Abstract
Environmental contamination with neonicotinoid insecticides represents an issue of wide concern due to their negative effects on pollinators. The goal of this work was to evaluate the potential use of biomixtures employed in biopurification systems (BPS) to remove two neonicotinoid pesticides, imidacloprid and thiamethoxam, from wastewater of agricultural origin. The removal was assayed by quantification of the parent compounds and the detection of putative transformation products of imidacloprid by means of LC-MS/MS, and mineralization of radiolabeled imidacloprid. Two biomixtures (B1, B2) were prepared using coconut fiber, compost and two soils pre-exposed to imidacloprid (volumetric composition 50:25:25). After spiking of neonicotinoids and 228 days of treatment, the removal ranged from 22.3%–30.3% and 38.6%–43.7% for imidacloprid and thiamethoxam, respectively. Transformation products imidacloprid-urea, desnitro-imidacloprid and desnitro-olefin-imidacloprid were detected in both biomixtures. The mineralization of 14C-imidacloprid revealed DT50 (mineralization half-lives) values of 3466 and 7702 days in the biomixtures B1 and B2, respectively, markedly lower than those in the soil used in their preparation (8667 and 9902 days, respectively). As demonstrated by these findings, the high persistence of these compounds in the BPS suggests that additional biological (or physicochemical) approaches should be explored in order to decrease the impact of neonicotinoid-containing wastewater of agricultural origin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Environmental contamination with pesticides is an undesired consequence of agricultural activities. Despite the development of new insecticide molecules, neonicotinoids have become the most widely used insecticides on the global market, with registrations in more than 120 countries since their introduction in the early 1990s (Casida and Durkin 2013; Simon-Delso et al. 2015; Tomizawa and Casida 2011). This type of pesticides is used to control insect pests in agricultural, commercial, residential, and veterinary settings, with activity attributed to the activation of post-synaptic nicotinic acetylcholine receptors (nAChR) in insects (Casida and Durkin 2013; Jeschke et al. 2010).
The specificity of neonicotinoids to insects, and their poor capacity of penetration in the mammalian blood–brain barrier, contributes to human safety associated with commercial uses (Tomizawa and Casida 2001, 2005); nonetheless, important risk exists for non-target insects exposed to these compounds. Several neonicotinoid compounds have shown high toxicity to bees in very small quantities, because they can be translocated into pollen and nectar (Cresswell 2011; Iwasa et al. 2004). Exposure to sublethal doses of these pesticides is known to play an important role in the decline of honey bee populations and colony losses, caused by the reduced learning, foraging and homing abilities (Fairbrother et al. 2014; Krupke et al. 2012; Yang et al. 2008). The concern for pollinators in the European Union has led to an interruption in the use of three neonicotinoids (clothianidin, thiamethoxam and imidacloprid), starting in 2013 for use on crops that are attractive to bees (corn, canola, sunflower and cotton) (Regulation No 485/2013). Other countries are now reviewing the registration status and guidelines for the use of neonicotinoid insecticides (Hussain et al. 2016).
Neonicotinoids are also persistent; imidacloprid and thiamethoxam offer long-term crop protection activity, with long DT50 values in aerobic soil conditions of around 3000 days (imidacloprid) and 353 days (thiamethoxam) (Goulson 2013). Moreover, imidacloprid and thiamethoxam are able to accumulate in the food chain (Gibbons et al. 2015; Morrissey et al. 2015) and contaminate ground and surface waters (Starner and Goh 2012); for these reasons, they also represent a significant risk to the biota these ecosystems support. To reduce ecological problems related to neonicotinoid residues, research has been focused on their degradation by physicochemical approaches such as adsorption on granular activated carbon, direct photolysis, incineration, and advanced oxidation process (AOP) such as heterogeneous photocatalysis by TiO2, and homogeneous photocatalysis by photo-Fenton, which have demonstrated promising results (Ahmed et al. 2011; Andreozzi et al. 1999; Kitsiou et al. 2009; Malato et al. 2001). However, these techniques are usually not a competitive option due to high cost and the production of several toxic and persistent subproducts (Ahmed et al. 2011). In contrast, and likely due to their high persistence in soil, few reports describe biological degradation of neonicotinoids (Bonmatin et al. 2005; Goulson 2013), and as of this moment, the mineralization of imidacloprid has not been demonstrated for a single microorganism. Therefore, the search for biological approaches for the elimination of neonicotinoids is a topic of interest.
