 277.3 to 1453.6 mg/kg and 585.7 to 2598.3 mg/kg, respectively, while the BC of the shoots and roots was 1.52 to 3.18, 1.97 to 3.22 and 1.33 to 3.33, and 9.02 to 52.1, 5.55 to 29.1 and 11.7 to 52.0, respectively. Simultaneously, the TF of P. notoginseng, C. comosum and C. officinalis was 0.03 to 0.34, 0.07 to 0.48 and 0.03 to 0.22, respectively. For P. notoginseng, when the Cu concentration of the solution was 10 mg/L (T2), the Cu concentration in the shoots reached the maximum value of 158.8 mg/kg while that in the roots was 700.1 mg/kg. When the Cu concentration was 80 mg/L (T5), the Cu concentration in the roots reached the maximum value of 2605.6 mg/kg while that in the shoots was small of 76.0 mg/kg. For C. comosum, when the Cu concentration of the solution was 10 mg/L (T2), the Cu concentration in the shoots reached the maximum value of 160.9 mg/kg while that in the roots was 412.9 mg/kg. When Cu concentration of the solution was 80 mg/L (T5), the Cu concentration in the roots reached the maximum value of 1453.6 mg/kg while that in the shoots was small of 98.4 mg/kg. For C. officinalis, when the Cu concentration in the solution was 10 mg/L (T2), the Cu concentration in the shoots reached the maximum value of 166.7 mg/kg while that in the roots was 744.8 mg/kg, respectively. When Cu concentration in the solution was 80 mg/L (T5), the Cu concentration in the roots reached the maximum value of 2598.3 mg/kg while the Cu concentration in the shoots was small of 72.6 mg/kg, respectively.
When the Pb concentration in the solution was 100 mg/L, the Cu accumulation in the shoots and roots of P. notoginseng, C. comosum and C. officinalis was 60.1 to 149.6 mg/kg, 74.6 to 169.7 mg/kg and 79.3 to 183.9 mg/kg, and 745.3 to 2645.5 mg/kg, 416.1 to 1489.3 mg/kg and 436.2 to 2762.8 mg/kg, respectively. For P. notoginseng, when the Cu concentration in the solution was 10 mg/L (T7), the Cu concentration in the shoots reached the maximum value of 149.6 mg/kg while that in the roots was 853.5 mg/kg. When the Cu concentration in the solution was 80 mg/L (T10), the Cu concentration in the roots reached the maximum accumulation of 2645.5 mg/kg while that in the shoots was small of 60.1 mg/kg. For C. comosum, when the Cu concentration in the solution was 10 mg/L (T7), the Cu concentration in the shoots reached the maximum accumulation of 169.7 mg/kg while that in the roots was 883.2 mg/kg. When the Cu concentration in the solution was 80 mg/L (T10), the Cu concentration in the roots reached the maximum accumulation of 1489.3 mg/kg while that in the shoots was small of 74.6 mg/kg. For C. officinalis, when the Cu concentration in the solution was 10 mg/L (T7), the Cu concentration in the shoots reached the maximum accumulation (183.9 mg/kg) while that in the roots was 816.0 mg/kg. When the Cu concentration in the solution was 80 mg/L (T10), the Cu concentration in the roots reached the maximum accumulation of 2762.8 mg/kg while that in the shoots was small of 79.3 mg/kg.
When the Pb concentrations in the solution were 50 mg/L and 100 mg/L, the Pb concentration in the shoots and roots of P. notoginseng reached the maximum accumulation of 639.3 mg/kg (Cu = 20 mg/L and Pb = 100 mg/L) and 4781.6 mg/kg (Cu = 5 mg/L and Pb = 100 mg/L), respectively. The Pb concentration in the shoots and roots of C. comosum reached the maximum accumulation of 865.3 mg/kg and 7635.2 mg/kg (Cu = 10 mg/L and Pb = 100 mg/L), respectively, and the Pb concentration in the shoots and roots of C. officinalis reached the maximum accumulation of 967.5 mg/kg (Cu = 5 mg/L and Pb = 100 mg/L) and 7639.7 mg/kg (Cu = 40 mg/L and Pb = 100 mg/L), respectively.