Biopurification systems (BPS) represent a biotechnological tool used to minimize point-source contamination with pesticides from on-farm practices such as handling of formulations (during preparation of application solutions), and disposal of residues from field application (Castillo et al. 2008). The active core of a BPS is the biomixture, a matrix where pesticide-containing wastewaters are disposed for fast degradation (Karanasios et al. 2012). The biomixture contains a lignocellulosic substrate, a humic rich component and soil at a typical volumetric proportion of 50:25:25 (Karanasios et al. 2013). The composition of the biomixtures depends on the availability of agricultural wastes, for this reason their components should be adapted to local availability; in particular, the use of soil pre-exposed to the target pesticide is desired to promote the establishment of microbial degrading communities. In the case of neonicotinoids, scarce reports describe their disposal in BPS, usually with unsuccessful results (Díaz et al. 2016; Huete-Soto et al. 2017).
The aim of this work was to evaluate the removal of imidacloprid and thiamethoxam in two different biomixtures prepared with tropical agroindustrial wastes and pre-exposed soils. In order to determine the ability of the matrices to completely oxidize imidacloprid, the mineralization of 14C-imidacloprid was assayed in the biomixtures and compared with its behavior in soil.
Materials and Methods
Analytical standards imidacloprid [(E)-1-(6-chloro-3-pyridylmethyl)-N-nitroimidazolidin-2-ylideneamine] (99.5% purity), thiamethoxam [(EZ)-3-(2-chloro-1,3-thiazol-5-ylmethyl)-5-methyl-1,3,5-oxadiazinan-4-ylidene(nitro)amine] (99.5%), and 6-chloronicotinic acid (6-chloropyridine-3-carboxylic acid) (99.2%) were obtained from Chem Service Inc. (West Chester, Pennsylvania, USA). Radiolabeled imidacloprid, (imidazolinona-2-14C-imidacloprid) (4.312 MBq mg−1; radiochemical purity 100%; chemical purity 100%) was obtained from Izotop (Institute of Isotopes Co., Budapest, Hungary). Commercial imidacloprid (Manager®, 35% w/v) and thiamethoxam (Engeo®, 24.7% w/v) were purchased from a local market. Carbofuran-d3 (surrogate standard, 98.0%) and linuron-d6 (internal standard, 98.5%) were purchased from Dr. Ehrenstorfer. Acetonitrile and methanol of HPLC grade, formic acid (purity 98%–100%), glacial acetic acid (purity ≥ 99.7%), and potassium hydroxide analytical grade were obtained from Merck (Darmstadt, Germany). Ultima Gold cocktail Liquid Scintillation Counting was purchased from Perkin Elmer (Waltham, Massachusetts, USA).
Soil was collected from the upper soil layer (0–20 cm) of two different watermelon fields with history of imidacloprid application (soil S1 and S2), in San Mateo, Alajuela, Costa Rica, and then air-dried and sieved (2 mm). Garden compost (used as the humic rich component) was collected from a composting station located at Universidad de Costa Rica and sieved (2 mm); coconut fiber (lignocellulosic substrate) was acquired at a local market. Biomixtures were prepared by mixing coconut fiber, compost and pre-exposed soil at a 50:25:25 volumetric ratio (Castillo et al. 2008; Karanasios et al. 2013); two matrices were prepared, each containing soil from one of the two fields (biomixtures B1 and B2, containing soil S1 or S2, respectively). The biomixtures were moistened to approximately 75% of maximum water-holding capacity and stored (aged) at 25°C during 1 month prior to use.
Removal assays were prepared by weighing 5 g of the biomixture into 12 polypropylene tubes (50 mL); the procedure was repeated for each biomixture. Each tube was spiked with commercial imidacloprid (7 mg kg−1) and thiamethoxam (5 mg kg−1), manually homogenized and incubated in the dark at (25 ± 1)°C during 228 days. The concentration of spiking was selected considering the recommended application indications in the pesticide formulations, the volume of wastewater usually discarded on the biomixture, and the mass of the biomixture in a cylindrical container (200 L). Water content losses were frequently adjusted according to the determination of the weight of each system. Pesticide concentrations were determined by sacrificing triplicate systems at times 0, 28, 192 and 228 days for analysis. Extraction of imidacloprid, thiamethoxam and transformation products from the biomixtures was carried out following a method described by Ruiz-Hidalgo et al. (2014). Carbofuran-d3 and linuron-d6 were added as surrogate and internal standard, respectively. Analyses were performed by LC–MS/MS using ultra high performance liquid chromatography (UPLC-1290 Infinity LC, Agilent Technologies, CA) coupled to a triple quadrupole mass spectrometer (model 6460). Chromatographic separation was done at 40 °C by injecting 6 µL samples in a Poroshell 120 EC-C18 column (100 mm × 2.1 mm i.d., particle size 2.7 µm), and using acidified water (formic acid 0.1% v/v, A) and acidified methanol (formic acid 0.1% v/v, B) as mobile phases. The mobile phase flow was 0.3 mL min−1 at the following conditions: 30% B for 3 min, followed by a 15 min linear gradient to 100% B, 4 min at 100% B and 0.1 min gradient back to 30% B, followed by 4 min at initial conditions. Selected transitions, limits of detection (LOD) and limits of quantification (LOQ) for imidacloprid, thiamethoxam and the transformation product 6-chloronicotinic acid are shown in Table 1; recoveries were: imidacloprid 96%; thiamethoxam 103%. Conditions of the mass spectrometry detector are described in Chin-Pampillo et al. (2015b). Detection of transformation products from imidacloprid (olefin-imidacloprid; 5-hydroxy-imidacloprid; imidacloprid urea; desnitro-imidacloprid HCl; desnitro-olefin-imidacloprid) was done using the ion transitions reported by Kamel (2010).
The mineralization of 14C-imidacloprid was determined through 14CO2 production in biometer flasks containing 14CO2 traps with 10 mL KOH (0.1 M) (Ruiz-Hidalgo et al. 2014). Fifty grams of either pre-exposed soil or biomixture were weighed into each biometer flask and spiked with commercial imidacloprid (50 mg kg−1) and 14C-imidacloprid (4.312 MBq mg−1); pre-exposed soil alone was used as a control to compare the mineralization in biomixtures. The systems were incubated in the dark at 25 ± 1°C for a period of 203 days. The KOH solution in the flasks was withdrawn at selected times and replaced with the same amount of fresh solution. Activity of 14C from the 14CO2 produced due to 14C-imidacloprid mineralization was analyzed in the KOH samples: scintillant liquid (8 mL) was added to 2 mL aliquots from samples and the 14C activity from the trapped 14CO2 was measured using a liquid scintillation counter (LS6000SC, Beckman Instruments Inc., USA). The total cumulative 14CO2 activity evolved from the pesticide and the initially added activity of 14C-imidacloprid were used to calculate the percentage of mineralized 14C-pesticide. Pesticide mineralization was fitted according to a first order model.
Results and Discussion
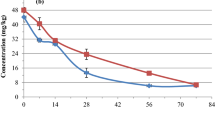
The removal profile of the neonicotinoid insecticides in the biomixtures is shown in Fig. 1. The biomixture B1 showed higher efficiency after 228 days of treatment, removing 30.3% imidacloprid and 43.7% thiamethoxam, compared to the biomixture B2, in which the elimination was 22.3% and 38.6% for imidacloprid and thiamethoxam, respectively. It is remarkable that most of the elimination took place in the first month of operation, time after which the removal became quite slow (as particularly observed for the biomixture B2). This can be partly explained due to the aging of the insecticides in the biomixture, a reason used to describe the difficult process of biodegradation of aged imidacloprid in soil (Anhalt et al. 2007), and to the sorption of imidacloprid to lignin (Díaz et al. 2016), which represents an important fraction of the biomixture. Moreover, the formation of pseudo-anaerobic microenvironments within the biomixture may have exerted a negative influence in the late elimination of the insecticides, as aerobic transformation of pollutants tends to be faster in most cases, except for multi-halogenated compounds (Bunge and Lechner 2009; Haritash and Kaushik 2009).