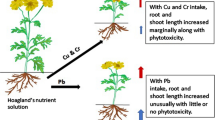
These findings showed that Cu at low solution concentrations could promote Cu accumulation in the three ornamental plants while excess levels caused toxicity to the ornamental plants, therefore, the Cu accumulation in the shoots of the three ornamental plants reduced the upward movement of Cu in the ornamental plant parts (Bona et al. 2007). In addition, the results indicated that the Pb concentration in ornamentals differed based on the different Cu concentrations and types of ornamentals, suggesting plant metal accumulation was related to not only metal concentrations in solution (Mahar et al. 2016) but also plant types and their tissues (Wilcke et al. 1998). When Cu was at a low concentration, the three ornamentals were promoted the absorption of heavy metal Pb, exhibiting a synergistic of ions. However, when the concentration of Cu in the nutrient solution was high, the absorption of Pb by the three ornamentals were suppressed and the antagonistic of ions was exhibited. Considering the growth response as well as the shoots and roots of Cu uptake in the three ornamental plants, the Cu accumulation ability order was C. officinalis > P. notoginseng > C. comosum. The analysis of variance showed that there were significant differences in Pb accumulation among the three ornamental plants (p < 0.05), indicating that the Pb accumulation in the three ornamental plants changed significantly under different Cu and Pb concentrations in the solution (Table 2). Similarly, the Pb concentration in the roots was much higher than that of the shoots and increased with increasing Cu and Pb concentration in the solution. In general, the Pb accumulation among the three ornamental plants was higher at a Pb treatment concentration of 50 mg/L than when the Pb treatment concentration was 100 mg/L. The Cu BC of the shoots and roots was 0.60 to 1.50, 0.75 to 1.70 and 0.79 to 1.84, and 7.45 to 26.5, 4.16 to 14.9 and 4.36 to 27.6, respectively. Simultaneously, and the TF of P. notoginseng, C. comosum and C. officinalis was 0.02 to 0.19, 0.05 to 0.32 and 0.03 to 0.27, respectively, which falls well within the range with previous research results (Tao et al. 2011; Youbao et al. 2011). The BC in the shoots and roots of P. notoginseng, C. comosum and C. officinalis ranged from 2.53 to 8.39, 4.05 to 10.5 and 4.54 to 13.4, and 12.9 to 95.2, 30.0 to 76.4 and 31.6 to 81.8, respectively. In the present study, the TF value was lower than that under soil-culture conditions (Li et al. 2010).
Recently, the use of phytoextraction plants (flowering plants) as phytoremediation technology in heavy metal-contaminated soils represented an important task among researchers (Wei et al. 2003). In general, the methods used for screening enriched phytoextraction plants are field sampling analysis, nutrient solution culture and pot experiments. Domestically, few field experiments have been performed on the remediation of metal-contaminated soils using ornamental plants. Although the experiments performed here were carried out on a trial basis, the test plants for such experiments were collected from heavy metal-contaminated polluted soils. The heavy metal content in the sample was used to identify the plant’s ability to enhance metal accumulation and determine whether it is a hyperaccumulator (Visoottiviseth et al. 2002). Internationally, the company Edenspace of the United States used Brassica juncea to phytoremediate Pb-contaminated soil in Bayonne, New Jersey. The use of this plant for phytoremediation showed great results, with the Pb content in the soil surface layer (0–15 cm) decreasing by 58.0%–64.6% (Lan et al. 2004). The plant C. officinalis isolated from pot experiments can be planted as urban greening plants in urban green belts, park squares, public green spaces and street flower beds, which can remedy contaminated environment and beautify it at the same time. Phytoremediation can alter the microbial community structure (Song et al. 2007) and increasing ecosystem diversity and stability (Tilman et al. 2006). Of course, the manual cleaning and harvesting of plant residues is carried out by artificial watering according to the water requirements of the restored plants. The disposal of harvested plant biomass after phytoremediation of heavy metal contaminated soils is still a debatable issue. Many approaches, including direct disposal, composting, compaction, incineration, and leaching, have been used for the treatment of heavy metal enriched biomass (Gomes 2012). Recently, fast pyrolysis has been regarded as a potential sustainable approach for the disposal of contaminated biomass, which can concentrate metals in a small volume, prevent metals volatilization into the atmosphere, and meanwhile provide some additional benefits (e.g., bioenergy production or phyto-mining) (Vocciante et al. 2019). Thus, we consider applying the fast pyrolysis technology to treat the contaminated ornamental plants harvested from the field in future.