The efficiency of BPS for the removal of pesticides relies on biomixture composition; soil, which should be preferentially pre-exposed to the target pesticide provides most of the degrading microbiota of the system (Chin-Pampillo et al. 2015a); in addition, the lignocellulosic substrate (coconut fiber in this case), promotes the growth and activity of lignocellulosic fungi, which exhibit the capacity to transform diverse organic pollutants (Asgher et al. 2008), and consequently the release of additional C-sources for other microbial communities. Therefore, the synergic effect of these populations theoretically translates into faster pesticide removal than using the soil alone. In a work by Díaz et al. (2016), biomixtures using vermicompost were not able to significantly remove imidacloprid (< 20% after 30 days in every case), even using bioaugmentation of the matrices with autochthonous microorganisms. Another study failed to demonstrate neonicotinoid elimination (imidacloprid and thiamethoxam) in compost-based biomixtures (Huete-Soto et al. 2017), nonetheless, that work used a matrix with a different composition and lasted only 120 days, in contrast to the 228 days in this work; moreover, the biomixture employed in those reports did not use soil pre-exposed to the neonicotinoids, a highly critical aspect to consider in order to attain an adapted degrading population in the biomixture (Sniegowski et al. 2011; Sniegowski and Springael 2015). Despite achieving some removal of both compounds, the use of pre-exposed soil (to imidacloprid in this case), did not warrant a complete or fast removal. Interestingly, the efficiency showed in biomixtures did not necessarily improve natural attenuation described in soil, in which DT50 values ranged from 28 to 1230 days and 7–353 days for imidacloprid and thiamethoxam, respectively (Goulson 2013), depending on the soil. Nonetheless, taking into account the highest DT50 values reported, it is clear that these compounds tend to be extremely persistent in the environment.
Several transformation products from imidacloprid were analyzed in the biomixture samples. Remarkably, neither imidacloprid-olefin, a relevant metabolite found in soil, nor 6-chloronicotinic acid, also described in soil (Lewis et al. 2016), were detected during the treatment process. 6-Chloronicotinic acid has been described as the major metabolite produced in the transformation of imidacloprid by a degrading strain of Mycobacterium in liquid media (Kandil et al. 2015). Similarly, 5-hydroxy-imidacloprid and olefin-imidacloprid, two of the most common metabolites described by bacterial transformation were not detected; in particular, the olefin metabolite is considered as a much more toxic compound to insects than the parent imidacloprid (Dai et al. 2006), reason why its absence from the biomixtures is highly desirable. Instead, three transformation products were detected as shown in Fig. 2, imidacloprid-urea, desnitro-imidacloprid (imidacloprid-guanidine) and desnitro-olefin-imidacloprid. Imidacloprid-urea has been reported in soil (Anhalt et al. 2008), and in the transformation by strains of Leifsonia sp. and Enterobacter sp. (Sharma et al. 2014). Desnitro-imidacloprid has been reported as the main metabolite found in tomato leaves developed in soil contaminated with imidacloprid (Alsayeda et al. 2008), in imidacloprid transformation in soil (Liu et al. 2011), and also in the transformation pathway of imidacloprid by degrading strains of Klebsiella pneumonia (Phugare et al. 2013) and Leifsonia sp. (Anhalt et al. 2007). Kandil et al. (2015) reported both compounds as minor metabolites from transformation by Mycobacterium. Two routes are known to produce desnitro-imidacloprid in soil: first, reduction of the nitro group to form the nitroso metabolite and subsequent loss of this group; and second, the direct denitration of the nitro group (Liu et al. 2011). Interestingly, this product is considered as a detoxification metabolite for insects (Nauen et al. 1998), and might suggest in the present case, that detoxification is taking place in the biomixtures. The oxidation of desnitro-imidacloprid is known to produce imidacloprid-urea in soil (Liu et al. 2011); moreover, this reaction has been also suggested in the transformation by a strain of Pseudomonas sp. in liquid medium (Pandey et al. 2009). Analogous desnitro- and urea- metabolites have been described during the transformation of thiamethoxam (Hussain et al. 2016; Pandey et al. 2009).
Time-course detection of transformation products from imidacloprid in the biomixtures B1 (filled circles) and B2 (open circles). Results are expressed as relative areas with respect to the largest area detected per metabolite. Transformation products: imidacloprid-urea (a), desnitro-imidacloprid (b), and desnitro-olefin-imidacloprid (c). Values plotted are means ± SD for triplicate systems
The results of 14C-imidacloprid mineralization over a period of 203 days are shown in Fig. 3. Overall, mineralization values were low, reaching 1.34% (S1), 1.35% (S2), 1.81% (B2) and a maximum of 2.56% in the biomixture B1. Estimation of mineralization DT50 revealed values of 8664 and 9902 days in soils. The mineralization was in both cases higher in the biomixture than in the soils used in their preparation, resulting in DT50 values of 3466 days for B1 and 7702 days in B2. Both mineralization and removal of imidacloprid were achieved at higher extent in biomixture B1; accordingly, soil S1 exhibited a lower mineralization DT50 value than S2. Mineralization DT50 values are expected to be longer than removal half-lives, given that the former process involves a more complex series of reactions to achieve the complete oxidation of the pesticide to 14CO2, while the removal only requires a minimal transformation of the parent compound to detect a decrese in its concentration.
Mineralization of 14C-imidacloprid represented as % 14CO2 evolved from the biomixture (or soil) over a period of 203 days. Biomixtures B1 (filled inverted triangles) and B2 (open diamonds); soil S1 (filled circles) and S2 (filled squares).Values plotted are means for triplicate systems; SD were < 10% in every case
Reports of imidacloprid mineralization in biological matrices are scarce in specialized literature; in a previous work by Anhalt et al. (2007), the authors reported no mineralization of 14C-imidacloprid in liquid medium after 21 days by a bacterial strain capable to transform the insecticide into products such as desnitro-imidacloprid, imidacloprid-urea and several unidentified metabolites. On the other hand, Diaz et al. (2017) achieved mineralization values ranging from 7% to 10% after 90 days in soils amended with vermicompost (higher than non-amended soils); such finding suggests that the addition of vermicompost could be an interesting way to enhance the performance of biomixtures.
Summarizing, two biomixtures prepared with soil pre-exposed to imidacloprid were capable to remove 22.3%–30.3% imidacloprid and 38.6%–43.7% thiamethoxam after 228 days (Fig. 1). The most toxic transformation products from imidacloprid were not detected; instead, the metabolites imidacloprid-urea, desnitro-imidacloprid and desnitro-olefin-imidacloprid were detected in both biomixtures. Additional ecotoxicological test would provide a more accurate estimation of the detoxification in the matrix. Consistently, the most efficient biomixture in terms of removal and mineralization of neonicotinoids was the same. Although biomixtures proved to achieve faster mineralization than the respective soils, elimination in these system remains quite slow and the search for alternate biological approaches to treat neonicotinoid-containing wastewater is highly recommended.
References
Ahmed S, Rasul MG, Brown R, Hashib MA (2011) Influence of parameters on the heterogeneous photocatalytic degradation of pesticides and phenolic contaminants in wastewater: a short review. J Environ Manage 92:311–330
Alsayeda H, Pascal-Lorber S, Nallanthigal C, Debrauwer L, Laurent F (2008) Transfer of the insecticide [14C] imidacloprid from soil to tomato plants. Environ Chem Lett 6:229–234
Andreozzi R, Caprio V, Insola A, Marotta R (1999) Advanced oxidation processes (AOP) for water purification and recovery. Catal Today 53:51–59
Anhalt JC, Moorman TB, Koskinen WC (2007) Biodegradation of imidacloprid by an isolated soil microorganism. J Environ Sci Health B 42:509–514
Anhalt JC, Moorman TB, Koskinen WC (2008) Degradation and sorption of imidacloprid in dissimilar surface and subsurface soils. J Environ Sci Health B 43:207–213