In conclusion, the results showed that these tested ornamental plants had higher tolerance to Cu–Pb combined pollution and could effectively accumulate the heavy metals. The Cu and Pb concentrations were higher in the roots of the ornamental plants than that in the shoots. The average concentration of the two heavy metals in the P. notoginseng, C. comosum, C. officinalis ornamental plants were 1402.1 mg/kg, 829.5 mg/kg and 1473.4 mg/kg for Cu and 2710.4 mg/kg, 4250.3 mg/kg and 4303.6 mg/kg for Pb, respectively. Although the three ornamental plants being of the accumulation and tolerance to Cu–Pb were demonstrated through the hydroponic-culture method in this study, the usage of these plants for the remediation of heavy metal contaminated soil needs to be further verified by field experiments.
References
Cameselle C (2015) Electrokinetic remediation and other physico-chemical remediation techniques for in situ treatment of soil from contaminated nuclear and NORM sites. Environ Remediat Restor Contam Nucl Norm Sites. https://doi.org/10.1016/b978-1-78242-231-0.00008-9
Dahmani-Muller H, Van OF, Gélie B, Balabane M (2000) Strategies of heavy metal uptake by three plant species growing near a metal smelter. Environ Pollut 109:231–238. https://doi.org/10.1016/S0269-7491(99)00262-6
Datko-Williams L, Wilkie A, Richmond-Bryant J (2014) Analysis of U.S. soil lead (Pb) studies from 1970 to 2012. Sci Total Environ 468–469:854–863. doi:https://doi.org/10.1016/j.scitotenv.2013.08.089
Doumett S, Fibbi D, Azzarello E, Mancuso S, Mugnai S, Petruzzelli G, Del Bubba M (2010) Influence of the application renewal of glutamate and tartrate on Cd, Cu, Pb and Zn distribution between contaminated soil and paulownia tomentosain a pilot-scale assisted phytoremediation study. Int J Phytorem 13:1–17.https://doi.org/10.1080/15226510903567455
Doumett S, Lamperi L, Checchini L, Azzarello E, Mugnai S, Mancuso S, Petruzzelli G, Del Bubba M (2008) Heavy metal distribution between contaminated soil and Paulownia tomentosa, in a pilot-scale assisted phytoremediation study: influence of different complexing agents. Chemosphere 72:1481–1490. https://doi.org/10.1016/j.chemosphere.2008.04.083
Gomes HI (2012) Phytoremediation for bioenergy: challenges and opportunities. Environ Technol Rev 1:59–66. https://doi.org/10.1080/09593330.2012.696715
Lan J (2004) Application status of phytoremediation technology in pollution control. Geol Hazards Environ Prot 15:46–51
Liu JN, Zhou QX, Sun T, Ma LQ, Wang S (2008) Growth responses of three ornamental plants to cd and cd–pb stress and their metal accumulation characteristics. J Hazard Mater 151:261–267. https://doi.org/10.1016/j.jhazmat.2007.08.016
Li A, Lin R, Lin C, He B, Zheng T, Lu L, Cao Y (2016) An environment-friendly and multi-functional absorbent from chitosan for organic pollutants and heavy metal ion. Carbohydr Polym 148:272–280. https://doi.org/10.1016/j.carbpol.2016.04.070
Li C, Shao Z, Wang Y, Zhang J (2010) Enrichment characteristics of Pb by several kinds of ornamental plants. J Northeast For Univ 39:49–51
Li Z, Ma Z, Kuijp T, Yuan Z, Huang L (2014) A review of soil heavy metal pollution from mines in China: pollution and health risk assessment. Sci Total Environ 468–469C:843–853. https://doi.org/10.1016/j.scitotenv.2013.08.090
Mahar A, Wang P, Ali A, Awasthi M, Lahori A, Wang Q, Li R, Zhang Z (2016) Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: a review. Ecotoxicol Environ Saf 126:111–121. doi:https://doi.org/10.1016/j.ecoenv.2015.12.023
Moreno N, Querol X, Alastuey A, Garcia-Sánchez A, Soler A, Ayora C (2006) Immobilization of heavy metals in polluted soils by the addition of zeolitic material synthesized from coal fly ash. Chemosphere 62:171–180. https://doi.org/10.1016/j.chemosphere.2005.05.029
Pereira BFF, De-Abreu CA, Herpin U, De-Abreu MF, Berton RS (2010) Phytoremediation of lead by jack beans on a rhodic hap-ludox amended with EDTA. Sci Agric 67:308–318. https://doi.org/10.1590/S0103-90162010000300009