Asgher M, Bhatti HN, Ashraf M, Legge RL (2008) Recent developments in biodegradation of industrial pollutants by white rot fungi and their enzyme system. Biodegradation 19:771–783
Bonmatin JM, Moineau I, Charvet R, Colin ME, Fleche C, Bengsch ER (2005) Behaviour of imidacloprid in fields. Toxicity for honey bees. In: Environmental chemistry. Springer, Berlin, pp 483–494
Bunge M, Lechner U (2009) Anaerobic reductive dehalogenation of polychlorinated dioxins. Appl Microbiol Biotechnol 84:429–444
Casida JE, Durkin KA (2013) Neuroactive insecticides: targets, selectivity, resistance, and secondary effects. Annu Rev Entomol 58:99–117
Castillo MDP, Torstensson L, Stenström J (2008) Biobeds for environmental protection from pesticide use: a review. J Agric Food Chem 56:6206–6219
Chin-Pampillo JS, Ruiz-Hidalgo K, Masís-Mora M, Carazo-Rojas E, Rodríguez-Rodríguez CE (2015a) Adaptation of biomixtures for carbofuran degradation in on-farm biopurification systems in tropical regions. Environ Sci Pollut Res 22:9839–9848
Chin-Pampillo JS, Ruiz-Hidalgo K, Masís-Mora M, Carazo-Rojas E, Rodríguez-Rodríguez CE (2015b) Design of an optimized biomixture for the degradation of carbofuran based on pesticide removal and toxicity reduction of the matrix. Environ Sci Pollut Res 22:19184–19193
Cresswell JE (2011) A meta-analysis of experiments testing the effects of a neonicotinoid insecticide (imidacloprid) on honey bees. Ecotoxicology 20:149–157
Dai YJ, Yuan S, Ge F, Chen T, Xu SC, Ni JP (2006) Microbial hydroxylation of imidacloprid for the synthesis of highly insecticidal olefin imidacloprid. Appl Microbiol Biotechnol 71:927–934
Diaz JMC, Delgado-Moreno L, Núñez R, Nogales R, Romero E (2016) Enhancing pesticide degradation using indigenous microorganisms isolated under high pesticide load in bioremediation systems with vermicomposts. Bioresour Technol 214:234–241
Diaz JMC, Martin-Laurent F, Beguet J, Nogales R, Romero E (2017) Fate and effect of imidacloprid on vermicompost-amended soils under dissimilar conditions: risk for soil functions, structure, and bacterial abundance. Sci Total Environ 579:1111–1119
Fairbrother A, Purdy J, Anderson T, Fell R (2014) Risks of neonicotinoid insecticides to honeybees. Environ Toxicol Chem 33:719–731
Gibbons D, Morrissey C, Mineau P (2015) A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environ Sci Pollut Res 22:103–118
Goulson D (2013) An overview of the environmental risks posed by neonicotinoid insecticides. J Appl Ecol 50:977–987
Haritash AK, Kaushik CP (2009) Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater 169:1–15
Huete-Soto A, Masís-Mora M, Lizano-Fallas V, Chin-Pampillo JS, Carazo-Rojas E, Rodríguez-Rodríguez CE (2017) Simultaneous removal of structurally different pesticides in a biomixture: detoxification and effect of oxytetracycline. Chemosphere 169:558–567
Hussain S, Hartley CJ, Shettigar M, Pandey G (2016) Bacterial biodegradation of neonicotinoid pesticides in soil and water systems. FEMS Microbiol Lett 363:1–13
Iwasa T, Motoyama N, Ambrose JT, Roe RM (2004) Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera. Crop Prot 23:371–378
Jeschke P, Nauen R, Schindler M, Elbert A (2010) Overview of the status and global strategy for neonicotinoids. J Agric Food Chem 59:2897–2908
Kamel A (2010) Refined methodology for the determination of neonicotinoid pesticides and their metabolites in honey bees and bee products by liquid chromatography – tandem mass spectrometry (LC-MS/MS). J Agric Food Chem 58:5926–5931
Kandil MM, Trigo C, Koskinen WC, Sadowsky MJ (2015) Isolation and characterization of a novel imidacloprid-degrading Mycobacterium sp. strain MK6 from an Egyptian soil. J Agric Food Chem 63:4721–4727
Karanasios E, Tsiropoulos NG, Karpouzas DG (2012) On-farm biopurification systems for the depuration of pesticide wastewaters: recent biotechnological advances and future perspectives. Biodegradation 23:787–802
Karanasios EC, Tsiropoulos NG, Karpouzas DG (2013) Quantitative and qualitative differences in the metabolism of pesticides in biobed substrates and soil. Chemosphere 93:20–28
Kitsiou V, Filippidis N, Mantzavinos D, Poulios I (2009) Heterogeneous and homogeneous photocatalytic degradation of the insecticide imidacloprid in aqueous solutions. Appl Catal B 86:27–35
Krupke CH, Hunt GJ, Eitzer BD, Andino G, Given K (2012) Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS ONE 7:e29268
Lewis KA, Tzilivakis J, Warner D, Green A (2016) An international database for pesticide risk assessments and management. Hum Ecol Risk Assess 22:1050–1064
Liu Z, Dai Y, Huang G, Gu Y, Ni J, Wei H, Yuan S (2011) Soil microbial degradation of neonicotinoid insecticides imidacloprid, acetamiprid, thiacloprid and imidaclothiz and its effect on the persistence of bioefficacy against horsebean aphid Aphis craccivora Koch after soil application. Pest Manag Sci 67:1245–1252
Malato S, Caceres J, Agüera A, Mezcua M, Hernando D, Vial J, Fernández-Alba AR (2001) Degradation of imidacloprid in water by photo-fenton and TiO2 photocatalysis at a solar pilot plant: a comparative study. Environ Sci Technol 35:4359–4366
Morrissey CA, Mineau P, Devries JH, Sanchez-Bayo F, Liess M, Cavallaro MC, Liber K (2015) Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: a review. Environ Int 74:291–303
Nauen R, Tietjen K, Wagner K, Elbert A (1998) Efficacy of plant metabolites of imidacloprid against Myzus persicae and Aphis gossypii (Homoptera: Aphididae). Pest Manag Sci 52:53–57
Pandey G, Dorrian SJ, Russell RJ, Oakeshott JG (2009) Biotransformation of the neonicotinoid insecticides imidacloprid and thiamethoxam by Pseudomonas sp. 1G. Biochem Biophys Res Commun 380:710–714
Phugare SS, Kalyani DC, Gaikwad YB, Jadhav JP (2013) Microbial degradation of imidacloprid and toxicological analysis of its biodegradation metabolites in silkworm (Bombyx mori). Chem Eng J 230:27–35
Regulation EU (2013) No 485/2013. Off J Eur Union 139:12–26
Ruiz-Hidalgo K, Chin-Pampillo JS, Masís-Mora M, Carazo E, Rodríguez-Rodríguez CE (2014) Degradation of carbofuran by Trametes versicolor in rice husk as a potential lignocellulosic substrate for biomixtures: from mineralization to toxicity reduction. Process Biochem 49:2266–2271
Sharma T, Rajor A, Toor AP (2014) Degradation of imidacloprid in liquid by Enterobacter sp. strain ATA1 using co-metabolism. Bioremediation J 18:227–235
Simon-Delso N, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Chagnon M, Downs C et al (2015) Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ Sci Pollut Res 22:5–34
Sniegowski K, Springael D (2015) Establishment of multiple pesticide biodegradation capacities from pesticide-primed materials in on-farm biopurification system microcosms treating complex pesticide-contaminated wastewater. Pest Manag Sci 71:986–995
Sniegowski K, Bers K, Van Goetem K, Ryckeboer J, Jaeken P, Spanoghe P, Springael D (2011) Improvement of pesticide mineralization in on-farm biopurification systems by bioaugmentation with pesticide-primed soil. FEMS Microbiol Ecol 76:64–73
Starner K, Goh KS (2012) Detections of the neonicotinoid insecticide imidacloprid in surface waters of three agricultural regions of California, USA, 2010–2011. Bull Environ Contam Toxicol 88:316–321
Tomizawa M, Casida JE (2001) Structure and diversity of insect nicotinic acetylcholine receptors. Pest Manag Sci 57:914–922
Tomizawa M, Casida JE (2005) Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu Rev Pharmacol Toxicol 45:247–268
Tomizawa M, Casida JE (2011) Neonicotinoid insecticides: highlights of a symposium on strategic molecular designs. J Agric Food Chem 59:2883–2886
Yang EC, Chuang YC, Chen YL, Chang LH (2008) Abnormal foraging behavior induced by sublethal dosage of imidacloprid in the honey bee (Hymenoptera: Apidae). J Econ Entomol 101:1743–1748
Acknowledgements
This work was supported by Vicerrectoría de Investigación, Universidad de Costa Rica (Project 802-B7-500).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodríguez-Castillo, G., Molina-Rodríguez, M., Pérez-Villanueva, M. et al. Removal of Two Neonicotinoid Insecticides and Mineralization of 14C-Imidacloprid in Biomixtures. Bull Environ Contam Toxicol 101, 137–143 (2018). https://doi.org/10.1007/s00128-018-2370-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-018-2370-0