Rajkumar M, Majeti P, Freitas H, Ae N (2009) Biotechnological applications of serpentine soil bacteria for phytoremediation of trace metals. Crit Rev Biotechnol 29:120–130. https://doi.org/10.1080/07388550902913772
Song Y, Marschner P, Li L, Bao X, Sun J, Zhang F (2007) Community composition of ammonia-oxidizing bacteria in the rhizosphere of intercropped wheat (triticum aestivuml.), maize (zea maysl.), and faba bean (vicia fabal.). Biol Fertil Soils 44:307–314. https://doi.org/10.1007/s00374-007-0205-y
Sun J, Gao J, Xu J, Xu M, Jiang R (2007a) Reviews on early warning of field heavy metal pollutions with molecular microbial ecological methods. Plant Nutr Fertili Sci 13:338–343
Sun Y, Zhou Q, Wei S, Ren L (2007b) Growth responses of the newly-discovered Cd-hyperaccumulator Rorippa globosa and its accumulation characteristics of Cd and As under joint stress of Cd and As. Front Environ Sci Eng 1:107–113. https://doi.org/10.1007/s11783-007-0020-6
Tao J, Wang Y, Dai J (2011) Accumulation and tolerance of Zinc in ornamental plant Chlorophytum comosum. Appl Mech Mater 66–68:524–527. doi:https://doi.org/10.4028/www.scientific.net/AMM.66-68.524
Tilman D, Reich P, Knops J (2006) Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature 441:629–632. doi:https://doi.org/10.1038/nature04742
Visoottiviseth P, Francesconi K, Sridokchan W (2002) The potential of Thai indigenous plant species for the phytoremediation of arsenic contaminated land. Environ Pollut 118:0–461. doi:https://doi.org/10.1016/s0269-7491(01)00293-7
Vocciante M, Caretta A, Bua L, Bagatin R, Franchi E, Petruzzelli G, Ferro S (2019) Enhancements in phytoremediation technology: Environmental assessment including different options of biomass disposal and comparison with a consolidated approach. J Environ Manage 237:560–568. doi:https://doi.org/10.1016/j.jenvman.2019.02.104
Wang XF (2005) Resource potential analysis of ornamentals applied in con-taminated soil remediation, A dissertation in Graduate School of Chinese Academy of Sciences, Beijing
Wei B, Yang L (2010) A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem J 94:99–107. https://doi.org/10.1016/j.microc.2009.09.014
Wei S, Zhou Q, Wang X (2003) Characteristics of 18 species of weed hyperaccumulating heavy metals in contaminated soils. J Basic Sci Eng 11:152–160 (in Chinese)
Wilcke W, Müller S, Kanchanakool N, Zech W (1998) Urban soil contamination in bangkok: heavy metal and aluminium partitioning in topsoils. Geoderma 86:211–228. https://doi.org/10.1016/S0016-7061(98)00045-7
Yan L, Li C, Zhang J, Moodley O, Liu S, Lan C, Gao Q, Zhang W (2017) Enhanced phytoextraction of lead from Artificially ontaminated soil by mirabilis jalapa with chelating Agents. Bull Environ Contam Toxicol 99:208–212. https://doi.org/10.1007/s00128-017-2127-1
Wang Y, Tao J, Dai J (2011) Lead tolerance and detoxification mechanism of Chlorophytum comosum. Afr J Biotechnol 10:14516–14521. https://doi.org/10.5897/ajb11.1496
Zaidi M, Asrar A, Mansoor A, Farooqui M (2005) The heavy metal concentration along roadside trees of quetta and its effects on public health. J Appl Sci 5:708–711. https://doi.org/10.3923/jas.2005.708.711
Zhao X, Zheng W, Dong D, Jiao L (2013) Temperature effect on fluorescence of PtOEP embedded in sol–gel membrane used in oxygen sensor. Optik-Int J Light Electron Opt 124:6799–6802. https://doi.org/10.1016/j.ijleo.2013.05.096
Acknowledgements
The authors acknowledge the National Natural Science Foundation of China (Grant No. 41471196) and National Training Programs of Innovation and Entrepreneurship for Undergraduates (Grant No. 201610193013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shao, Z., Lu, W., Nasar, J. et al. Growth Responses and Accumulation Characteristics of Three Ornamentals Under Copper and Lead Contamination in a Hydroponic-Culture Experiment. Bull Environ Contam Toxicol 103, 854–859 (2019). https://doi.org/10.1007/s00128-019-02724-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-019-02724-9